Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy
Abstract
1. Introduction
2. Materials and Methods
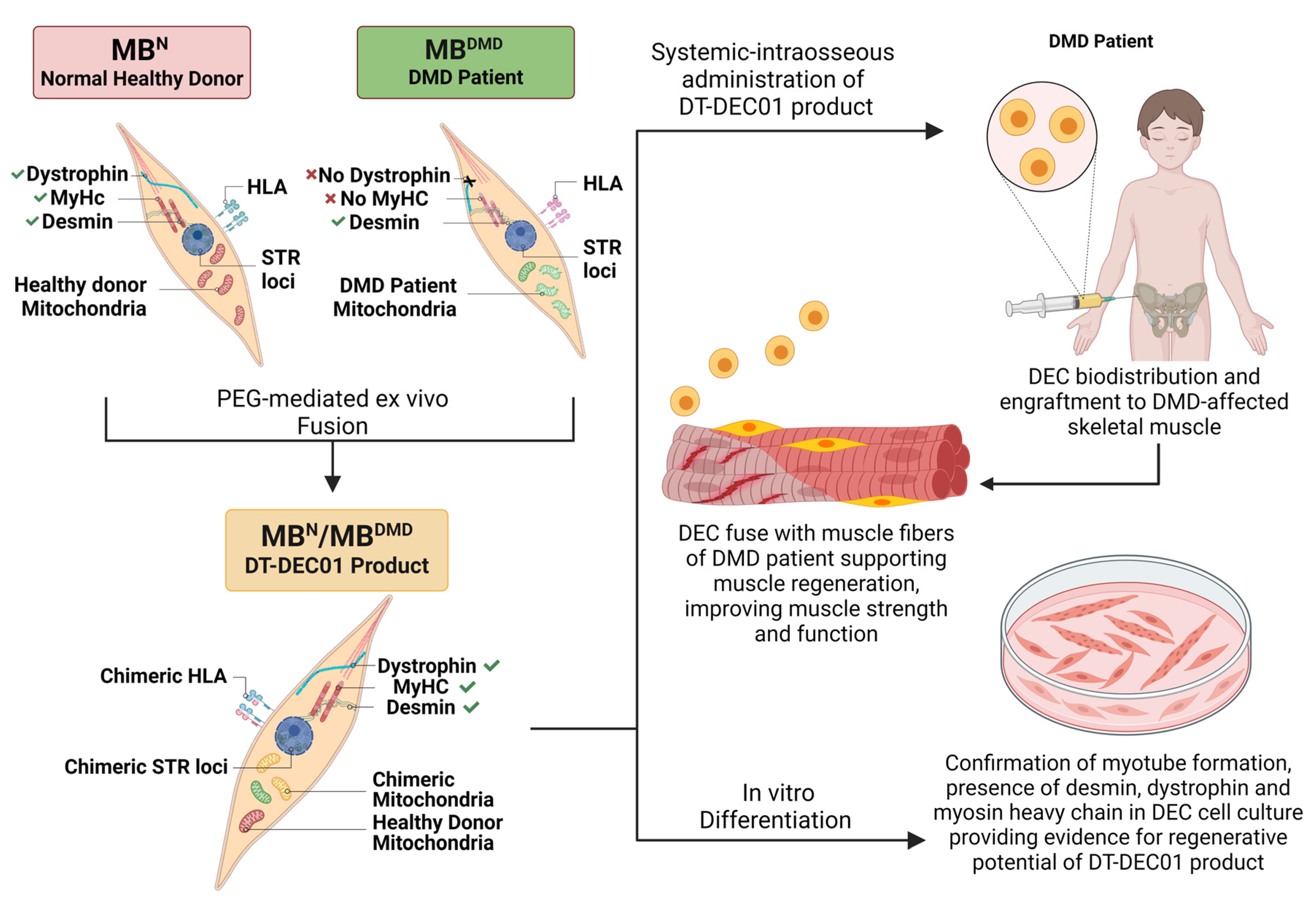
2.1. Creation of Human DT-DEC01 Product by PEG-Mediated Fusion of Myoblasts Derived from Normal, Healthy Donor and DMD Patient
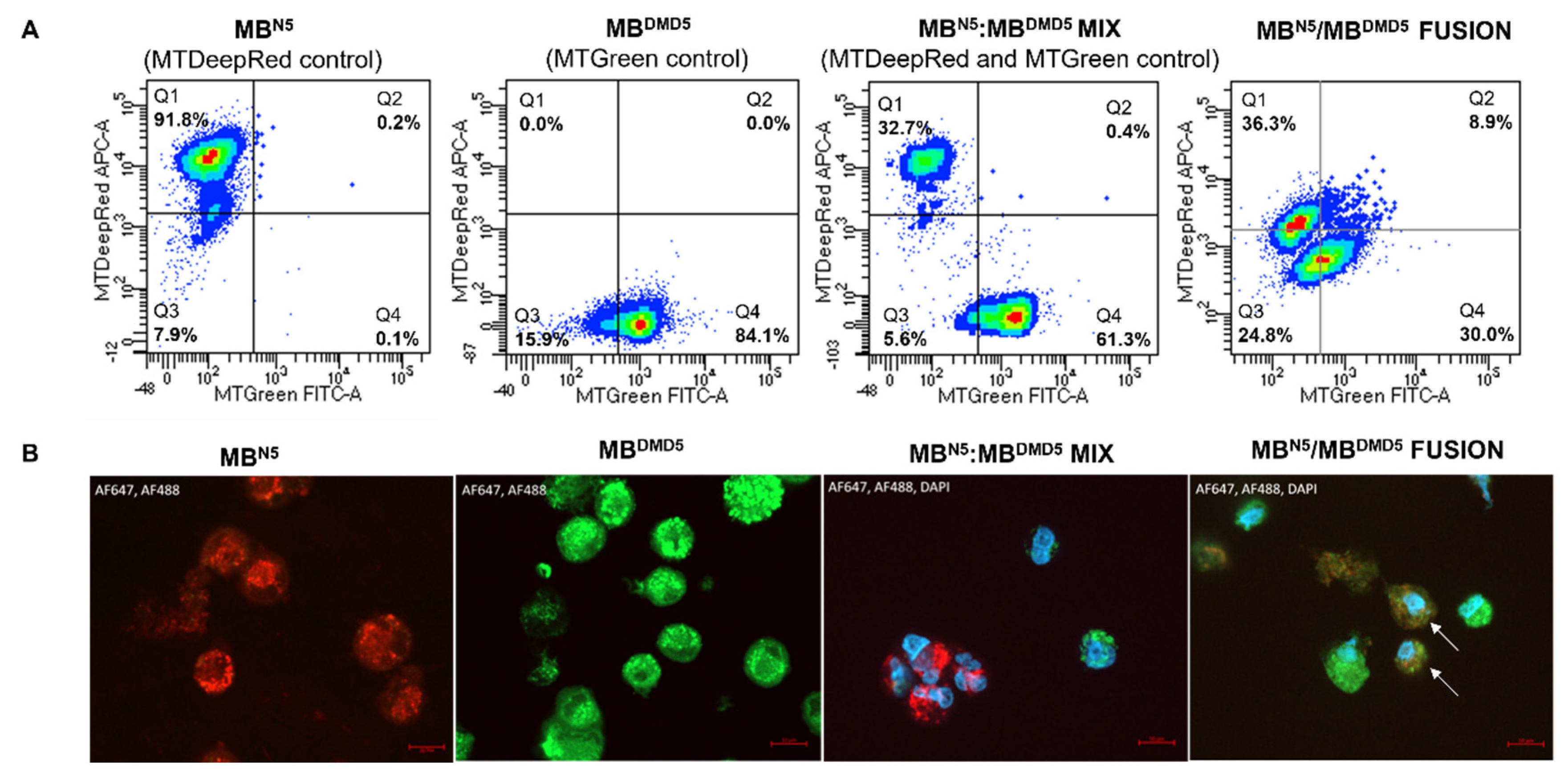
2.2. Assessment of Donor-Specific Chimerism by STR-PCR Analysis of Donor Specific STR Loci
2.3. Characterization of the Human DT-DEC01 Product In Vitro by Immunofluorescence Detection of Desmin, Dystrophin and Myosin Heavy Chain
2.4. Assessment of the Intercellular Mitochondrial Transfer and Mitochondrial Chimeric State in the Created DT-DEC01 Product
2.5. Characterization of the Human DT-DEC01 Product In Vitro by Detection of Myotube Formation
3. Results
3.1. Confirmation of Creation of Patient-Derived Chimeric (MBN/MBDMD) DT-DEC01 Product by PEG-Mediated Fusion of Myoblasts from Normal, Healthy Donor and DMD Patient
3.2. Confirmation of Donor-Specific Chimerism in Patient-Derived DT-DEC01 Product through Detection of the STR Loci Specific for the Normal Myoblast Donor
3.3. Confirmation of Myogenic Potential of the Patient-Derived DT-DEC01 Product through Expression of Desmin, Dystrophin and Myosin Heavy Chain
3.4. Confirmation of Transfer of Healthy Donor Mitochondria and Presence of Chimeric Mitochondria in the Patients-Derived DT-DEC01 Product
3.5. Confirmation of Myogenic Potential of the Patient-Derived DT-DEC01 Product by Detection of Myotube Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzur, A.Y.; Kinali, M.; Muntoni, F. Update on the Management of Duchenne Muscular Dystrophy. Arch. Dis. Child. 2008, 93, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Govoni, A.; D’Angelo, M.G.; Del Bo, R.; Ghezzi, S.; Sandra, G.; Turconi, A.C.; Sciacco, M.; Ciscato, P.; Bordoni, A.; et al. Genotype and Phenotype Characterization in a Large Dystrophinopathic Cohort with Extended Follow-Up. J. Neurol. 2011, 258, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Salgado, D.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; Dawkins, H.; Lamont, L.; Roy, A.J.; Chamova, T.; et al. The TREAT-NMD DMD Global Database: Analysis of More than 7,000 Duchenne Muscular Dystrophy Mutations. Hum. Mutat. 2015, 36, 395–402. [Google Scholar] [CrossRef]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; Aartsma-Rus, A.; Vieira, N.M.; Davies, K.E.; van Ommen, G.-J.B.; Kunkel, L.M. The Pathogenesis and Therapy of Muscular Dystrophies. Annu. Rev. Genom. Hum. Genet. 2015, 16, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; Davies, K.E. Regenerative Biomarkers for Duchenne Muscular Dystrophy. Neural Regen. Res. 2019, 14, 1317–1320. [Google Scholar] [CrossRef]
- Paramsothy, P.; Wang, Y.; Cai, B.; Conway, K.M.; Johnson, N.E.; Pandya, S.; Ciafaloni, E.; Mathews, K.D.; Romitti, P.A.; Howard, J.F.; et al. Selected Clinical and Demographic Factors and All-Cause Mortality among Individuals with Duchenne Muscular Dystrophy in the Muscular Dystrophy Surveillance, Tracking, and Research Network. Neuromuscul. Disord. 2022, 32, 468–476. [Google Scholar] [CrossRef]
- Lechner, A.; Herzig, J.J.; Kientsch, J.G.; Kohler, M.; Bloch, K.E.; Ulrich, S.; Schwarz, E.I. Cardiomyopathy as Cause of Death in Duchenne Muscular Dystrophy: A Longitudinal Observational Study. ERJ Open Res. 2023, 9, 00176–02023. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Neuromuscular, Rehabilitation, Endocrine, and Gastrointestinal and Nutritional Management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 2: Respiratory, Cardiac, Bone Health, and Orthopaedic Management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 3: Primary Care, Emergency Management, Psychosocial Care, and Transitions of Care across the Lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and Management of Duchenne Muscular Dystrophy, Part 1: Diagnosis, and Pharmacological and Psychosocial Management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Kumar, A.; West, N.A.; DiRienzo, A.G.; James, K.A.; Oleszek, J. Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) Trends with Corticosteroid Use in Males with Duchenne Muscular Dystrophy Born 1982–2001. J. Child. Neurol. 2015, 30, 21–26. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the Treatment of Duchenne Muscular Dystrophy. Cochrane Database Syst. Rev. 2016, 2016, CD003725. [Google Scholar] [CrossRef]
- Koeks, Z.; Bladen, C.L.; Salgado, D.; van Zwet, E.; Pogoryelova, O.; McMacken, G.; Monges, S.; Foncuberta, M.E.; Kekou, K.; Kosma, K.; et al. Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of 5345 Patients from the TREAT-NMD DMD Global Database. J. Neuromuscul. Dis. 2017, 4, 293–306. [Google Scholar] [CrossRef]
- Bönnemann, C.G.; Belluscio, B.A.; Braun, S.; Morris, C.; Singh, T.; Muntoni, F. Dystrophin Immunity after Gene Therapy for Duchenne’s Muscular Dystrophy. N. Engl. J. Med. 2023, 388, 2294–2296. [Google Scholar] [CrossRef]
- Mendell, J.R.; Sahenk, Z.; Lehman, K.J.; Lowes, L.P.; Reash, N.F.; Iammarino, M.A.; Alfano, L.N.; Lewis, S.; Church, K.; Shell, R.; et al. Long-Term Safety and Functional Outcomes of Delandistrogene Moxeparvovec Gene Therapy in Patients with Duchenne Muscular Dystrophy: A Phase 1/2a Nonrandomized Trial. Muscle Nerve 2024, 69, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.C.; Lee, Y.I.; Xie, J.; Gao, G.; Lin, B.L.; Hammers, D.W.; Sweeney, H.L. Potential Limitations of Microdystrophin Gene Therapy for Duchenne Muscular Dystrophy. JCI Insight 2024, 9, e165869. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, C.M.; Goedeker, N.L.; Aqul, A.A.; Butterfield, R.J.; Connolly, A.M.; Crystal, R.G.; Godwin, K.E.; Hor, K.N.; Mathews, K.D.; Proud, C.M.; et al. Management of Select Adverse Events Following Delandistrogene Moxeparvovec Gene Therapy for Patients With Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2024, 11, 687–699. [Google Scholar] [CrossRef]
- Philippidis, A. Pfizer Weighs Next Steps after DMD Therapy Linked to Boy’s Death Fails Phase III Trial. Hum. Gene Ther. 2024, 35, 413–415. [Google Scholar] [CrossRef]
- Pfizer. Pfizer Provides Update on Phase 3 Study of Investigational Gene Therapy for Ambulatory Boys with Duchenne Muscular Dystrophy. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provides-update-phase-3-study-investigational-gene (accessed on 26 July 2024).
- Viltolarsen (NS-065/NCNP-01) for the Treatment of Duchenne Muscular Dystrophy Preliminary Results of the Analysis of the Phase III Trial (RACER53 Study). Available online: https://www.nippon-shinyaku.co.jp/english/ir/ir_news/ns2024/ (accessed on 26 July 2024).
- Gussoni, E.; Blau, H.M.; Kunkel, L.M. The Fate of Individual Myoblasts after Transplantation into Muscles of DMD Patients. Nat. Med. 1997, 3, 970–977. [Google Scholar] [CrossRef]
- Skuk, D.; Goulet, M.; Roy, B.; Piette, V.; Côté, C.H.; Chapdelaine, P.; Hogrel, J.-Y.; Paradis, M.; Bouchard, J.-P.; Sylvain, M.; et al. First Test of a “High-Density Injection” Protocol for Myogenic Cell Transplantation throughout Large Volumes of Muscles in a Duchenne Muscular Dystrophy Patient: Eighteen Months Follow-Up. Neuromuscul. Disord. 2007, 17, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Collins-Hooper, H.; Patel, K. The Origin, Molecular Regulation and Therapeutic Potential of Myogenic Stem Cell Populations. J. Anat. 2009, 215, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Skuk, D.; Tremblay, J.P. Clarifying Misconceptions About Myoblast Transplantation in Myology. Mol. Ther. 2014, 22, 897–898. [Google Scholar] [CrossRef]
- Skuk, D.; Tremblay, J.P. Intramuscular Cell Transplantation as a Potential Treatment of Myopathies: Clinical and Preclinical Relevant Data. Expert Opin. Biol. Ther. 2011, 11, 359–374. [Google Scholar] [CrossRef]
- Vilquin, J.-T.; Catelain, C.; Vauchez, K. Cell Therapy for Muscular Dystrophies: Advances and Challenges. Curr. Opin. Organ. Transplant. 2011, 16, 640–649. [Google Scholar] [CrossRef]
- Sampath, S.C.; Sampath, S.C.; Millay, D.P. Myoblast Fusion Confusion: The Resolution Begins. Skelet. Muscle 2018, 8, 3. [Google Scholar] [CrossRef]
- Millay, D.P. Regulation of the Myoblast Fusion Reaction for Muscle Development, Regeneration, and Adaptations. Exp. Cell Res. 2022, 415, 113134. [Google Scholar] [CrossRef]
- Siemionow, M.; Cwykiel, J.; Heydemann, A.; Garcia-Martinez, J.; Siemionow, K.; Szilagyi, E. Creation of Dystrophin Expressing Chimeric Cells of Myoblast Origin as a Novel Stem Cell Based Therapy for Duchenne Muscular Dystrophy. Stem Cell Rev. Rep. 2018, 14, 189–199. [Google Scholar] [CrossRef]
- Siemionow, M.; Langa, P.; Brodowska, S.; Kozlowska, K.; Zalants, K.; Budzynska, K.; Heydemann, A. Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy. Stem Cell Rev. Rep. 2022, 18, 2872–2892. [Google Scholar] [CrossRef]
- Siemionow, M.; Brodowska, S.; Langa, P.; Zalants, K.; Kozlowska, K.; Grau-Kazmierczak, W.; Heydemann, A. Long-Term Biodistribution and Safety of Human Dystrophin Expressing Chimeric Cell Therapy After Systemic-Intraosseous Administration to Duchenne Muscular Dystrophy Model. Arch. Immunol. Ther. Exp. 2022, 70, 20. [Google Scholar] [CrossRef]
- Siemionow, M.; Budzynska, K.; Zalants, K.; Langa, P.; Brodowska, S.; Siemionow, K.; Heydemann, A. Amelioration of Morphological Pathology in Cardiac, Respiratory, and Skeletal Muscles Following Intraosseous Administration of Human Dystrophin Expressing Chimeric (DEC) Cells in Duchenne Muscular Dystrophy Model. Biomedicines 2024, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A.; Bieganski, G.; Wachowiak, J.; Czarnota, J.; Niezgoda, A.; Siemionow, K.; Ziemiecka, A.; Sikorska, M.H.; Bozyk, K.; Tullius, S.G.; et al. Dystrophin Expressing Chimeric (DEC) Cell Therapy for Duchenne Muscular Dystrophy: A First-in-Human Study with Minimum 6 Months Follow-Up. Stem Cell Rev. Rep. 2023, 19, 1340–1359. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, A.; Biegański, G.; Wachowiak, J.; Czarnota, J.; Siemionow, K.; Heydemann, A.; Ziemiecka, A.; Sikorska, M.H.; Bożyk, K.; Siemionow, M. Assessment of Motor Unit Potentials Duration as the Biomarker of DT-DEC01 Cell Therapy Efficacy in Duchenne Muscular Dystrophy Patients up to 12 Months After Systemic-Intraosseous Administration. Arch. Immunol. Ther. Exp. 2023, 71, 24. [Google Scholar] [CrossRef]
- Siemionow, M.; Bocian, K.; Bozyk, K.T.; Ziemiecka, A.; Siemionow, K. Chimeric Cell Therapy Transfers Healthy Donor Mitochondria in Duchenne Muscular Dystrophy. Stem Cell Rev. Rep. 2024. [Google Scholar] [CrossRef]
- Markati, T.; Oskoui, M.; Farrar, M.A.; Duong, T.; Goemans, N.; Servais, L. Emerging Therapies for Duchenne Muscular Dystrophy. Lancet Neurol. 2022, 21, 814–829. [Google Scholar] [CrossRef]
- Bez Batti Angulski, A.; Hosny, N.; Cohen, H.; Martin, A.A.; Hahn, D.; Bauer, J.; Metzger, J.M. Duchenne Muscular Dystrophy: Disease Mechanism and Therapeutic Strategies. Front. Physiol. 2023, 14, 1183101. [Google Scholar] [CrossRef]
- Berling, E.; Nicolle, R.; Laforêt, P.; Ronzitti, G. Gene Therapy Review: Duchenne Muscular Dystrophy Case Study. Rev. Neurol. 2023, 179, 90–105. [Google Scholar] [CrossRef]
- Kupatt, C.; Windisch, A.; Moretti, A.; Wolf, E.; Wurst, W.; Walter, M.C. Genome Editing for Duchenne Muscular Dystrophy: A Glimpse of the Future? Gene Ther. 2021, 28, 542–548. [Google Scholar] [CrossRef]
- Happi Mbakam, C.; Tremblay, J.P. Gene Therapy for Duchenne Muscular Dystrophy: An Update on the Latest Clinical Developments. Expert Rev. Neurother. 2023, 23, 905–920. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Recommends Non-Renewal of Authorisation of Duchenne Muscular Dystrophy Medicine Translarna. Available online: https://www.ema.europa.eu/en/news/ema-recommends-non-renewal-authorisation-duchenne-muscular-dystrophy-medicine-translarna (accessed on 28 March 2024).
- European Medicines Agency. EMA Confirms Recommendation for Non-Renewal of Authorisation of Duchenne Muscular Dystrophy Medicine Translarna. Available online: https://www.ema.europa.eu/en/news/ema-confirms-recommendation-non-renewal-authorisation-duchenne-muscular-dystrophy-medicine-translarna (accessed on 28 March 2024).
- European Medicines Agency. EMA Recommends Non-Renewal of Authorisation of Duchenne Muscular Dystrophy Medicine Translarna. Available online: https://www.ema.europa.eu/en/news/ema-recommends-non-renewal-authorisation-duchenne-muscular-dystrophy-medicine-translarna-0 (accessed on 25 July 2024).
- Duan, D. Duchenne Muscular Dystrophy Gene Therapy in 2023: Status, Perspective, and Beyond. Hum. Gene Ther. 2023, 34, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, F.; Wein, N. Personalized Gene and Cell Therapy for Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2018, 28, 803–824. [Google Scholar] [CrossRef]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S303–S316. [Google Scholar] [CrossRef]
- Chung Liang, L.; Sulaiman, N.; Yazid, M.D. A Decade of Progress in Gene Targeted Therapeutic Strategies in Duchenne Muscular Dystrophy: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 833833. [Google Scholar] [CrossRef]
- Lentz, B.R. PEG as a Tool to Gain Insight into Membrane Fusion. Eur. Biophys. J. 2007, 36, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Paskal, A.; Paskal, W.; Pietruski, P.; Wlodarski, P. Polyethylene Glycol: The Future of Posttraumatic Nerve Repair? Systemic Review. Int. J. Mol. Sci. 2019, 20, 1478. [Google Scholar] [CrossRef]
- Ferry, A.; Messéant, J.; Parlakian, A.; Lemaitre, M.; Roy, P.; Delacroix, C.; Lilienbaum, A.; Hovhannisyan, Y.; Furling, D.; Klein, A.; et al. Desmin Prevents Muscle Wasting, Exaggerated Weakness and Fragility, and Fatigue in Dystrophic Mdx Mouse. J. Physiol. 2020, 598, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic Developments for Duchenne Muscular Dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef]
- Marini, J.F.; Pons, F.; Leger, J.; Loffreda, N.; Anoal, M.; Chevallay, M.; Fardeau, M.; Leger, J.J. Expression of Myosin Heavy Chain Isoforms in Duchenne Muscular Dystrophy Patients and Carriers. Neuromuscul. Disord. 1991, 1, 397–409. [Google Scholar] [CrossRef]
- Paulin, D.; Li, Z. Desmin: A Major Intermediate Filament Protein Essential for the Structural Integrity and Function of Muscle. Exp. Cell Res. 2004, 301, 1–7. [Google Scholar] [CrossRef]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental Myosins: Expression Patterns and Functional Significance. Skelet. Muscle 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.C.; Rayavarapu, S.; Hogarth, M.W.; Van der Meulen, J.H.; Horn, A.; Defour, A.; Takeda, S.; Brown, K.J.; Hathout, Y.; Nagaraju, K.; et al. Mitochondria Mediate Cell Membrane Repair and Contribute to Duchenne Muscular Dystrophy. Cell Death Differ. 2017, 24, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R. The Role of Mitochondria in Mediation of Skeletal Muscle Repair. Muscles 2023, 2, 119–163. [Google Scholar] [CrossRef]
- Chen, T.-H.; Koh, K.-Y.; Lin, K.M.-C.; Chou, C.-K. Mitochondrial Dysfunction as an Underlying Cause of Skeletal Muscle Disorders. Int. J. Mol. Sci. 2022, 23, 12926. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Mikheeva, I.B.; Stepanova, A.E.; Igoshkina, A.D.; Cherepanova, A.A.; Semenova, A.A.; Sharapov, V.A.; Kireev, I.I.; Belosludtsev, K.N. Mitochondrial Transplantation Therapy Ameliorates Muscular Dystrophy in Mdx Mouse Model. Biomolecules 2024, 14, 316. [Google Scholar] [CrossRef]
- Rybalka, E.; Timpani, C.A.; Stathis, C.G.; Hayes, A.; Cooke, M.B. Metabogenic and Nutriceutical Approaches to Address Energy Dysregulation and Skeletal Muscle Wasting in Duchenne Muscular Dystrophy. Nutrients 2015, 7, 9734–9767. [Google Scholar] [CrossRef] [PubMed]
- Vu Hong, A.; Sanson, M.; Richard, I.; Israeli, D. A Revised Model for Mitochondrial Dysfunction in Duchenne Muscular Dystrophy. Eur. J. Transl. Myol. 2021, 31, 10012. [Google Scholar] [CrossRef]
- Budzinska, M.; Zimna, A.; Kurpisz, M. The Role of Mitochondria in Duchenne Muscular Dystrophy. J. Physiol. Pharmacol. 2021, 72, 157–166. [Google Scholar] [CrossRef]
- Lehka, L.; Rędowicz, M.J. Mechanisms Regulating Myoblast Fusion: A Multilevel Interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Castello, S.; Podestà, M.; Menditto, V.G.; Ibatici, A.; Pitto, A.; Figari, O.; Scarpati, D.; Magrassi, L.; Bacigalupo, A.; Piaggio, G.; et al. Intra-Bone Marrow Injection of Bone Marrow and Cord Blood Cells: An Alternative Way of Transplantation Associated with a Higher Seeding Efficiency. Exp. Hematol. 2004, 32, 782–787. [Google Scholar] [CrossRef]
- Frassoni, F.; Gualandi, F.; Podestà, M.; Raiola, A.M.; Ibatici, A.; Piaggio, G.; Sessarego, M.; Sessarego, N.; Gobbi, M.; Sacchi, N.; et al. Direct Intrabone Transplant of Unrelated Cord-Blood Cells in Acute Leukaemia: A Phase I/II Study. Lancet Oncol. 2008, 9, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Metheny, L.; Eid, S.; Lingas, K.; Reese, J.; Meyerson, H.; Tong, A.; de Lima, M.; Huang, A.Y. Intra-Osseous Co-Transplantation of CD34-Selected Umbilical Cord Blood and Mesenchymal Stromal Cells. Hematol. Med. Oncol. 2016, 1, 25–29. [Google Scholar] [CrossRef][Green Version]
- Murata, M.; Maeda, Y.; Masuko, M.; Onishi, Y.; Endo, T.; Terakura, S.; Ishikawa, Y.; Iriyama, C.; Ushijima, Y.; Goto, T.; et al. Phase II Study of Intrabone Single Unit Cord Blood Transplantation for Hematological Malignancies. Cancer Sci. 2017, 108, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Agata, H.; Sumita, Y.; Hidaka, T.; Iwatake, M.; Kagami, H.; Asahina, I. Intra-Bone Marrow Administration of Mesenchymal Stem/Stromal Cells Is a Promising Approach for Treating Osteoporosis. Stem Cells Int. 2019, 2019, 4214281. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, F.; Dan, E.; Labopin, M.; Sessa, M.; Guadagnuolo, V.; Ferioli, M.; Rizzi, S.; De Carolis, S.; Sinigaglia, B.; Motta, M.R.; et al. Intrabone Transplant Provides Full Stemness of Cord Blood Stem Cells with Fast Hematopoietic Recovery and Low GVHD Rate: Results from a Prospective Study. Bone Marrow Transplant. 2019, 54, 717–725. [Google Scholar] [CrossRef]
- Skuk, D.; Tremblay, J.P. Myoblast Transplantation: The Current Status of a Potential Therapeutic Tool for Myopathies. J. Muscle Res. Cell Motil. 2003, 24, 287–302. [Google Scholar] [CrossRef]
- Torrente, Y.; Belicchi, M.; Marchesi, C.; D’Antona, G.; Cogiamanian, F.; Pisati, F.; Gavina, M.; Giordano, R.; Tonlorenzi, R.; Fagiolari, G.; et al. Autologous Transplantation of Muscle-Derived CD133+ Stem Cells in Duchenne Muscle Patients. Cell Transplant. 2007, 16, 563–577. [Google Scholar] [CrossRef]
- Marktel, S.; Scaramuzza, S.; Cicalese, M.P.; Giglio, F.; Galimberti, S.; Lidonnici, M.R.; Calbi, V.; Assanelli, A.; Bernardo, M.E.; Rossi, C.; et al. Intrabone Hematopoietic Stem Cell Gene Therapy for Adult and Pediatric Patients Affected by Transfusion-Dependent ß-Thalassemia. Nat. Med. 2019, 25, 234–241. [Google Scholar] [CrossRef]
- Döring, M.; Kluba, T.; Cabanillas Stanchi, K.M.; Kahle, P.; Lenglinger, K.; Tsiflikas, I.; Treuner, C.; Vaegler, M.; Mezger, M.; Erbacher, A.; et al. Longtime Outcome After Intraosseous Application of Autologous Mesenchymal Stromal Cells in Pediatric Patients and Young Adults with Avascular Necrosis After Steroid or Chemotherapy. Stem Cells Dev. 2020, 29, 811–822. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.B.; Lee, S.; Baek, S.; Kim, H.; Kim, S.J. Intra-Osseous Injection of Donor Mesenchymal Stem Cell (MSC) into the Bone Marrow in Living Donor Kidney Transplantation; a Pilot Study. J. Transl. Med. 2013, 11, 96. [Google Scholar] [CrossRef]



| ID of the MBN/MBDMD DT-DEC01 Product | Donor (MBN)-Specific STR Loci |
|---|---|
| MBN1/MBDMD1 | 27% |
| MBN2/MBDMD2 | 21% |
| MBN3/MBDMD3 | 6% |
| MBN4/MBDMD4 | 35% |
| MBN5/MBDMD5 | 15% |
| MBN6/MBDMD6 | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemionow, M.; Ziemiecka, A.; Bożyk, K.; Siemionow, K. Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy. Biomedicines 2024, 12, 1996. https://doi.org/10.3390/biomedicines12091996
Siemionow M, Ziemiecka A, Bożyk K, Siemionow K. Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy. Biomedicines. 2024; 12(9):1996. https://doi.org/10.3390/biomedicines12091996
Chicago/Turabian StyleSiemionow, Maria, Anna Ziemiecka, Katarzyna Bożyk, and Krzysztof Siemionow. 2024. "Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy" Biomedicines 12, no. 9: 1996. https://doi.org/10.3390/biomedicines12091996
APA StyleSiemionow, M., Ziemiecka, A., Bożyk, K., & Siemionow, K. (2024). Mechanisms of Chimeric Cell Therapy in Duchenne Muscular Dystrophy. Biomedicines, 12(9), 1996. https://doi.org/10.3390/biomedicines12091996






