Von Willebrand Factor Collagen-Binding Activity and Von Willebrand Factor-Mediated Platelet Adhesion in Patients with Coronary Artery Disease
Abstract
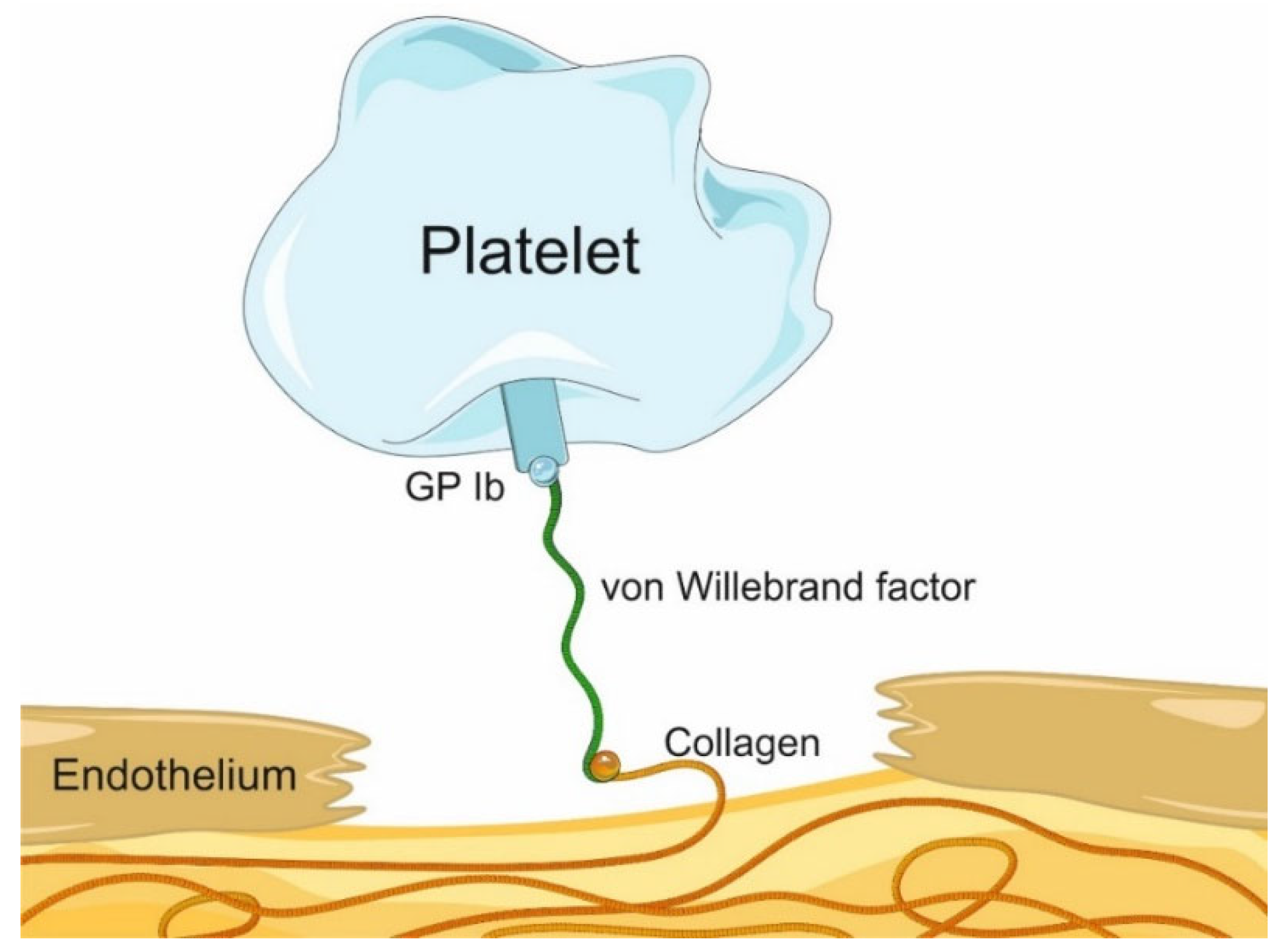
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval
2.3. Blood Sample Collection
2.4. Determination of VWF:Ag Levels, VWF:RCo Activity, and VWF:CB Activity
2.5. Measurement of Platelet Adhesion to the Collagen Surface
2.6. Statistical Analysis
3. Results
3.1. Univariate Logistic Regression Analysis
3.2. Multivariate Logistic Regression Analysis
3.3. Receiver Operating Characteristic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.; Okhota, S.; Avtaeva, Y.; Melnikov, I.; Matroze, E.; Gabbasov, Z. Von Willebrand factor in diagnostics and treatment of cardiovascular disease: Recent advances and prospects. Front. Cardiovasc. Med. 2022, 9, 1038030. [Google Scholar] [CrossRef]
- Sonneveld, M.A.; Franco, O.H.; Ikram, M.A.; Hofman, A.; Kavousi, M.; de Maat, M.P.; Leebeek, F.W. Von Willebrand Factor, ADAMTS13, and the Risk of Mortality: The Rotterdam Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2446–2451. [Google Scholar] [CrossRef]
- Folsom, A.R.; Wu, K.K.; Rosamond, W.D.; Sharrett, A.R.; Chambless, L.E. Prospective study of hemostatic factors and incidence of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 1997, 96, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Patel, R.S.; Eshtehardi, P.; Dhawan, S.; McDaniel, M.C.; Rab, S.T.; Vaccarino, V.; Zafari, A.M.; Samady, H.; Quyyumi, A.A. Coronary angiographic scoring systems: An evaluation of their equivalence and validity. Am. Heart J. 2012, 164, 547–552.e1. [Google Scholar] [CrossRef]
- Okhota, S.; Kozlov, S.; Avtaeva, Y.; Melnikov, I.; Saburova, O.; Guria, K.; Matroze, E.; Gabbasov, Z. Platelet Adhesion Mediated by von Willebrand Factor at High Shear Rates Is Associated with Premature Coronary Artery Disease. Biomedicines 2023, 11, 1916. [Google Scholar] [CrossRef]
- Roberts, J.C.; Flood, V.H. Laboratory diagnosis of von Willebrand disease. Int. J. Lab. Hematol. 2015, 37 (Suppl. S1), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, N.; Moret, A.; Caunedo, P.; Cid, A.R.; Vila, V.; España, F.; Aznar, J.A. Comparison of a new chemiluminescent immunoassay for von Willebrand factor activity with the ristocetin cofactor-induced platelet agglutination method. Haemophilia 2013, 19, 920–925. [Google Scholar] [CrossRef]
- Gabbasov, Z.A.; Avtaeva, Y.N.; Melnikov, I.S.; Okhota, S.D.; Caprnda, M.; Mozos, I.; Prosecky, R.; Rodrigo, L.; Kruzliak, P.; Zozulya, N.I. Kinetics of platelet adhesion to a fibrinogen-coated surface in whole blood under flow conditions. J. Clin. Lab. Anal. 2021, 35, e23939. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feghhi, S.; Munday, A.D.; Tooley, W.W.; Rajsekar, S.; Fura, A.M.; Kulman, J.D.; López, J.A.; Sniadecki, N.J. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophys. J. 2016, 111, 601–608. [Google Scholar] [CrossRef]
- Massberg, S.; Konrad, I.; Bültmann, A.; Schulz, C.; Münch, G.; Peluso, M.; Lorenz, M.; Schneider, S.; Besta, F.; Müller, I.; et al. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 2004, 18, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Krämer, B.; Daub, K.; Stellos, K.; Gawaz, M. Molecular pathways used by platelets to initiate and accelerate atherogenesis. Curr. Opin. Lipidol. 2007, 18, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Seaman, C.D.; Yabes, J.; Comer, D.M.; Ragni, M.V. Does deficiency of von Willebrand factor protect against cardiovascular disease? Analysis of a national discharge register. J. Thromb. Haemost. 2015, 13, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Kienast, J.; Pyke, S.D.; Haverkate, F.; van de Loo, J.C. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N. Engl. J. Med. 1995, 332, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Danesh, J.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Rumley, A.; Lowe, G.D. von Willebrand factor and coronary heart disease: Prospective study and meta-analysis. Eur. Heart J. 2002, 23, 1764–1770. [Google Scholar] [CrossRef]
- Willeit, P.; Thompson, A.; Aspelund, T.; Rumley, A.; Eiriksdottir, G.; Lowe, G.; Gudnason, V.; Di Angelantonio, E. Hemostatic factors and risk of coronary heart disease in general populations: New prospective study and updated meta-analyses. PLoS ONE 2013, 8, e55175. [Google Scholar] [CrossRef]
- Rumley, A.; Lowe, G.D.; Sweetnam, P.M.; Yarnell, J.W.; Ford, R.P. Factor VIII, von Willebrand factor and the risk of major ischaemic heart disease in the Caerphilly Heart Study. Br. J. Haematol. 1999, 105, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.B.; Lee, A.J.; Fowkes, F.G.; Price, J.F.; Rumley, A.; Lowe, G.D. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3321–3325. [Google Scholar] [CrossRef] [PubMed]
- Conlan, M.G.; Folsom, A.R.; Finch, A.; Davis, C.E.; Sorlie, P.; Marcucci, G.; Wu, K.K. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Thromb. Haemost. 1993, 70, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin. Thromb. Hemost. 2007, 33, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, C.Z.; Zhang, X.; Springer, T.A. A mechanically stabilized receptor–ligand flex-bond important in the vasculature. Nature 2010, 466, 992–995. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Patients with CAD (n = 30) | Control Group (n = 50) | p |
|---|---|---|---|
| Age, years | 55 (50; 57) | 51 (46; 61) | 0.286 |
| Males/females | 19 (63%)/11 (37%) | 20 (40%)/30 (60%) | 0.074 |
| Family history of CAD | 9 (30%) | 7 (14%) | 0.093 |
| Arterial hypertension | 25 (83%) | 41 (82%) | 0.989 |
| Diabetes mellitus | 7 (23%) | 5 (10%) | 0.119 |
| Smoking | 21 (70%) | 17 (34%) | 0.002 * |
| LDL cholesterol > 3 mmol/L | 23 (77%) | 30 (60%) | 0.314 |
| HDL cholesterol < 1.0 mmol/L for males and <1.2 mmol/L for females | 15 (50%) | 10 (20%) | 0.022 * |
| Obesity (BMI ≥ 30.0 kg/m2) | 16 (53%) | 21 (42%) | 0.452 |
| Patients | Platelet Adhesion at Baseline, mV | Platelet Adhesion after GPIb Inhibition, mV | Relative Decrease in Platelet Adhesion (∆), % | p |
|---|---|---|---|---|
| Control group | 12.6 (9.6; 15.7) * | 10.3 (4.3; 14.5) * | 29.3 (0.0; 60.4) | <0.001 * |
| Patients with CAD | 7.9 (5.0; 11.7) * | 1.8 (1.5; 2.7) * | 76.0 (60.6; 82.1) | <0.001 * |
| Patients | Platelet Adhesion at Baseline, mV | Platelet Adhesion after PGE1 Addition, mV | Relative Decrease in Platelet Adhesion (∆), % | p |
|---|---|---|---|---|
| Control group | 12.6 (9.6; 15.7) * | 5.9 (3.7; 7.9) * | 55.8 (40.7; 67.3) | <0.001 * |
| Patients with CAD | 7.9 (5.0; 11.7) * | 6.8 (4.7; 10.3) * | 3.9 (−44.0; 51.1) | 0.50 * |
| Parameters | Patients with CAD (n = 30) | Control Group (n = 50) | p |
|---|---|---|---|
| VWF:Ag, % 50–150% normal range | 135.2 (108.8; 194.2) | 152.0 (114.0; 191.1) | 0.58 |
| VWF:RCo, % 50–150% normal range | 134.1 (109.0; 185.7) | 140.3 (111.8; 175.1) | 0.93 |
| VWF:CB, % 50–250% normal range | 106.7 (82.1; 131.6) | 160.4 (112.5; 218.1) | <0.001 |
| Variable | Coefficient (β) | OR (95% CI) | p |
|---|---|---|---|
| Decrease in platelet adhesion after GPIb inhibition, per 1% | 0.049 | 1.05 (1.03–1.07) | <0.001 |
| VWF:CB, per 1% | −0.016 | 0.98 (0.97–0.99) | 0.002 |
| Smoking | 1. 511 | 4.53 (1.71– 12.02) | 0.002 |
| HDL cholesterol < 1.0 mmol/L for males and <1.2 mmol/L for females | 1.293 | 3.64 (1.32–10.04) | 0.012 |
| Male sex | 0.95 | 2.59 (1.00–6.69) | 0.046 |
| Family history of CAD | 0.968 | 2.63 (0.86–8.05) | 0.089 |
| Diabetes mellitus | 1.008 | 2.74 (0.78–9.59) | 0.115 |
| Age | 0.03 | 1.03 (0.98–1.09) | 0.211 |
| LDL cholesterol > 3 mmol/L | 0.622 | 1.86 (0.66–5.24) | 0.239 |
| Obesity | 0.456 | 1.58 (0.64–3.93) | 0.326 |
| Arterial hypertension | 0.093 | 1.10 (0.33–3.65) | 0.879 |
| Variable | Coefficient (β) | aOR (95% CI) | p |
|---|---|---|---|
| Decrease in platelet adhesion after GPIb inhibition, per 1% | 0.054 | 1.06 (1.03–1.09) | <0.001 |
| VWF:CB, per 1% | −0.019 | 0.98 (0.97–0.99) | 0.011 |
| Smoking | 1.591 | 4.91 (1.28–18.79) | 0.020 |
| Age | 0.084 | 1.09 (1.01–1.18) | 0.037 |
| Intercept | −6.184 | 0.002 | 0.019 |
| Variables | AUC | 95% CI | p |
|---|---|---|---|
| Logistic regression model | 91.6% ± 3.1% | 85.6–97.6% | <0.001 |
| Decrease in platelet adhesion after GPIb inhibition | 82.0% ± 4.7% | 72.9–91.1% | <0.001 |
| VWF:CB | 27.0% ± 5.6% | 16.1–37.9% | 0.001 |
| Smoking | 68.0% ± 6.2% | 55.8–80.2% | 0.007 |
| Age | 57.1% ± 6.5% | 44.5–69.8% | 0.288 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbasov, Z.; Okhota, S.; Avtaeva, Y.; Saburova, O.; Melnikov, I.; Shtelmakh, V.; Bazanovich, S.; Guria, K.; Kozlov, S. Von Willebrand Factor Collagen-Binding Activity and Von Willebrand Factor-Mediated Platelet Adhesion in Patients with Coronary Artery Disease. Biomedicines 2024, 12, 2007. https://doi.org/10.3390/biomedicines12092007
Gabbasov Z, Okhota S, Avtaeva Y, Saburova O, Melnikov I, Shtelmakh V, Bazanovich S, Guria K, Kozlov S. Von Willebrand Factor Collagen-Binding Activity and Von Willebrand Factor-Mediated Platelet Adhesion in Patients with Coronary Artery Disease. Biomedicines. 2024; 12(9):2007. https://doi.org/10.3390/biomedicines12092007
Chicago/Turabian StyleGabbasov, Zufar, Sergey Okhota, Yuliya Avtaeva, Olga Saburova, Ivan Melnikov, Valentina Shtelmakh, Sergey Bazanovich, Konstantin Guria, and Sergey Kozlov. 2024. "Von Willebrand Factor Collagen-Binding Activity and Von Willebrand Factor-Mediated Platelet Adhesion in Patients with Coronary Artery Disease" Biomedicines 12, no. 9: 2007. https://doi.org/10.3390/biomedicines12092007







