Angular Trajectory of the Vestibular Aqueduct in a Cohort of Chinese Patients with Unilateral Ménière’s Disease: Association with Other Imaging Indices and Clinical Profiles
Abstract
1. Introduction
2. Patients and Methods
2.1. Participants
2.2. Radiological Evaluation
2.2.1. Computer Tomography (CT) Protocols
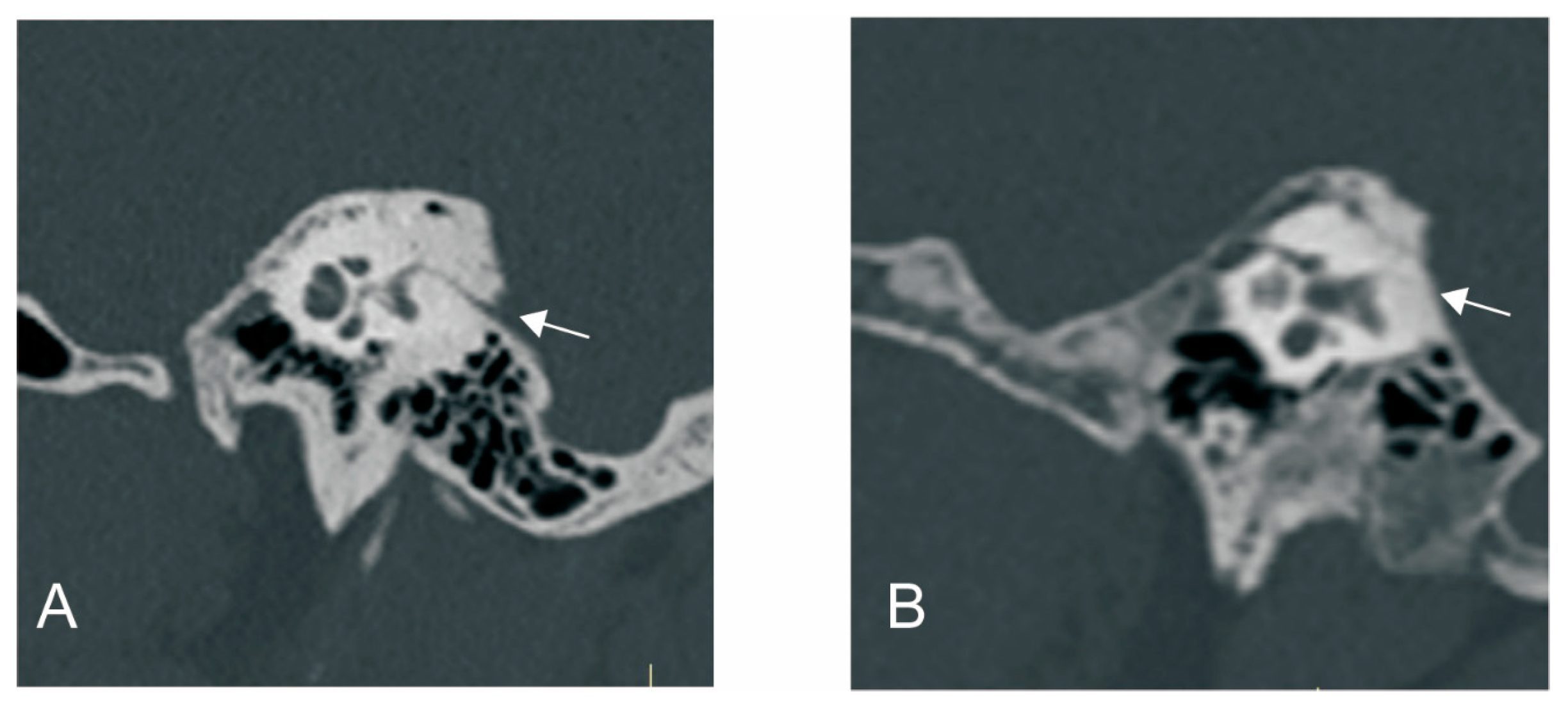
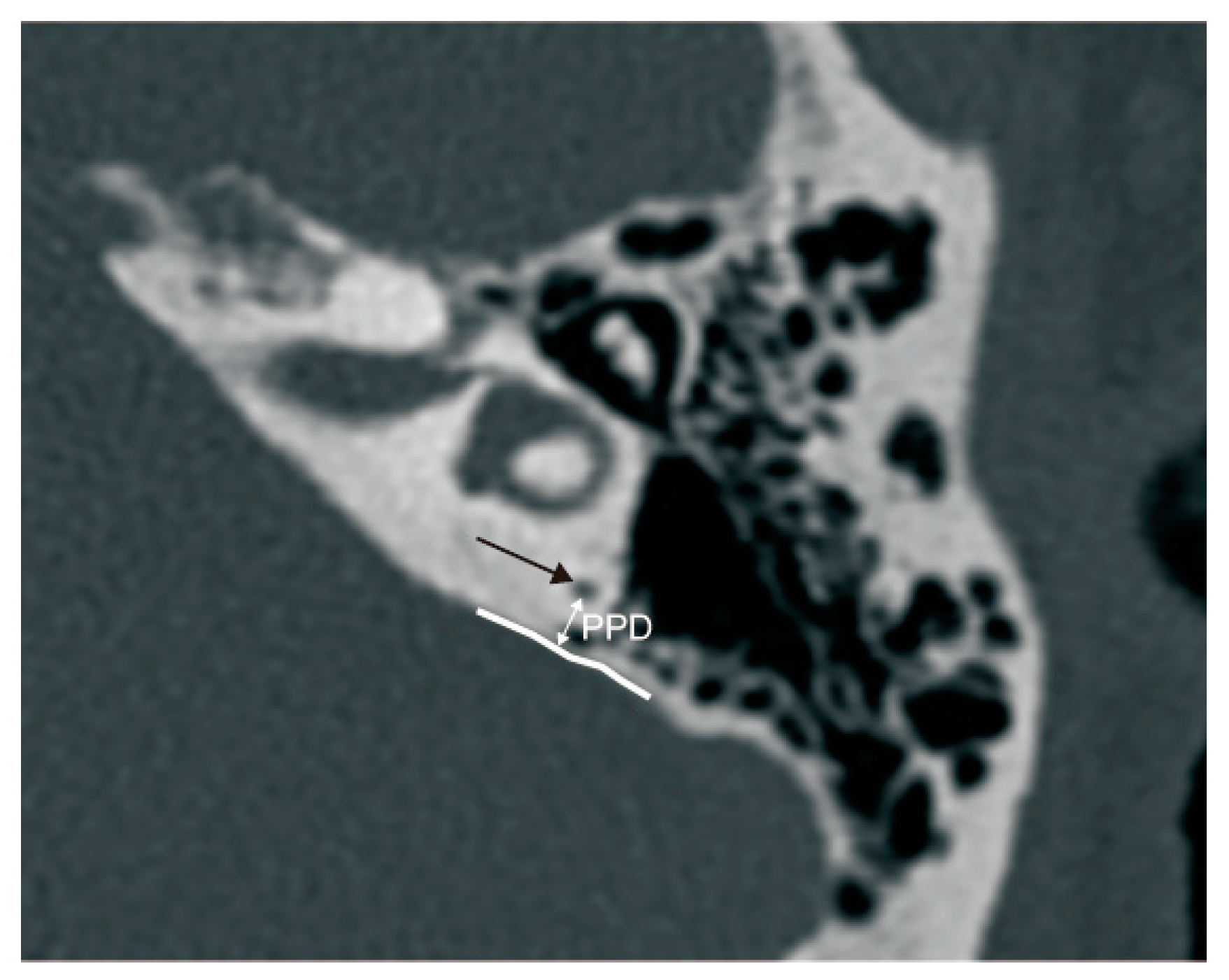
2.2.2. Radiological Measurement
2.3. Audio-Vestibular Examinations
2.3.1. Caloric Test
2.3.2. Electrocochleogram (ECochG)
2.3.3. Glycerol Test
2.4. Statistical Analysis
3. Results
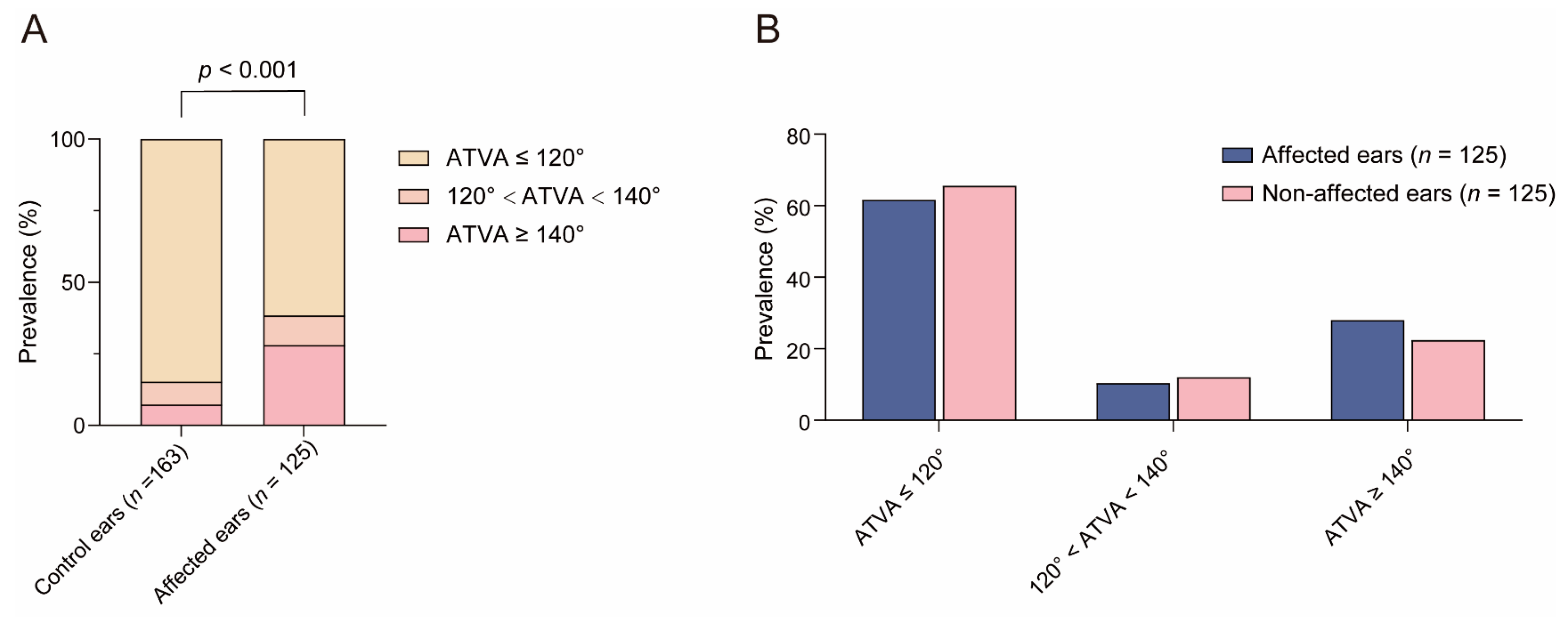
3.1. Distribution of ATVA Subgroups in Control Subjects and MD Patients
3.2. The Relationship between ATVA and Other Radiological Variations in Temporal Bone
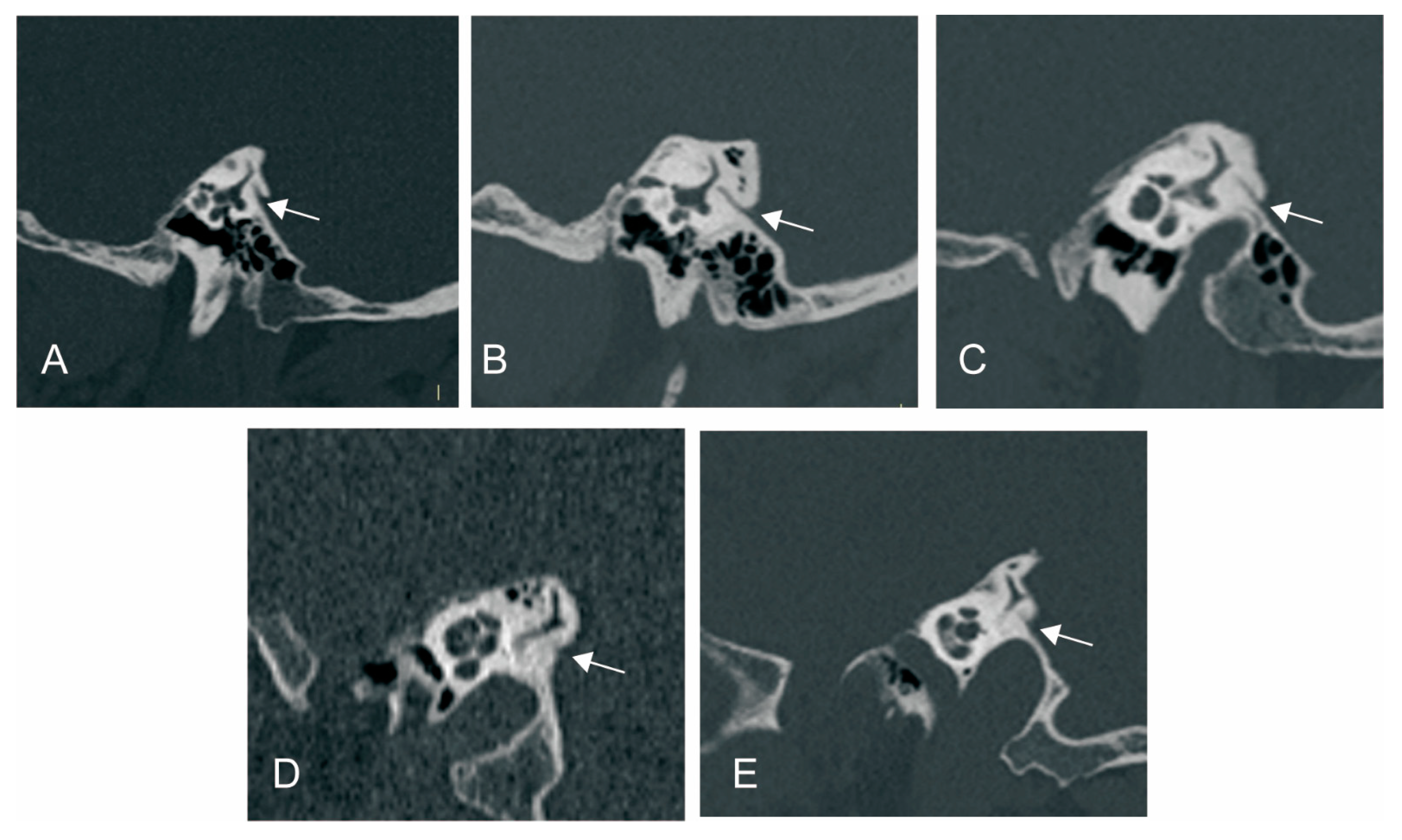
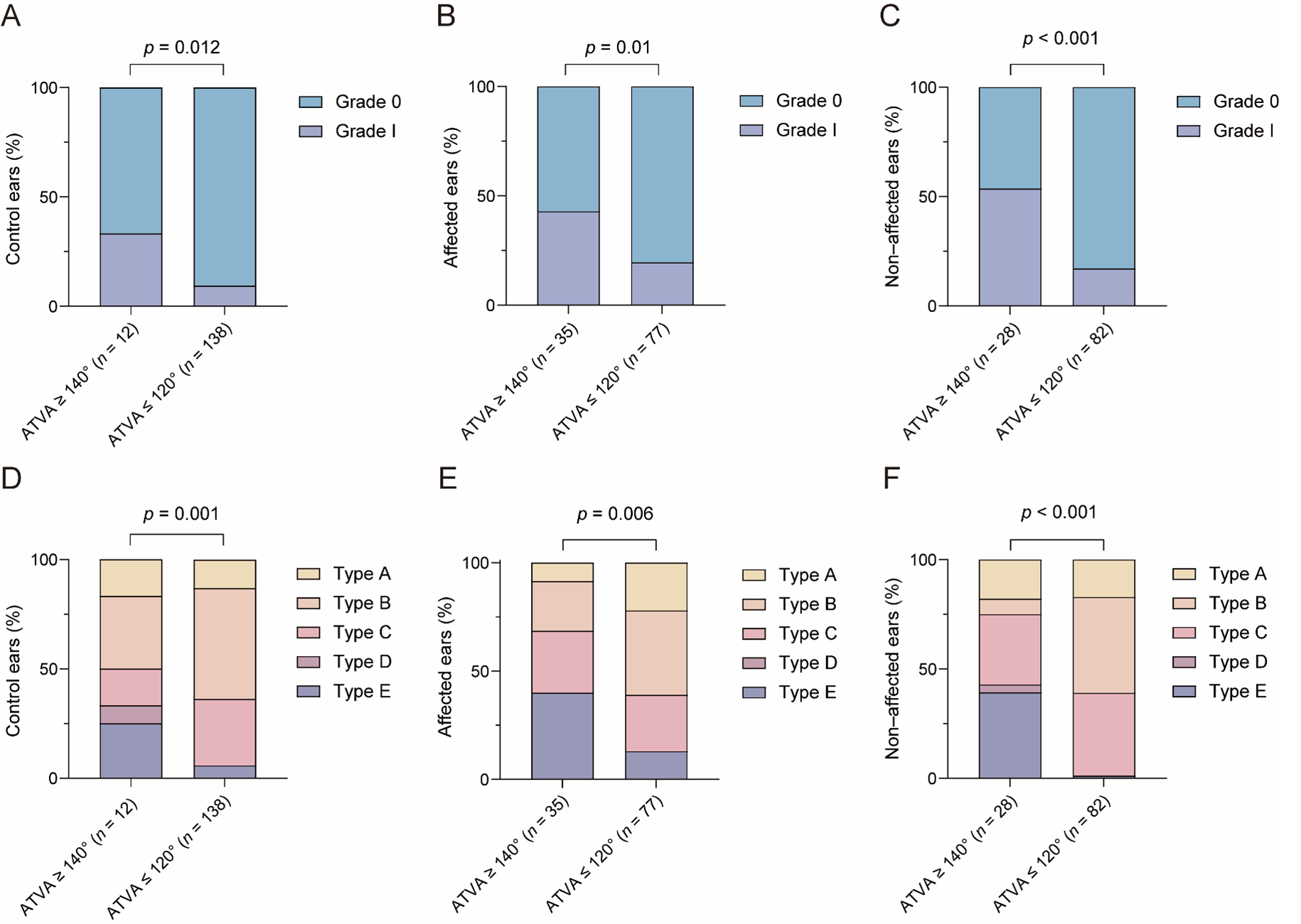
3.2.1. The Relationship between ATVA and VA Visibility and Morphology
3.2.2. The Relationship between ATVA and PPD
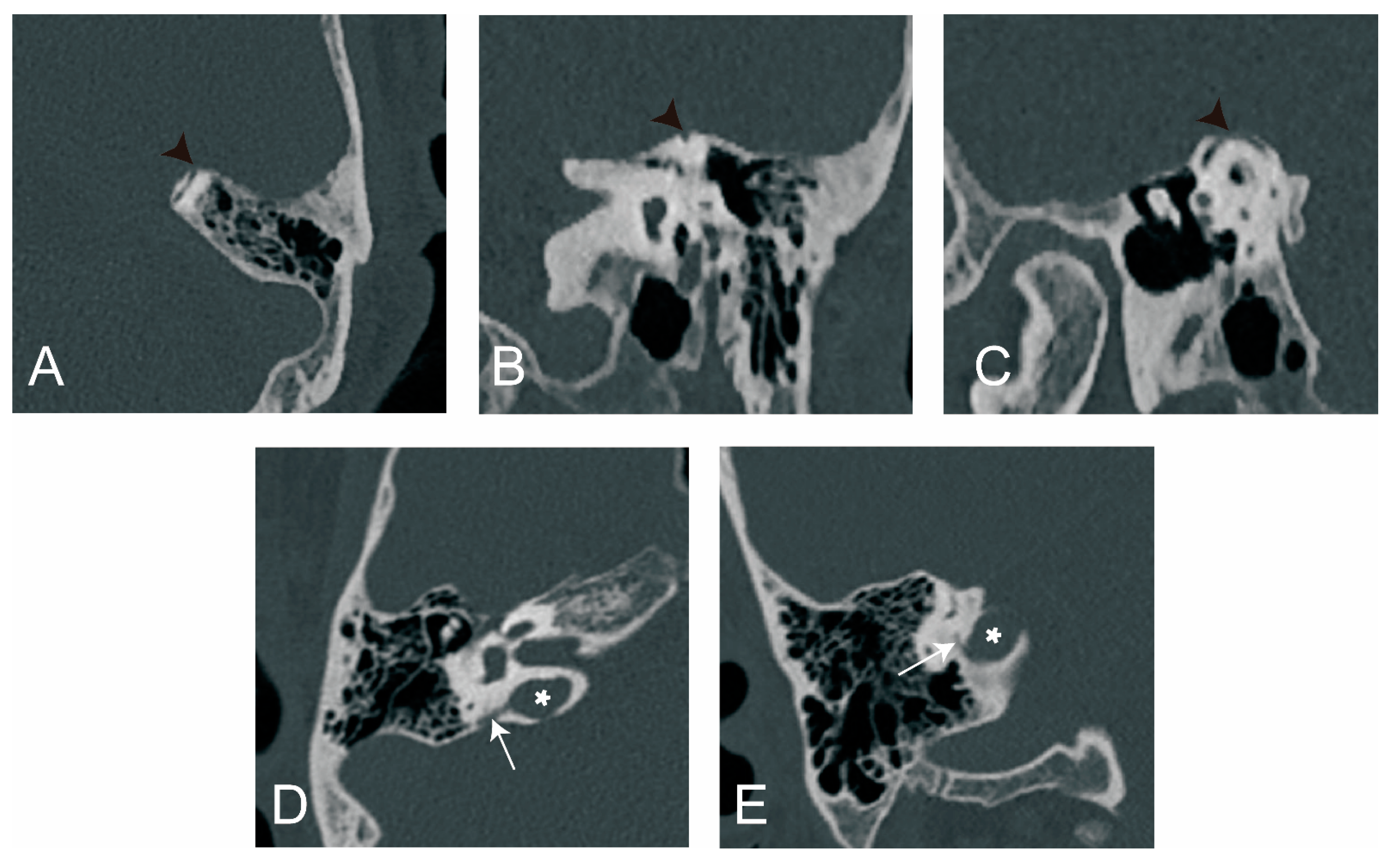
3.2.3. The Relationship between ATVA and Peri-VA Pneumatization
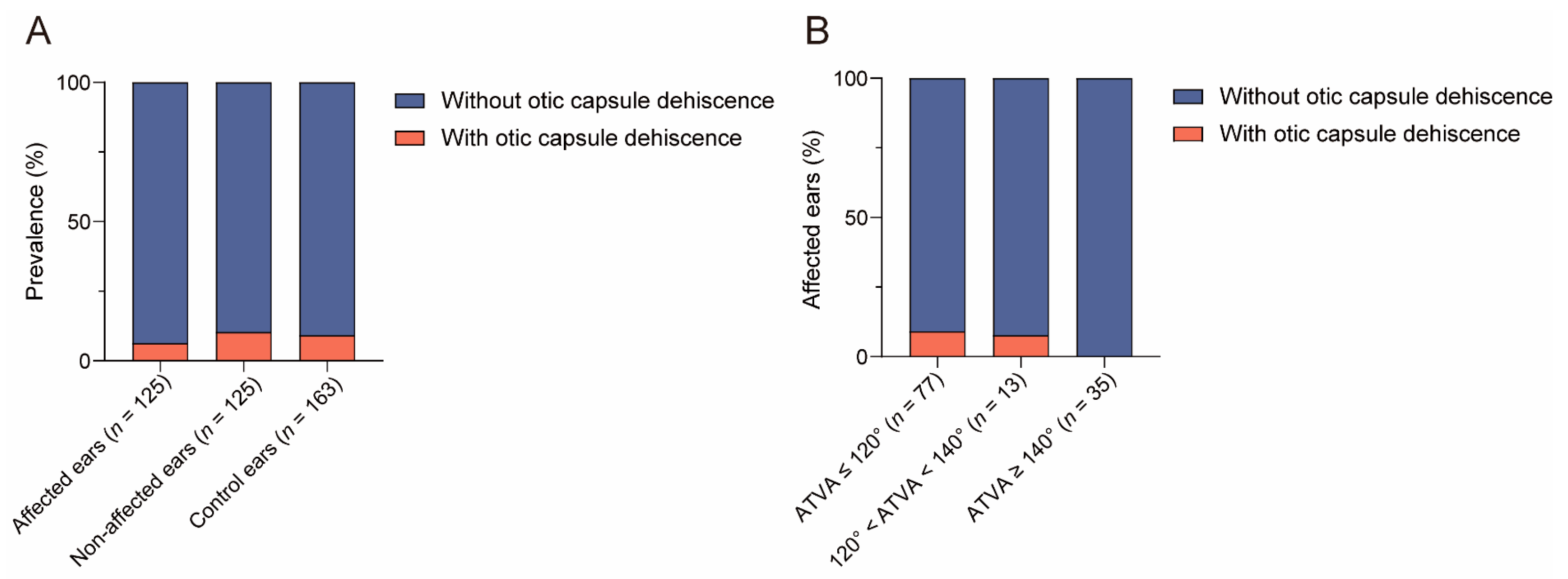
3.2.4. The Relationship between ATVA and Otic Capsule Dehiscence
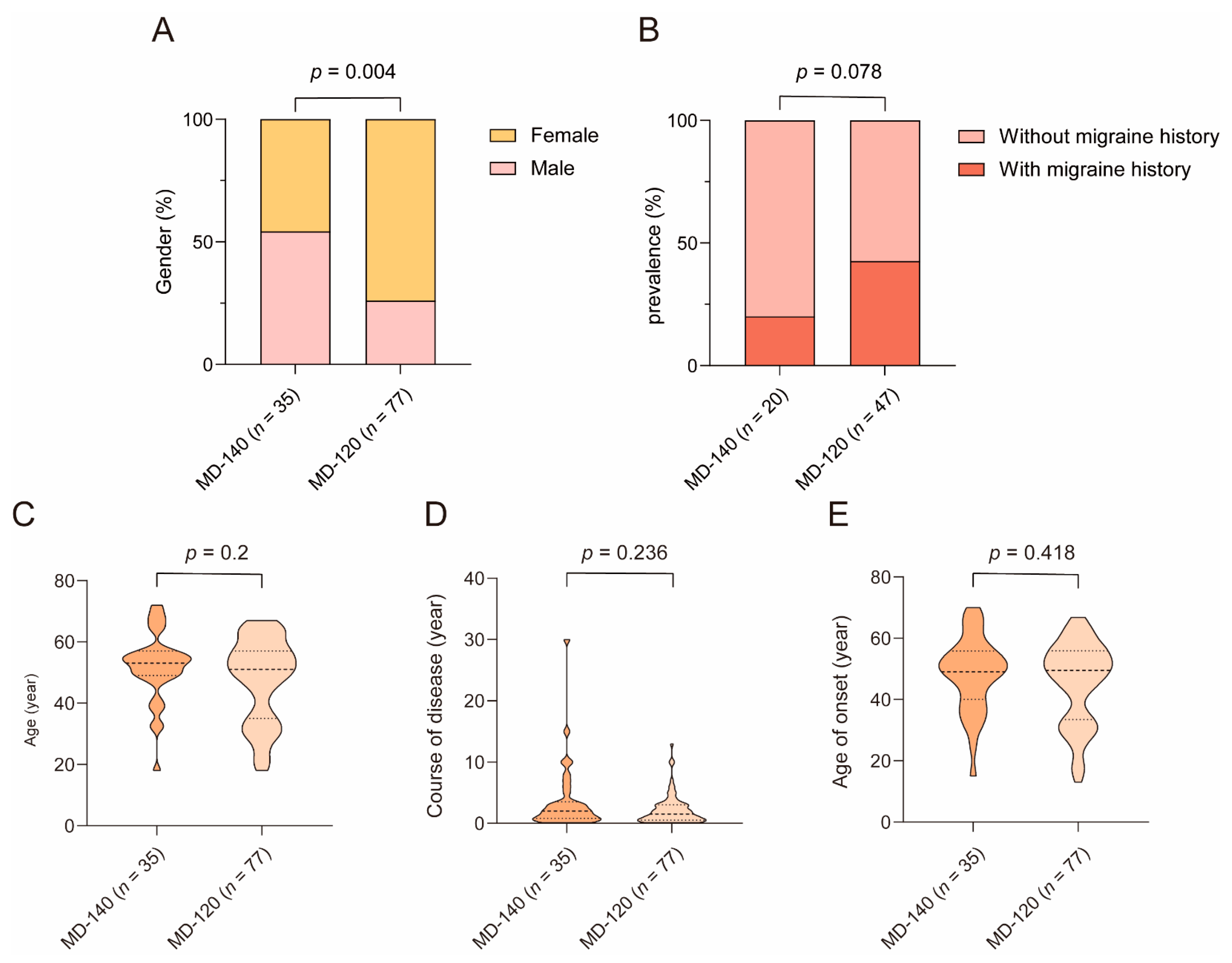
3.3. The Relationship between Clinical Features and ATVA
4. Discussion
4.1. ATVA in MD Patients
4.2. The Relationship between ATVA and Other Radiological Parameters of VA and ES
4.2.1. The Relationship between ATVA and Other Radiological Parameters of VA
4.2.2. The Relationship between ATVA and PPD
4.2.3. The Relationship between ATVA and Peri-VA Pneumatization
4.2.4. The Relationship between ATVA and Otic Capsule Dehiscence
4.3. The Difference in Clinical Feature between MD-120 Subgroup and MD-140 Subgroup
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere’s Disease. Otolaryngol. Neck Surg. 1995, 113, 181–185. [Google Scholar] [CrossRef]
- Arenberg, I.K.; Lemke, C.; Shambaugh, G.E. Viral Theory for Ménière’s Disease and Endolymphatic Hydrops: Overview and New Therapeutic Options for Viral Labyrinthitis. Ann. N. Y. Acad. Sci. 1997, 830, 306–313. [Google Scholar] [CrossRef]
- Bloch, S.L.; Friis, M. Objective Measurement of the Human Endolymphatic Sac Dimensions in Ménière’s Disease. Otol. Neurotol. 2011, 32, 1364–1369. [Google Scholar] [CrossRef]
- Ikeda, M.; Sando, I. Endolymphatic Duct and Sac in Patients with Meniere’s Disease. Ann. Otol. Rhinol. Laryngol. 1984, 93, 540–546. [Google Scholar] [CrossRef]
- Friberg, U.; Rask-Andersen, H. Vascular Occlusion in the Endolymphatic Sac in Meniere’s Disease. Ann. Otol. Rhinol. Laryngol. 2002, 111, 237–245. [Google Scholar] [CrossRef]
- Wackym, P.A. Histopathologic findings in meniere’s disease. Otolaryngol. Neck Surg. 1995, 112, 90–100. [Google Scholar] [CrossRef]
- Xia, K.; Lei, P.; Liu, Y.; Chen, C.; Pan, H.; Leng, Y.; Liu, B. Comparison of vestibular aqueduct visualization on computed tomography and magnetic resonance imaging in patients with Ménière’s disease. BMC Med. Imaging 2024, 24, 1–9. [Google Scholar] [CrossRef]
- Mainnemarre, J.; Hautefort, C.; Toupet, M.; Guichard, J.-P.; Houdart, E.; Attyé, A.; Eliezer, M. The vestibular aqueduct ossification on temporal bone CT: An old sign revisited to rule out the presence of endolymphatic hydrops in Menière’s disease patients. Eur. Radiol. 2020, 30, 6331–6338. [Google Scholar] [CrossRef]
- Yamane, H.; Konishi, K.; Sakamaoto, H.; Yamamoto, H.; Matsushita, N.; Oishi, M.; Iguchi, H.; Inoue, Y. Practical 3DCT imaging of the vestibular aqueduct for Meniere’s disease. Acta Oto-Laryngol. 2015, 135, 799–806. [Google Scholar] [CrossRef]
- Albers, F.W.J.; Van Weissenbruch, R.; Casselman, J.W. 3DFT-magnetic Resonance Imaging of the Inner Ear in Meniere’s Disease. Acta Oto-Laryngologica 1994, 114, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Redfern, R.E.; Brown, M.; Benson, A.G. High jugular bulb in a cohort of patients with definite Ménière’s disease. J. Laryngol. Otol. 2014, 128, 759–764. [Google Scholar] [CrossRef]
- Hall, S.F.; O’Connor, A.F.; Thakkar, C.H.; Wylie, I.G.; Morrison, A.W. Significance of tomography in meniere’s disease: Periaqueductal pneumatization. Laryngoscope 1983, 93, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, A.H.; Zhu, M.; O’malley, J.T.; Williams, G.H.; Loffing, J.; Rauch, S.D.; Nadol, J.B.; Liberman, M.C.; Adams, J.C. Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol. 2018, 137, 343–357. [Google Scholar] [CrossRef]
- Bächinger, D.; Luu, N.-N.; Kempfle, J.S.; Barber, S.; Zürrer, D.; Lee, D.J.; Curtin, H.D.; Rauch, S.D.; Nadol, J.B.; Adams, J.C.; et al. Vestibular Aqueduct Morphology Correlates With Endolymphatic Sac Pathologies in Menière’s Disease—A Correlative Histology and Computed Tomography Study. Otol. Neurotol. 2019, 40, e548–e555. [Google Scholar] [CrossRef]
- Bächinger, D.; Brühlmann, C.; Honegger, T.; Michalopoulou, E.; Naldi, A.M.; Wettstein, V.G.; Muff, S.; Schuknecht, B.; Eckhard, A.H. Endotype-Phenotype Patterns in Meniere’s Disease Based on Gadolinium-Enhanced MRI of the Vestibular Aqueduct. Front. Neurol. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Bächinger, D.; Filidoro, N.; Naville, M.; Juchler, N.; Kurtcuoglu, V.; Nadol, J.B.; Schuknecht, B.; Kleinjung, T.; Veraguth, D.; Eckhard, A.H. Radiological feature heterogeneity supports etiological diversity among patient groups in Meniere’s disease. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Bächinger, D.; Goosmann, M.M.; Schuknecht, B.; Nadol, J.B.; Adams, J.C.; Huber, A.; Eckhard, A.H. Clinical Imaging Findings of Vestibular Aqueduct Trauma in a Patient With Posttraumatic Meniere’s Syndrome. Front. Neurol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Bächinger, D.; Schuknecht, B.; Dlugaiczyk, J.; Eckhard, A.H. Radiological Configuration of the Vestibular Aqueduct Predicts Bilateral Progression in Meniere’s Disease. Front. Neurol. 2021, 12, 674170. [Google Scholar] [CrossRef]
- Jung, D.; Nagururu, N.; Hui, F.; Pearl, M.S.; Carey, J.P.; Ward, B.K. 2D Measurements of the Angle of the Vestibular Aqueduct Using CT Imaging. Brain Sci. 2022, 13, 47. [Google Scholar] [CrossRef]
- de Pont, L.M.H.; Houben, M.T.P.M.; Verhagen, T.O.; Verbist, B.M.; van Buchem, M.A.; Bommeljé, C.C.; Blom, H.M.; Hammer, S. Visualization and clinical relevance of the endolymphatic duct and sac in Ménière’s disease. Front. Neurol. 2023, 14, 1239422. [Google Scholar] [CrossRef] [PubMed]
- Stahle, J.; Wilbrand, H.F. The Temporal Bone in Patients with Meniere’s Disease. Acta Oto-Laryngol. 1982, 95, 81–94. [Google Scholar] [CrossRef]
- Zarandy, M.M.; Kouhi, A.; Emami, H.; Amirzargar, B.; Kazemi, M.A. Prevalence of otic capsule dehiscence in temporal bone computed tomography scan. Eur. Arch. Oto-Rhino-Laryngology 2022, 280, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Grosser, D.; Willenborg, K.; Dellani, P.; Avallone, E.; Götz, F.; Böthig, D.; Warnecke, A.; Lanfermann, H.; Lenarz, T.; Giesemann, A. Vestibular Aqueduct Size Correlates With the Degree of Cochlear Hydrops in Patients With and Without Menière’s Disease. Otol. Neurotol. 2021, 42, e1532–e1536. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, R.; Xu, N.; Ding, H.; Pu, W.; Xie, J.; Yang, Z.; Liu, Y.; Gong, S.; Wang, Z.; et al. Association Between Vestibular Aqueduct Morphology and Meniere’s Disease. Laryngoscope 2024, 134, 3349–3354. [Google Scholar] [CrossRef]
- Boston, M.; Halsted, M.; Meinzen-Derr, J.; Bean, J.; Vijayasekaran, S.; Arjmand, E.; Choo, D.; Benton, C.; Greinwald, J. The large vestibular aqueduct: A new definition based on audiologic and computed tomography correlation. Otolaryngol. Neck Surg. 2007, 136, 972–977. [Google Scholar] [CrossRef]
- Noyalet, L.; Ilgen, L.; Bürklein, M.; Shehata-Dieler, W.; Taeger, J.; Hagen, R.; Neun, T.; Zabler, S.; Althoff, D.; Rak, K. Vestibular Aqueduct Morphology and Meniere’s Disease—Development of the “Vestibular Aqueduct Score” by 3D Analysis. Front. Surg. 2022, 9, 747517. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Sando, I. Three-dimensional course of the vestibular aqueduct. Eur. Arch. Oto-Rhino-Laryngol. 1996, 253, 122–125. [Google Scholar] [CrossRef]
- Watzke, D.; Bast, T.H. The development and structure of the otic (endolymphatic) sac. Anat. Rec. 1950, 106, 361–379. [Google Scholar] [CrossRef]
- Fujita, S.; Sando, I. Postnatal Development of the Vestibular Aqueduct in Relation to the Internal Auditory Canal. Ann. Otol. Rhinol. Laryngol. 1994, 103, 719–722. [Google Scholar] [CrossRef]
- Merchant, S.N.; Rosowski, J.J. Conductive Hearing Loss Caused by Third-Window Lesions of the Inner Ear. Otol. Neurotol. 2008, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-L.; Moonis, G.; Halpin, C.; Curtin, H. Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond. Am. J. Neuroradiol. 2016, 38, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Johanis, M.; De Jong, R.; Miao, T.; Hwang, L.; Lum, M.; Kaur, T.; Willis, S.; Arsenault, J.J.; Duong, C.; Yang, I.; et al. Concurrent superior semicircular canal dehiscence and endolymphatic hydrops: A novel case series. Int. J. Surg. Case Rep. 2021, 78, 382–386. [Google Scholar] [CrossRef]
- Sone, M.; Yoshida, T.; Morimoto, K.; Teranishi, M.; Nakashima, T.; Naganawa, S. Endolymphatic hydrops in superior canal dehiscence and large vestibular aqueduct syndromes. Laryngoscope 2016, 126, 1446–1450. [Google Scholar] [CrossRef]
- Lorente-Piera, J.; Prieto-Matos, C.; Manrique-Huarte, R.; Garaycochea, O.; Domínguez, P.; Manrique, M. Otic Capsule Dehiscences Simulating Other Inner Ear Diseases: Characterization, Clinical Profile, and Follow-Up—Is Ménière’s Disease the Sole Cause of Vertigo and Fluctuating Hearing Loss? Audiol. Res. 2024, 14, 372–385. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, C.H.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Park, B.; Lee, H.-J.; Choi, H.G. Association Between Meniere Disease and Migraine. Arch. Otolaryngol. Neck Surg. 2022, 148, 457–464. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Lempert, T.; Gresty, M.; Brookes, G.; Bronstein, A.; Neuhauser, H. Migraine and Ménière’s disease. Neurology 2002, 59, 1700–1704. [Google Scholar] [CrossRef]
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.-H. Meniere’s disease. Nat. Rev. Dis. Prim. 2016, 2, 16028. [Google Scholar] [CrossRef]
- Margolis, R.H.; Levine, S.C.; Foamier, E.M.; Hunter, L.L.; Smith, S.L.; Lilly, D.J. Tympanic Electrocochleography: Normal and Abnormal Patterns of Response. Int. J. Audiol. 1992, 31, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Frejo, L.; Martin-Sanz, E.; Teggi, R.; Trinidad, G.; Soto-Varela, A.; Santos-Perez, S.; Manrique, R.; Perez, N.; Aran, I.; Almeida-Branco, M.; et al. Extended phenotype and clinical subgroups in unilateral Meniere disease: A cross-sectional study with cluster analysis. Clin. Otolaryngol. 2017, 42, 1172–1180. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, K.; Lei, P.; Liu, Y.; Chen, C.; Xiao, H.; Leng, Y.; Liu, B. Angular Trajectory of the Vestibular Aqueduct in a Cohort of Chinese Patients with Unilateral Ménière’s Disease: Association with Other Imaging Indices and Clinical Profiles. Biomedicines 2024, 12, 2008. https://doi.org/10.3390/biomedicines12092008
Xia K, Lei P, Liu Y, Chen C, Xiao H, Leng Y, Liu B. Angular Trajectory of the Vestibular Aqueduct in a Cohort of Chinese Patients with Unilateral Ménière’s Disease: Association with Other Imaging Indices and Clinical Profiles. Biomedicines. 2024; 12(9):2008. https://doi.org/10.3390/biomedicines12092008
Chicago/Turabian StyleXia, Kaijun, Ping Lei, Yingzhao Liu, Cen Chen, Hongjun Xiao, Yangming Leng, and Bo Liu. 2024. "Angular Trajectory of the Vestibular Aqueduct in a Cohort of Chinese Patients with Unilateral Ménière’s Disease: Association with Other Imaging Indices and Clinical Profiles" Biomedicines 12, no. 9: 2008. https://doi.org/10.3390/biomedicines12092008
APA StyleXia, K., Lei, P., Liu, Y., Chen, C., Xiao, H., Leng, Y., & Liu, B. (2024). Angular Trajectory of the Vestibular Aqueduct in a Cohort of Chinese Patients with Unilateral Ménière’s Disease: Association with Other Imaging Indices and Clinical Profiles. Biomedicines, 12(9), 2008. https://doi.org/10.3390/biomedicines12092008




