Does Eosinophil Heterogeneity Translate into Functional Diversity? A Review of the Evolving Paradigm of Eosinophil Heterogeneity in Asthma
Abstract
:1. Introduction
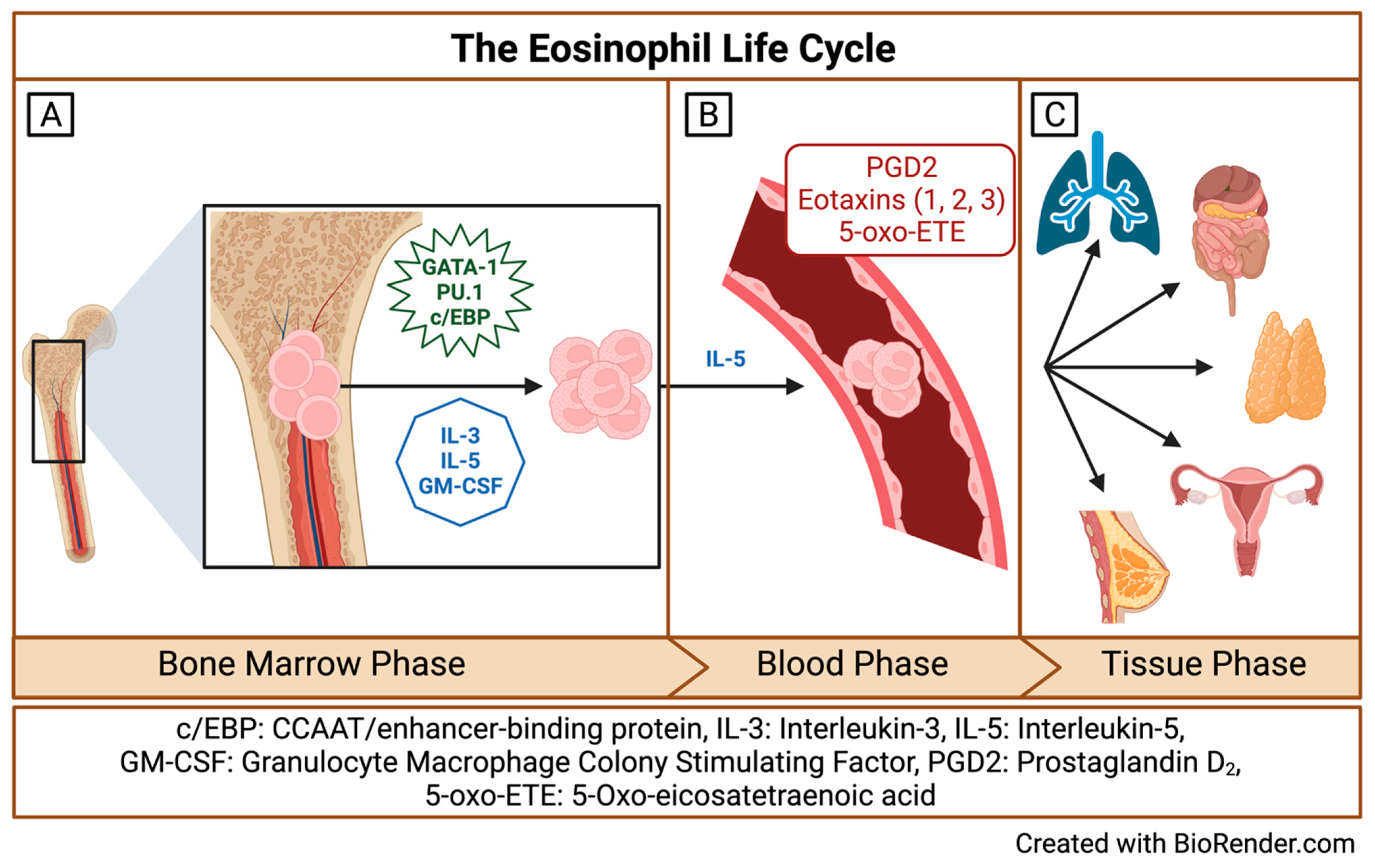
2. Origins and Multiple Fates of Eosinophils
3. The Evolution of Eosinophil Subtypes in the Literature
4. The Significance of Eosinophil Subpopulations in Asthma
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef]
- Ravin, K.A.; Loy, M. The Eosinophil in Infection. Clin. Rev. Allergy Immunol. 2016, 50, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Investig. 1999, 103, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Abdala-Valencia, H.; Coden, M.E.; Chiarella, S.E.; Jacobsen, E.A.; Bochner, B.S.; Lee, J.J.; Berdnikovs, S. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018, 104, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Yun, Y.; Bui, D.V.; Nguyen, L.M.; Kobayashi, Y.; Suzuki, K.; Mitani, A.; Sawada, S.; Hamada, S.; Asako, M.; et al. The multiple functions and subpopulations of eosinophils in tissues under steady-state and pathological conditions. Allergol. Int. 2021, 70, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Bochner, B.S. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol. Res. 2010, 2, 87–101. [Google Scholar] [CrossRef]
- Shah, K.; Ignacio, A.; McCoy, K.D.; Harris, N.L. The emerging roles of eosinophils in mucosal homeostasis. Mucosal Immunol. 2020, 13, 574–583. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef]
- Goh, Y.P.; Henderson, N.C.; Heredia, J.E.; Red Eagle, A.; Odegaard, J.I.; Lehwald, N.; Nguyen, K.D.; Sheppard, D.; Mukundan, L.; Locksley, R.M.; et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9914–9919. [Google Scholar] [CrossRef]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef]
- Chu, V.T.; Frohlich, A.; Steinhauser, G.; Scheel, T.; Roch, T.; Fillatreau, S.; Lee, J.J.; Lohning, M.; Berek, C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011, 12, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Throsby, M.; Herbelin, A.; Pleau, J.M.; Dardenne, M. CD11c+ eosinophils in the murine thymus: Developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J. Immunol. 2000, 165, 1965–1975. [Google Scholar] [CrossRef]
- Mitre, E.; Klion, A.D. Eosinophils and helminth infection: Protective or pathogenic? Semin. Immunopathol. 2021, 43, 363–381. [Google Scholar] [CrossRef]
- Gaur, P.; Zaffran, I.; George, T.; Rahimli Alekberli, F.; Ben-Zimra, M.; Levi-Schaffer, F. The regulatory role of eosinophils in viral, bacterial, and fungal infections. Clin. Exp. Immunol. 2022, 209, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Cohn, L.; Herrick, C.; Niu, N.; Homer, R.; Bottomly, K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: Defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J. Immunol. 2001, 166, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Wallrapp, A.; Riesenfeld, S.J.; Burkett, P.R.; Kuchroo, V.K. Type 2 innate lymphoid cells in the induction and resolution of tissue inflammation. Immunol. Rev. 2018, 286, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- Campbell, C.D.; Gleeson, M.; Sulaiman, I. The role of the respiratory microbiome in asthma. Front. Allergy 2023, 4, 1120999. [Google Scholar] [CrossRef]
- Winqvist, I.; Olofsson, T.; Olsson, I.; Persson, A.M.; Hallberg, T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology 1982, 47, 531–539. [Google Scholar] [PubMed]
- Prin, L.; Capron, M.; Tonnel, A.B.; Bletry, O.; Capron, A. Heterogeneity of human peripheral blood eosinophils: Variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int. Arch. Allergy Appl. Immunol. 1983, 72, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Dunnette, S.L.; Reed, C.E.; Ackerman, S.J.; Peters, M.S.; Gleich, G.J. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am. Rev. Respir. Dis. 1985, 132, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.P.; Yu, T.R.; Yu, C.T. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. Eur. Respir. J. 1994, 7, 1452–1459. [Google Scholar] [CrossRef]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy 2016, 71, 267–271. [Google Scholar] [CrossRef]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Kachler, K.; Llerins Perez, M.; Kirchner, P.; Kolle, J.; Giessl, A.; Rauber, S.; Song, R.; Aust, O.; et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 451–468. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.Y.; Yi, H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef]
- Dolitzky, A.; Shapira, G.; Grisaru-Tal, S.; Hazut, I.; Avlas, S.; Gordon, Y.; Itan, M.; Shomron, N.; Munitz, A. Transcriptional Profiling of Mouse Eosinophils Identifies Distinct Gene Signatures Following Cellular Activation. Front. Immunol. 2021, 12, 802839. [Google Scholar] [CrossRef]
- Yun, Y.; Kanda, A.; Kobayashi, Y.; Van Bui, D.; Suzuki, K.; Sawada, S.; Baba, K.; Yagi, M.; Asako, M.; Okazaki, H.; et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 2020, 69, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef] [PubMed]
- Barretto, K.T.; Swanson, C.M.; Nguyen, C.L.; Annis, D.S.; Esnault, S.J.; Mosher, D.F.; Johansson, M.W. Control of cytokine-driven eosinophil migratory behavior by TGF-beta-induced protein (TGFBI) and periostin. PLoS ONE 2018, 13, e0201320. [Google Scholar] [CrossRef]
- Munitz, A.; Bachelet, I.; Finkelman, F.D.; Rothenberg, M.E.; Levi-Schaffer, F. CD48 is critically involved in allergic eosinophilic airway inflammation. Am. J. Respir. Crit. Care Med. 2007, 175, 911–918. [Google Scholar] [CrossRef]
- Gurtner, A.; Borrelli, C.; Gonzalez-Perez, I.; Bach, K.; Acar, I.E.; Nunez, N.G.; Crepaz, D.; Handler, K.; Vu, V.P.; Lafzi, A.; et al. Active eosinophils regulate host defence and immune responses in colitis. Nature 2023, 615, 151–157. [Google Scholar] [CrossRef]
- Januskevicius, A.; Jurkeviciute, E.; Janulaityte, I.; Kalinauskaite-Zukauske, V.; Miliauskas, S.; Malakauskas, K. Blood Eosinophils Subtypes and Their Survivability in Asthma Patients. Cells 2020, 9, 1248. [Google Scholar] [CrossRef]
- Hughes, J.M.; Arthur, C.A.; Baracho, S.; Carlin, S.M.; Hawker, K.M.; Johnson, P.R.; Armour, C.L. Human eosinophil-airway smooth muscle cell interactions. Mediat. Inflamm. 2000, 9, 93–99. [Google Scholar] [CrossRef]
- Halwani, R.; Vazquez-Tello, A.; Sumi, Y.; Pureza, M.A.; Bahammam, A.; Al-Jahdali, H.; Soussi-Gounni, A.; Mahboub, B.; Al-Muhsen, S.; Hamid, Q. Eosinophils induce airway smooth muscle cell proliferation. J. Clin. Immunol. 2013, 33, 595–604. [Google Scholar] [CrossRef]
- Jurkeviciute, E.; Januskevicius, A.; Rimkunas, A.; Palacionyte, J.; Malakauskas, K. alpha(4)beta(1) and alpha(M)beta(2) Integrin Expression and Pro-Proliferative Properties of Eosinophil Subtypes in Asthma. J. Pers. Med. 2021, 11, 829. [Google Scholar] [CrossRef]
- Cabrera Lopez, C.; Sanchez Santos, A.; Lemes Castellano, A.; Cazorla Rivero, S.; Brena Atienza, J.; Gonzalez Davila, E.; Celli, B.; Casanova Macario, C. Eosinophil Subtypes in Adults with Asthma and Adults with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2023, 208, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Nencini, F.; Maggiore, G.; Chiccoli, F.; Accinno, M.; Vivarelli, E.; Bruno, C.; Locatello, L.G.; Palomba, A.; Nucci, E.; et al. High proportion of inflammatory CD62L(low) eosinophils in blood and nasal polyps of severe asthma patients. Clin. Exp. Allergy 2022, 53, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Accinno, M.; Vivarelli, E.; Mecheri, V.; Maggiore, G.; Cosmi, L.; Parronchi, P.; Rossi, O.; Maggi, E.; Gallo, O.; et al. Blood CD62L(low) inflammatory eosinophils are related to the severity of asthma and reduced by mepolizumab. Allergy 2023, 78, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Bacharier, L.B.; Gergen, P.J.; Gagalis, L.; Calatroni, A.; Wellford, S.; Gill, M.A.; Stokes, J.; Liu, A.H.; Gruchalla, R.S.; et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet 2022, 400, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.E.; Knight, J.; Liu, Q.; Shelar, A.; Stewart, E.; Wang, X.; Yan, X.; Sanders, J.; Visness, C.; Gill, M.; et al. Activated sputum eosinophils associated with exacerbations in children on mepolizumab. J. Allergy Clin. Immunol. 2024, 154, 297–307.e13. [Google Scholar] [CrossRef]
| Study | Eosinophil Subtypes Identified (Method; Sample Type) | Main Findings and Conclusions |
|---|---|---|
| Winqvist et al. Immunology, 1982 Prin et al. Int Arch Allergy Appl Immunol, 1983 Fukuda et al. Am Rev Respir Dis, 1985 Kuo et al. Eur Respir J, 1994 | “Normodense” and “Hypodense” eosinophils (density-gradient centrifugation; human blood) | “Hypodense” eosinophils exhibit an activated phenotype, with decreased eosinophil cationic protein (ECP) content, increased oxygen consumption, increased expression of Fc-IgG and complement receptors, and higher capacity to induce cytotoxicity compared to “normodense” eosinophils [21,22]. “Hypodense” eosinophils are increased in the blood in patients with asthma and correlates with asthma severity and airway hyper-responsiveness [23,24]. |
| Abdala Valencia et al. Allergy, 2016 | Interstitial eosinophils and airway eosinophils (multicolor flow cytometry; digested lung tissue and BAL specimens from BALBc/J mice) | Airway eosinophils exhibit a Siglec-FhighCD11clow phenotype compared to Siglec-FmedCD11c- interstitial eosinophils, indicating that airway eosinophils are phenotypically distinct and that CD11c plays an important role in integrin-mediated trans-epithelial migration of eosinophils to the airways during allergic inflammation [25]. |
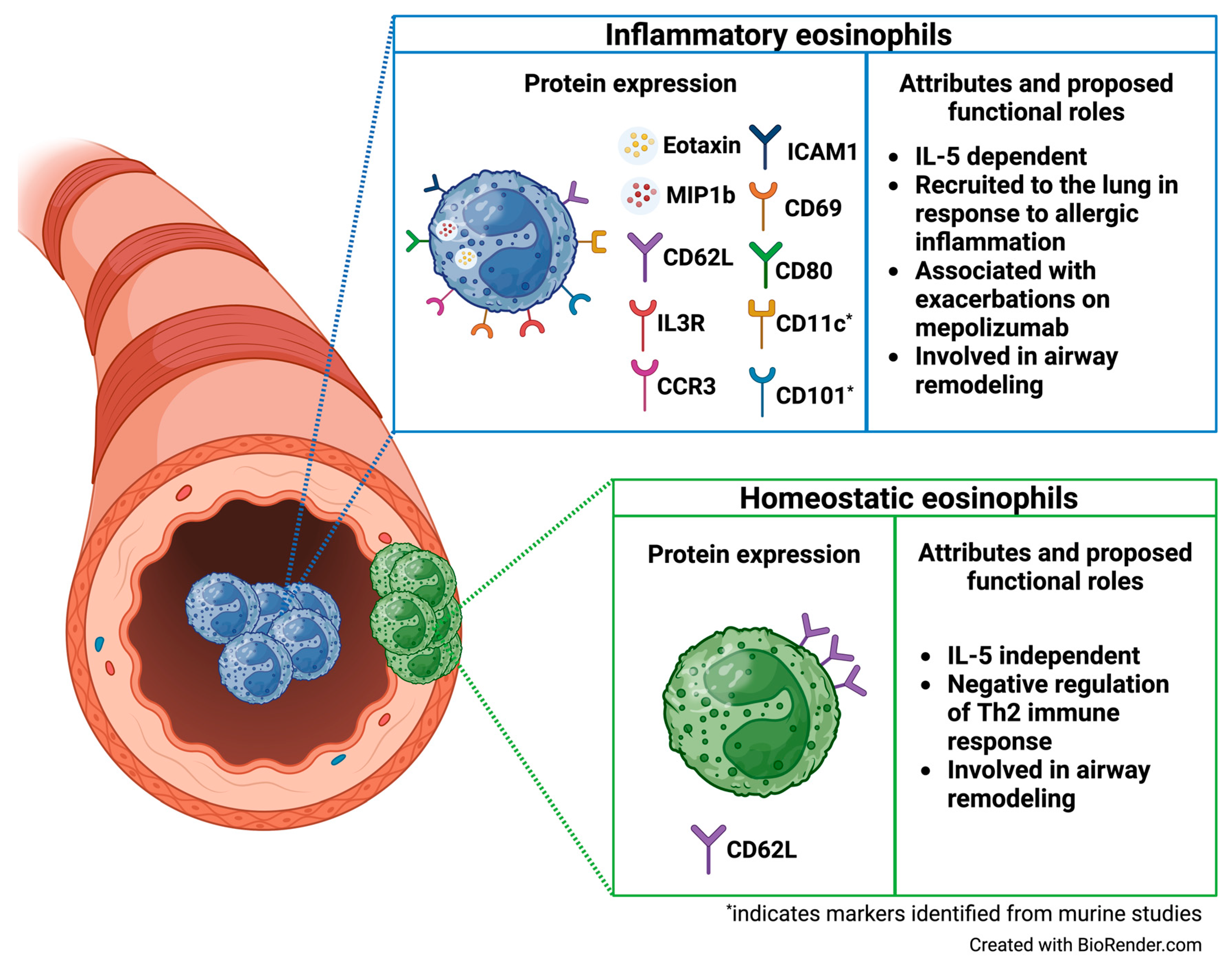
| Mesnil et al. J Clin Invest, 2016 | “rEos” (resident eosinophils) and “iEos” (inflammatory eosinophils) (flow cytometry and FACS sorting; digested lung tissue C57BL/6 mice) | Murine rEos are CD62L+ CD101−, IL-5-independent eosinophils that reside in the lung parenchyma and exhibit high expression of genes involved in negative regulation of the Th2 immune response. In contrast, iEos are CD62L−CD101+, IL-5 dependent inflammatory eosinophils that are recruited to the peribronchial areas in response to allergen challenge and exhibit a pro-inflammatory transcriptomic signature. Limited human data indicates variable CD62L expression and low IL3R expression among rEos, whereas iEos express low levels of CD62L and high levels of IL3R [26]. |
| Andreev et al. Ann Rheum Dis, 2021 | Synovial regulatory eosinophils (“rEos”) and inflammatory lung eosinophils “iEos” (flow cytometry and FACS sorting; digested lung and ankle tissue from WT, ∆dblGATA, and IL-5tf/4Get mice on BALB/cJRj background) | Synovial regulatory eosinophils (“rEos”) accumulate in the synovium in response to allergic asthma and are associated with resolution of arthritis and preservation of joint structure in an IL-5 dependent manner. In a case series of eight patients with rheumatoid arthritis in remission and eosinophilic asthma, six experienced a flare of their RA after initiation of mepolizumab therapy, indicating an immunomodulatory and potentially regenerative role for synovial rEos [27]. |
| Dolitzky et al. Front Immunol, 2021 | “Type 1-activated” versus “Type 2-activated” eosinophils (peritoneal cavity, IC57BL/6 NJ.1638 Il5Tg mice) | Type 2 stimulation with IL-4 leads to a distinct transcriptomic signature including upregulation of CD101 and CD69, whereas type 1 stimulation with E. Coli and/or IFNɣ led to increased expression of several pro-inflammatory cytokines distinct from that seen with type 2 stimulation, including IL6, IL1b, and TNFα, IL-13, and IL-9 [30]. |
| Gurtner et al. Nature, 2023 | A-Eos (active eosinophils) and B-Eos (basal eosinophils) (flow cytometry and MACS sorting; digested lung, stomach, colon, and small intestine from C57BL/6J mice) | Active eosinophils (A-Eos) and basal eosinophils (B-Eos) demonstrate tissue-specific localization in the GI tract and distinct transcriptomic signatures, with B-Eos expressing effector molecules involved in tissue morphogenesis and remodeling and A-Eos expressing co-stimulatory molecules involved in immune modulation. A-Eos express high levels of PD-L1 and CD80 and exhibit higher secretory activity, granularity, and activation relative to B-Eos. In gastrointestinal inflammation, A-Eos are increased compared to B-Eos and demonstrate upregulation of genes involved in granulogenesis, antimicrobial activity, immune modulation, IFNɣ signaling, and MHC-1-restricted antigen processing and presentation. A-Eos and B-Eos subpopulations exist on a differentiation continuum, with A-Eos representing the predicted terminal state for all eosinophil subsets. IL-33 is an important factor in inducing maturation of eosinophils to A-Eos [36]. |
| Januskevicius et al. Cells, 2020 Jurkeviciute et al. J Pers Med, 2021 | CD62L+ rEos and CD62L− iEos (MACS, peripheral blood from patients with asthma and healthy controls) | CD62L+ rEos and CD62L− iEos are present in the blood of patients with severe non-eosinophilic asthma (SNEA), allergic asthma (AA), and healthy subjects (HS). While HS exhibit roughly equal proportions of blood rEos and iEos, rEos predominate in the blood of SNEA patients. iEos predominate in the blood of AA patients, but this is attenuated following allergen challenge, suggesting migration of iEos out of the bloodstream and into the tissue in response to allergen challenge. rEos demonstrate increased adhesive properties compared to iEos, supporting their role as resident cells within tissue [37]. Eosinophil subtype integrin expression analysis demonstrates that rEos exhibit higher expression of β1 integrin compared to iEos, which exhibited higher expression of the ɑMβ2 integrin subunits. In co-culture, both eosinophil subtypes significantly increase ASM proliferation, indicating that both may play a role in airway remodeling [40]. |
| Cabrera Lopez et al. Am J Respir Crit Care Med, 2023 | Siglec-8+CD62L+IL3Rlo rEos and Siglec-8+ CD62Llo IL3Rhi iEos, (flow cytometry, peripheral blood from patients with asthma, COPD, smokers without COPD, and healthy volunteers). | Siglec-8+CD62L+IL3Rlo rEos and Siglec-8+ CD62Llo IL3Rhi iEos are present in patients with asthma, COPD, smokers without COPD, and healthy volunteers. Circulating iEos are significantly higher in patients with asthma compared to the other groups, regardless of eosinophil count and exhibit increased expression of IL-5Rɑ compared to healthy subjects, smokers without COPD, and patients with COPD [41]. |
| Matucci et al. Clin Exp Allergy, 2023 Vultaggio et al. Allergy, 2023 | CD62Lbright rEos and CD62Llow iEos (FACS, peripheral blood and nasal polyp tissue from patients with severe eosinophilic asthma with or without chronic rhinosinusitis with nasal polyposis) | CD62Llow iEos are increased in the blood of patients with severe eosinophilic asthma compared to healthy donors and are highly concentrated in nasal polyp tissue from patients with concomitant chronic rhinosinusitis with nasal polyposis. CD62Llow iEos from the blood demonstrate increased expression of CCR3 and CD69, and decreased expression of IL-5Rɑ, CRTH2, CD86, and CD28 when compared to CD62Lbright rEos, supporting the notion that iEos reflect an activated and functional subset of eosinophils. The proportion of CD62Llow iEos correlate with asthma and nasal polyp scores and number of asthma exacerbations, implicating iEos as a potential biomarker for risk of exacerbations and poor asthma control [42]. Stimulation with IL-5 significantly increases the percentage of iEos in the blood, and exposure to IL-5 blockade with mepolizumab, and an anti-IL-5R antibody abrogates this expansion of iEos. In patients with severe eosinophilic asthma, treatment with mepolizumab results in a significant and sustained reduction in the proportion of CD62Llow eosinophils in the blood. This reduction in iEos following mepolizumab directly correlates with improvement in post-treatment asthma control scores, indicating that mepolizumab may improve asthma control through a direct effect on reducing circulating iEos [43]. |
| Wilson et al. J Allergy Clin Immunol, 2024 | CD62Lhi eosinophils, CD62Lint eosinophils, and CD62Llo eosinophils (mass cytometry (CyTOF), sputum from children with exacerbation-prone eosinophilic asthma enrolled in the MUPPITS2 clinical trial and treated with mepolizumab or placebo) | Three distinct airway eosinophil subpopulations exist and express low, intermediate, and high levels of CD62L (“CD62Llo”, “CD62Lint”, and “CD62Lhi” eosinophils). CD62Lint eosinophils express increased levels of several chemokine receptors and ligands involved in eosinophil activation and mobilization (including CD69, CD80, ICAM1, MIP1β, Eotaxin, and CCR3) compared to the other airway eosinophil subpopulations. Among children treated with mepolizumab, CD62Lint and CD62Lhi eosinophils are increased in those who experience exacerbations on treatment compared to exacerbation-free children, indicating that these eosinophil subpopulations may play a role in break-through exacerbations on mepolizumab [45]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, G.E.; Gautam, S.; Chupp, G.L. Does Eosinophil Heterogeneity Translate into Functional Diversity? A Review of the Evolving Paradigm of Eosinophil Heterogeneity in Asthma. Biomedicines 2024, 12, 2011. https://doi.org/10.3390/biomedicines12092011
Wilson GE, Gautam S, Chupp GL. Does Eosinophil Heterogeneity Translate into Functional Diversity? A Review of the Evolving Paradigm of Eosinophil Heterogeneity in Asthma. Biomedicines. 2024; 12(9):2011. https://doi.org/10.3390/biomedicines12092011
Chicago/Turabian StyleWilson, Gabriella E., Samir Gautam, and Geoffrey L. Chupp. 2024. "Does Eosinophil Heterogeneity Translate into Functional Diversity? A Review of the Evolving Paradigm of Eosinophil Heterogeneity in Asthma" Biomedicines 12, no. 9: 2011. https://doi.org/10.3390/biomedicines12092011





