The Potential Diagnostic Utility of SMAD4 and ACCS in the Context of Inflammation in Type 2 Diabetes Mellitus Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. Results of Multivariate GLM Analysis
3.3. Correlation Matrix
3.4. Neural Network Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Sah, S.P.; Singh, B.; Choudhary, S.; Kumar, A. Animal models of insulin resistance: A review. Pharmacol. Rep. 2016, 68, 1165–1177. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care. 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards of Medical Care in Diabetes. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; Chetty, R. Smad4/DPC4. J. Clin. Pathol. 2018, 71, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, E.; Jaureguizar, A.; Casitas, R.; García-Tovar, S.; Sánchez-Sánchez, B.; Zamarrón, E.; López-Collazo, E.; García-Río, F.; Cubillos-Zapata, C. SMAD4 Overexpression in Patients with Sleep Apnoea May Be Associated with Cardiometabolic Comorbidities. J. Clin. Med. 2020, 9, 2378. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2008, 1782, 197–228. [Google Scholar] [CrossRef]
- Herder, C.; Zierer, A.; Koenig, W.; Roden, M.; Meisinger, C.; Thorand, B. Transforming Growth Factor-β1 and Incident Type 2 Diabetes: Results from the MONICA/KORA case-cohort study, 1984–2002. Diabetes Care 2009, 32, 1921–1923. [Google Scholar] [CrossRef]
- Tan, C.K.; Chong, H.C.; Tan, E.H.P.; Tan, N.S. Getting ‘Smad’ about obesity and diabetes. Nutr. Diabetes 2012, 2, e29. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Wang, L.; Zhao, C.-Y.; Lan, H.-Y. Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules 2022, 12, 373. [Google Scholar] [CrossRef]
- Thomas, J.; Liu, F.; Link, D.C. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr. Opin. Hematol. 2002, 9, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Klimontov, V.V.; Korbut, A.I.; Orlov, N.B.; Dashkin, M.V.; Konenkov, V.I. Multiplex Bead Array Assay of a Panel of Circulating Cytokines and Growth Factors in Patients with Albuminuric and Non-Albuminuric Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 3006. [Google Scholar] [CrossRef]
- Lim, Y.-H.; Joe, J.-H.; Jang, K.-S.; Song, Y.-S.; So, B.-I.; Fang, C.-H.; Shin, J.; Kim, J.-H.; Lim, H.-K.; Kim, K.-S. Effects of granulocyte-colony stimulating factor (G-CSF) on diabetic cardiomyopathy in Otsuka Long-Evans Tokushima Fatty rats. Cardiovasc. Diabetol. 2011, 10, 92. [Google Scholar] [CrossRef]
- Erbaş, O.; Solmaz, V.; Taşkıran, D. Granulocyte colony-stimulating factor provides protection against cardiovascular autonomic neuropathy in streptozotocin-induced diabetes in rats. Diabetes Res. Clin. Pract. 2015, 107, 377–383. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Li, J.-F.; Qu, L.-H.; Li, N. Tyr152 plays a central role in the catalysis of 1-aminocyclopropane-1-carboxylate synthase. J. Exp. Bot. 2005, 56, 2203–2210. [Google Scholar] [CrossRef]
- Chae, H.S.; Faure, F.; Kieber, J.J. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 2003, 15, 545–559. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; Bogaart, G.v.D.; Kox, M.; Dingjan, I.; Neerincx, A.H.; Bendix, M.B.; ter Beest, M.; Harren, F.J.M.; Risby, T.; Pickkers, P.; et al. Ethylene, an early marker of systemic inflammation in humans. Sci. Rep. 2017, 7, 6889. [Google Scholar] [CrossRef]
- Berkelmans, H.; Moeskops, B.; Bominaar, J.; Scheepers, P.; Harren, F. Pharmacokinetics of ethylene in man by on-line laser photoacoustic detection. Toxicol. Appl. Pharmacol. 2003, 190, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hagyousif, Y.A.; Sharaf, B.M.; Zenati, R.A.; El-Huneidi, W.; Bustanji, Y.; Abu-Gharbieh, E.; Alqudah, M.A.Y.; Giddey, A.D.; Abuhelwa, A.Y.; Alzoubi, K.H.; et al. Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms. Int. J. Mol. Sci. 2023, 24, 1604. [Google Scholar] [CrossRef]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- WHO. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Al-Hakeim, H.K.; Abdulzahra, M.S. Correlation Between Glycated Hemoglobin and Homa Indices in Type 2 Diabetes Mellitus: Prediction of Beta-Cell Function from Glycated Hemoglobin / Korelacija Između Glikoliziranog Hemoglobina I Homa Indeksa U Dijabetes Melitusu Tipa 2: Predviđanje Funkcije Beta Ćelija Na Osnovu Glikoliziranog Hemoglobina. J. Med. Biochem. 2015, 34, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rammahi, D.A.; Al-Dujaili, A.H. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Morrish, N.J.; WHO Multinational Study Group; Wang, S.-L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 2001, 44, S14–S21. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Abaffy, T.; Möller, M.; Riemer, D.D.; Milikowski, C.; DeFazio, R.A. A case report-Volatile metabolomic signature of malignant melanoma using matching skin as a control. J. Cancer Sci. Ther 2011, 3, 140. [Google Scholar] [CrossRef]
- Xiao, B.; Lu, C.; Link, H. Cell biology and clinical promise of G-CSF: Immunomodulation and neuroprotection. J. Cell. Mol. Med. 2007, 11, 1272–1290. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.; Liu, R.; Alikhani, M.; Al-Mashat, H.; Trackman, P. Diabetes-enhanced Inflammation and Apoptosis—Impact on Periodontal Pathology. J. Dent. Res. 2006, 85, 15–21. [Google Scholar] [CrossRef]
- Barbu, E.; Popescu, M.-R.; Popescu, A.-C.; Balanescu, S.-M. Inflammation as A Precursor of Atherothrombosis, Diabetes and Early Vascular Aging. Int. J. Mol. Sci. 2022, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Nellaiappan, K.; Preeti, K.; Khatri, D.K.; Singh, S.B. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Curr. Diabetes Rev. 2022, 18, e030821192146. [Google Scholar] [CrossRef]
- Jain, C.; Ansarullah; Bilekova, S.; Lickert, H. Targeting pancreatic β cells for diabetes treatment. Nat. Metab. 2022, 4, 1097–1108. [Google Scholar] [CrossRef]
- Dong, C.; Gongora, R.; Sosulski, M.L.; Luo, F.; Sanchez, C.G. Regulation of transforming growth factor-beta1 (TGF-β1)-induced pro-fibrotic activities by circadian clock gene BMAL1. Respir. Res. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2015, 167, 228–256. [Google Scholar] [CrossRef]
- Clementini, M.; Rossetti, P.; Penarrocha, D.; Micarelli, C.; Bonachela, W.; Canullo, L. Systemic risk factors for peri-implant bone loss: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Arefanian, H.; Abu Al-Roub, A.; Ali, S.; Wilson, A.; Al-Hubail, A.; Al-Beloushi, S.; Al-Zanki, S.; Ahmad, R. Increased circulatory levels of fractalkine (CX3CL1) are associated with inflammatory chemokines and cytokines in individuals with type-2 diabetes. J. Diabetes Metab. Disord. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ordelheide, A.-M.; Gommer, N.; Böhm, A.; Hermann, C.; Thielker, I.; Machicao, F.; Fritsche, A.; Stefan, N.; Häring, H.-U.; Staiger, H. Granulocyte colony-stimulating factor (G-CSF): A saturated fatty acid-induced myokine with insulin-desensitizing properties in humans. Mol. Metab. 2016, 5, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Ehses, J.A.; Perren, A.; Eppler, E.; Ribaux, P.; Pospisilik, J.A.; Maor-Cahn, R.; Gueripel, X.; Ellingsgaard, H.; Schneider, M.K.; Biollaz, G.; et al. Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes 2007, 56, 2356–2370. [Google Scholar] [CrossRef]
- Welte, K.; Gabrilove, J.; Bronchud, M.H.; Platzer, E.; Morstyn, G. Filgrastim (r-metHuG-CSF): The first 10 years. Blood 1996, 88, 1907–1929. [Google Scholar] [CrossRef]
- Edelsberg, J.; Weycker, D.; Bensink, M.; Bowers, C.; Lyman, G.H. Prophylaxis of febrile neutropenia with colony-stimulating factors: The first 25 years. Curr. Med. Res. Opin. 2019, 36, 483–495. [Google Scholar] [CrossRef]
- Porte, D.; Sherwin, R.S.; Baron, A.; Ellenberg, M.; Rifkin, H. Ellenberg and Rifkin’s Diabetes Mellitus; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Moeskops, B.; Steeghs, M.; Van Swam, K.; Cristescu, S.; Scheepers, P.; Harren, F. Real-time trace gas sensing of ethylene, propanal and acetaldehyde from human skin in vivo. Physiol. Meas. 2006, 27, 1187. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Kiss, R.; Hekkert, S.T.L.; Dalby, M.; Harren, F.J.; Risby, T.H.; Marczin, N. Real-time monitoring of endogenous lipid peroxidation by exhaled ethylene in patients undergoing cardiac surgery. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L509–L515. [Google Scholar] [CrossRef]
- Tanito, M.; Agbaga, M.-P.; Anderson, R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free. Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef]
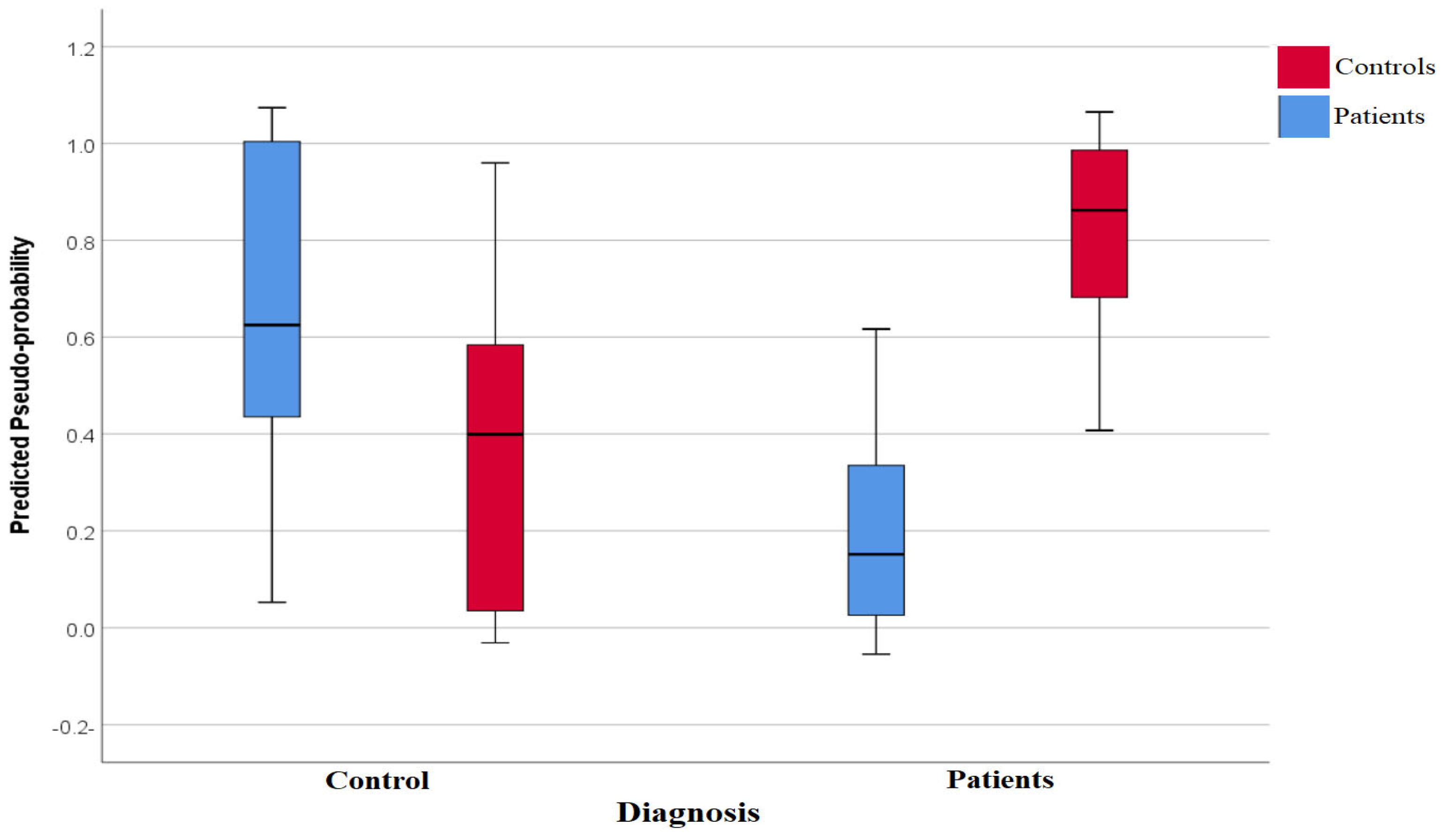
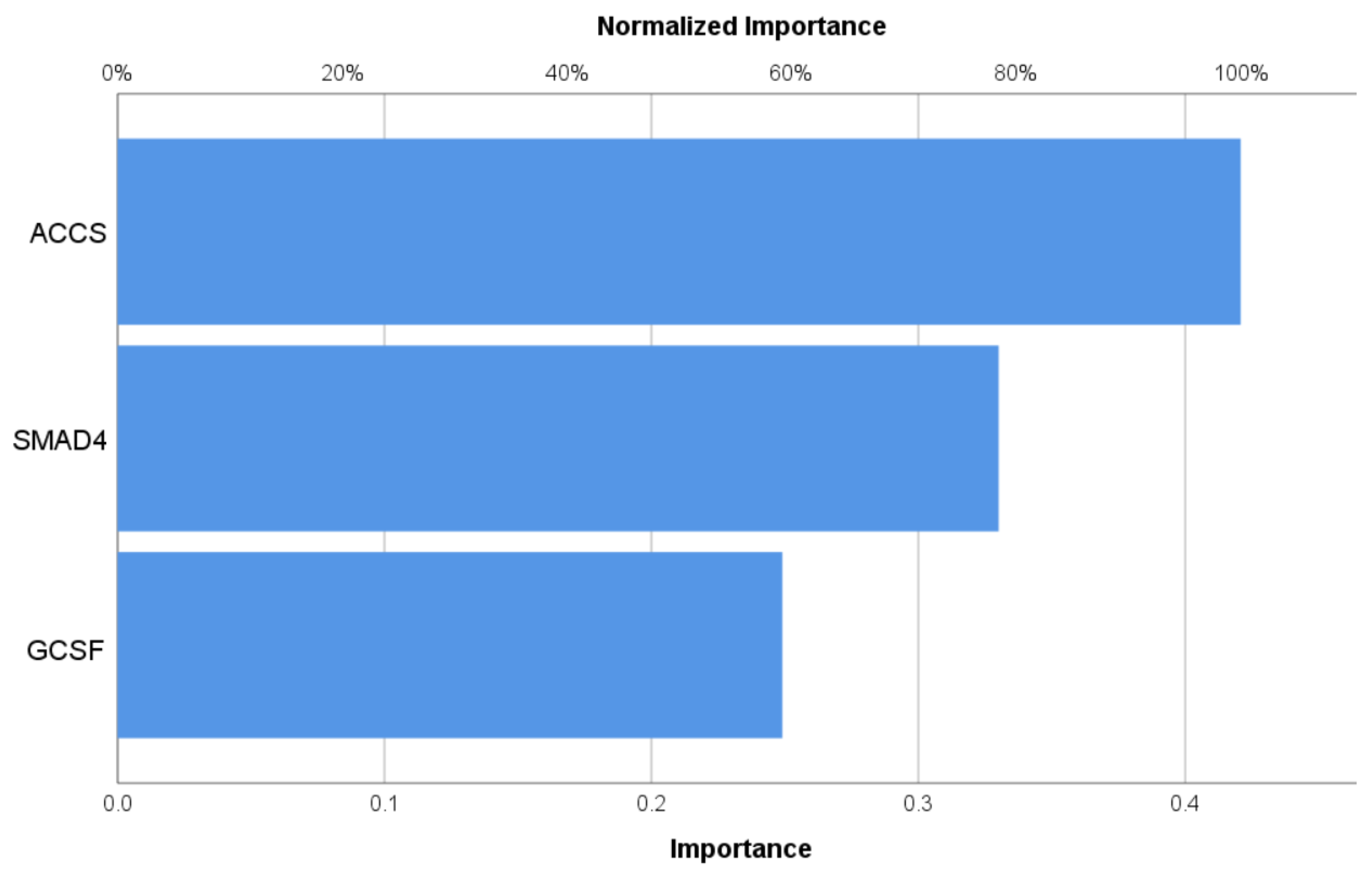
| Variables | Control n = 30 | T2DM n = 60 | df | F/χ2 | p | |
|---|---|---|---|---|---|---|
| Age | years | 44.67 ± 9.279 | 46.62 ± 8.279 | 1/88 | 1.023 | 0.315 |
| Sex | (Female/Male) | 19/11 | 37/23 | 1 | 0.024 | 0.878 |
| BMI | (kg/m2) | 26.778 ± 4.819 | 26.837 ± 3.052 | 1/88 | 0.005 | 0.944 |
| Single/Married | 2/28 | 4/56 | 1 | 0 | 1 | |
| Residency Rural/Urban | 3/27 | 6/54 | 1 | 0 | 1 | |
| Family History | (No/Yes) | 29/1 | 35/25 | 1 | 14.306 | <0.001 |
| Exercise | (No/Yes) | 8/22 | 11/49 | 1 | 1.092 | 0.311 |
| TUD | (No/Yes) | 19/11 | 34/26 | 1 | 0.367 | 0.545 |
| Duration of DM | Years | - | 12.411 ± 5.363 | - | - | - |
| Glucose | mM | 5.532 ± 0.686 | 8.182 ± 2.742 | 1/88 | 27.038 | <0.001 |
| Insulin | pM | 60.228 ± 10.731 | 42.592 ± 13.171 | 1/88 | 40.329 | <0.001 |
| (Insulin/Glucose) ×10−9 | 11.018 ± 2.324 | 5.884 ± 2.774 | 1/88 | 75.975 | <0.001 | |
| HOMA2%B | 83.250 (71.825–102.925) | 37.650 (21.650–57.625) | 1/88 | 75.008 | <0.001 | |
| HOMA2%S | 86.800 (76.025–102.000) | 114.850 (91.825–135.850) | 1/88 | 14.652 | <0.001 | |
| HOMA2IR | 1.151 ± 0.208 | 0.884 ± 0.252 | 1/88 | 25.136 | <0.001 | |
| SMAD4 | ng/mL | 63.335 (34.757–106.058) | 177.793 (84.827–284.376) | 1/88 | 37.916 | <0.001 |
| ACCS | pg/mL | 4.0188 (3.149–5.965) | 6.061 (5.680–7.303) | 1/88 | 39.313 | <0.001 |
| G-CSF | pg/mL | 67.059 (34.344–110.080) | 143.992 (89.753–210.583) | 1/88 | 30.207 | <0.001 |
| Sitagliptin 100 mg | (No/Yes) | 30/0 | 42/18 | 1 | 11.250 | 0.001 |
| Amaryl 4 mg | (No/Yes) | 30/0 | 42/18 | 1 | 11.250 | 0.001 |
| Daonil 5 mg | (No/Yes) | 30/0 | 36/24 | 1 | 16.364 | <0.001 |
| Test | Dependent Variables | Explanatory Variables | F | df | p | Partial η2 |
|---|---|---|---|---|---|---|
| Multivariate | All measured biomarkers | Diagnosis | 12.959 | 9/75 | <0.001 | 0.637 |
| Age | 0.721 | 9/75 | 0.702 | 0.089 | ||
| Sex | 1.258 | 9/75 | 0.288 | 0.156 | ||
| TUD | 0.575 | 9/75 | 0.829 | 0.072 | ||
| Exercise | 0.485 | 9/75 | 0.894 | 0.062 | ||
| BMI | 1.023 | 9/75 | 0.432 | 0.122 | ||
| Between-subject effects | Diagnosis | Insulin/Glucose | 71.983 | 1 | <0.001 | 0.464 |
| HOMA2%B | 71.811 | 1 | <0.001 | 0.487 | ||
| LnACCS | 40.722 | 1 | <0.001 | 0.329 | ||
| Insulin | 36.129 | 1 | <0.001 | 0.303 | ||
| LnSMAD4 | 35.426 | 1 | <0.001 | 0.299 | ||
| LnGCSF | 30.418 | 1 | <0.001 | 0.268 | ||
| Glucose | 27.320 | 1 | <0.001 | 0.248 | ||
| HOMA2IR | 21.768 | 1 | <0.001 | 0.208 | ||
| HOMA2%S | 12.629 | 1 | 0.001 | 0.132 |
| ACCS | G-CSF | SMAD4 | |
|---|---|---|---|
| Glucose | 0.273 ** | 0.344 ** | 0.341 ** |
| Insulin pM | −0.221 * | −0.274 ** | −0.215 * |
| In/G | −0.282 ** | −0.336 ** | −0.306 ** |
| HOMA2%B | −0.292 ** | −0.356 ** | −0.346 ** |
| HOMA2%S | 0.176 | 0.214 * | 0.175 |
| HOMA2IR | −0.176 | −0.214 * | −0.175 |
| ACCS | 1.000 | 0.370 ** | 0.430 ** |
| G-CSF | 0.370 ** | 1.000 | 0.745 ** |
| SMAD4 | 0.430 ** | 0.745 ** | 1.000 |
| Models | T2DM vs. Control | |
|---|---|---|
| Input Layer | Number of units | 3 |
| Rescaling method | Normalized | |
| Hidden layers | Number of hidden layers | 2 |
| Number of units in hidden layer 1 | 1 | |
| Number of units in hidden layer 2 | 2 | |
| Activation Function | Hyperbolic tangent | |
| Output layer | Dependent variables | T2DM vs. Control |
| Number of units | 2 | |
| Activation function | Identity | |
| Error function | Sum of squares | |
| Training | Sum of squares error term | 7.36 |
| % Incorrect or relative error | 22.0% | |
| Prediction (sensitivity, specificity) | 50%, 91.0% | |
| Testing | Sum of squares error | 2.15 |
| Percent Incorrect Predictions | 13.0% | |
| Prediction (sensitivity, specificity) | 71.4%, 93.8% | |
| AUC ROC | 0.902 | |
| Holdout | Percent Incorrect Predictions | 11.8% |
| Prediction (sensitivity, specificity) | 85.7%, 90.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalsada, H.K.; Abdulsaheb, Y.S.; Zolghadri, S.; Al-Hakeim, H.K.; Stanek, A. The Potential Diagnostic Utility of SMAD4 and ACCS in the Context of Inflammation in Type 2 Diabetes Mellitus Patients. Biomedicines 2024, 12, 2015. https://doi.org/10.3390/biomedicines12092015
Abdalsada HK, Abdulsaheb YS, Zolghadri S, Al-Hakeim HK, Stanek A. The Potential Diagnostic Utility of SMAD4 and ACCS in the Context of Inflammation in Type 2 Diabetes Mellitus Patients. Biomedicines. 2024; 12(9):2015. https://doi.org/10.3390/biomedicines12092015
Chicago/Turabian StyleAbdalsada, Habiba Khdair, Yusra Sebri Abdulsaheb, Samaneh Zolghadri, Hussein Kadhem Al-Hakeim, and Agata Stanek. 2024. "The Potential Diagnostic Utility of SMAD4 and ACCS in the Context of Inflammation in Type 2 Diabetes Mellitus Patients" Biomedicines 12, no. 9: 2015. https://doi.org/10.3390/biomedicines12092015







