Interleukin-37 Inhibits Interleukin-1β-Induced Articular Chondrocyte Apoptosis by Suppressing Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Preparation and Culture
2.2. Cell Proliferation Assay
2.3. Quantitative Real-Time PCR
2.4. Western Blot Assay
2.5. Reactive Oxygen Species Measurement
2.6. Apoptosis Analysis
2.7. Hoechst 33342 Staining
2.8. Cell Cycle Analysis
2.9. Statistical Analysis
3. Results
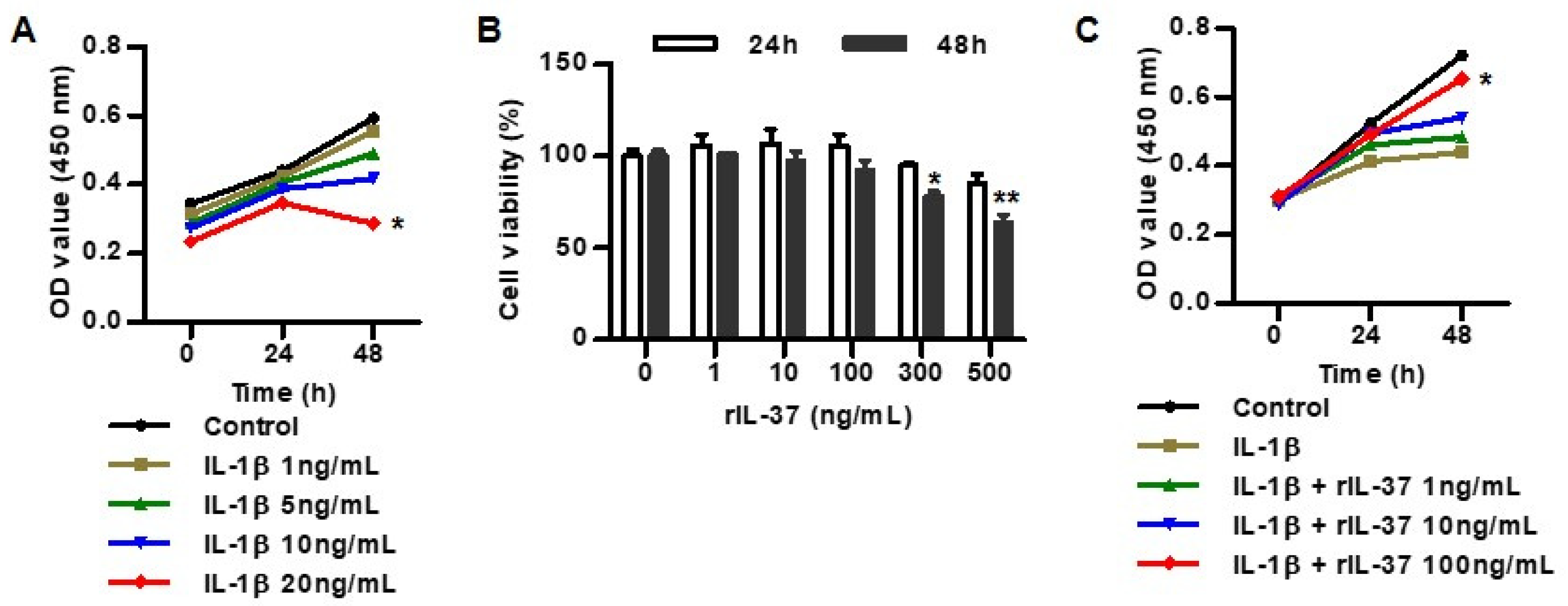
3.1. IL-37 Inhibits IL-1β-Induced Cytotoxicity in Rat Chondrocytes
3.2. IL-37 Inhibits Inflammatory Cytokines and Extracellular Matrix Degradation in IL-1β-Mediated Rat Chondrocytes
3.3. IL-37 Suppresses Mitochondrial ROS Generation in IL-1β-Stimulated Chondrocytes
3.4. IL-1β Induced Apoptosis in Rat Chondrocytes
3.5. IL-37 Inhibits IL-1β-Induced Chondrocyte Apoptosis
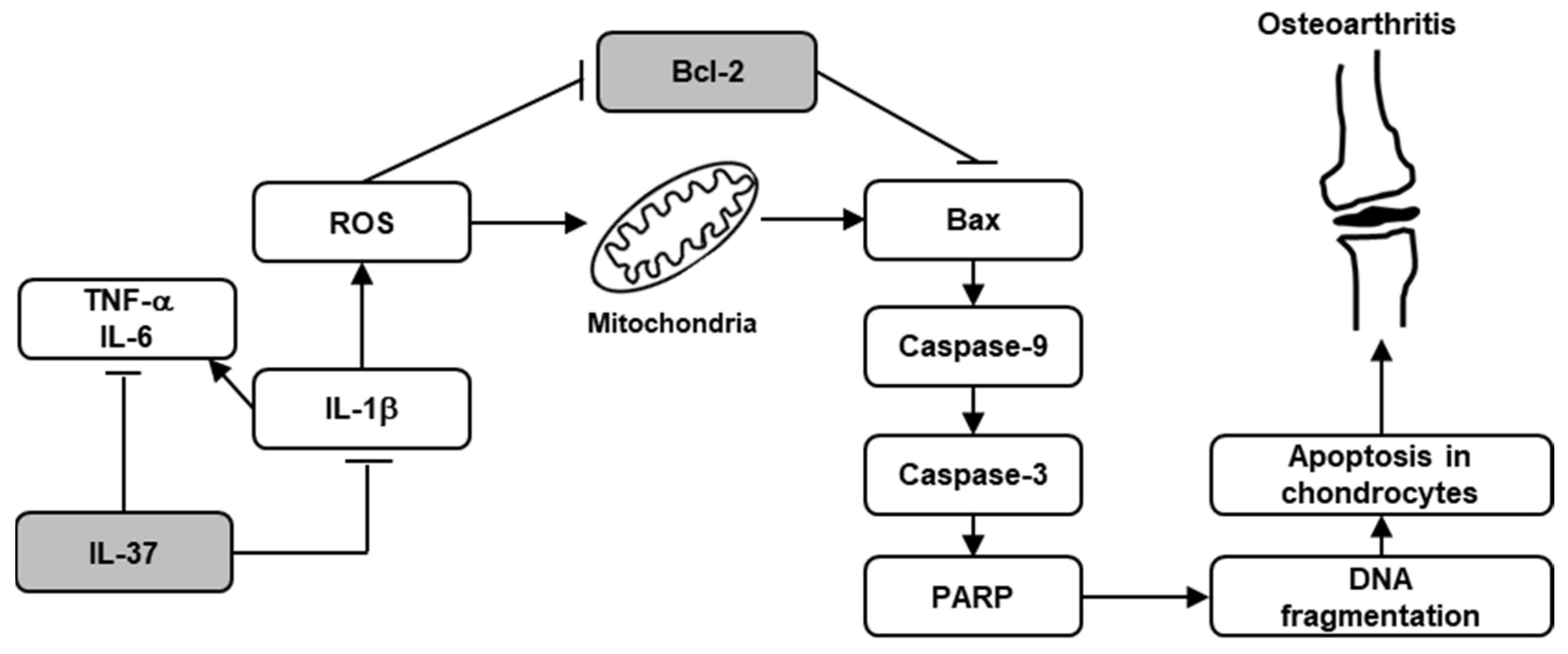
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, L.A.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Price, A. Osteoarthritis: A narrative review of molecular approaches to disease management. Arthritis Res. Ther. 2023, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Favero, M.; Ruggieri, P.; Macchi, V.; Carniel, E.L. Mechanical behavior of infrapatellar fat pad of patients affected by osteoarthritis. J. Biomech. 2022, 131, 110931. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L.; et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-Dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Guitian, R.; Vázquez-Martul, E.; de Toro, F.J.; Galdo, F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998, 41, 284–289. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Falcieri, E.; Battistelli, M. Chondrocyte death involvement in osteoarthritis. Cell. Tissue. Res. 2022, 389, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, E.; Griffin, T.M.; Humphrey, M.B. Innate Immune Responses and Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 45. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; van Lent, P.L.E.M.; van der Kraan, P.M. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Stöve, J.; Huch, K.; Günther, K.P.; Scharf, H.P. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology 2000, 68, 144–149. [Google Scholar] [CrossRef]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed]
- Schlaak, J.F.; Schwarting, A.; Knolle, P.; Meyer zum Büschenfelde, K.H.; Mayet, W. Effects of Th1 and Th2 cytokines on cytokine production and ICAM-1 expression on synovial fibroblasts. Ann. Rheum. Dis. 1995, 54, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Calich, A.L.; Domiciano, D.S.; Fuller, R. Osteoarthritis: Can anti-cytokine therapy play a role in treatment. Clin. Rheumatol. 2010, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology 2017, 56, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhou, K.; Ye, Z. Biology of interleukin-37 and its role in autoimmune diseases (Review). Exp. Ther. Med. 2022, 24, 495. [Google Scholar] [CrossRef]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- van Geffen, E.W.; van Caam, A.P.; van Beuningen, H.M.; Vitters, E.L.; Schreurs, W.; van de Loo, F.A.; van Lent, P.L.; Koenders, M.I.; Blaney Davidson, E.N.; van der Kraan, P.M. IL37 dampens the IL1β-induced catabolic status of human OA chondrocytes. Rheumatology 2017, 56, 351–361. [Google Scholar] [CrossRef]
- van Geffen, E.W.; van Caam, A.P.M.; Schreurs, W.; van de Loo, F.A.; van Lent, P.L.E.M.; Koenders, M.I.; Thudium, C.S.; Bay-Jensen, A.C.; Blaney Davidson, E.N.; van der Kraan, P.M. IL-37 diminishes proteoglycan loss in human OA cartilage: Donor-specific link between IL-37 and MMP-3. Osteoarthr. Cartil. 2019, 27, 148–157. [Google Scholar] [CrossRef]
- van Geffen, E.W.; van Caam, A.P.M.; Vitters, E.L.; van Beuningen, H.M.; van de Loo, F.A.; van Lent, P.L.E.M.; Koenders, M.I.; van der Kraan, P.M. Interleukin-37 Protects Stem Cell-Based Cartilage Formation in an Inflammatory Osteoarthritis-Like Microenvironment. Tissue. Eng. Part A 2019, 25, 1155–1166. [Google Scholar] [CrossRef]
- Rai, V.; Dilisio, M.F.; Samadi, F.; Agrawal, D.K. Counteractive Effects of IL-33 and IL-37 on Inflammation in Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 5690. [Google Scholar] [CrossRef]
- Ding, L.; Hong, X.; Sun, B.; Huang, Q.; Wang, X.; Liu, X.; Li, L.; Huang, Z.; Liu, D. IL-37 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Sci. Rep. 2017, 7, 11601. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Giraudeau, B.; Conrozier, T.; Marliere, J.; Kiefer, P.; Goupille, P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: A multicenter study. J. Rheumatol. 2005, 32, 1317–1323. [Google Scholar]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Caron, J.P.; Evans, C.; Robbins, P.D.; Georgescu, H.I.; Jovanovic, D.; Fernandes, J.C.; Martel-Pelletier, J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997, 40, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Tardif, G.; Martel-Pelletier, J.; Lascau-Coman, V.; Dupuis, M.; Moldovan, F.; Sheppard, M.; Krishnan, B.R.; Pelletier, J.P. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: Prevention of osteoarthritis progression. Am. J. Pathol. 1999, 154, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Liu, H.; Cheng, Q.; Li, H.; Huang, H. Mitochondrial pathology in osteoarthritic chondrocytes. Curr. Drug. Targets 2014, 15, 710–719. [Google Scholar] [CrossRef]
- Reed, K.N.; Wilson, G.; Pearsall, A.; Grishko, V.I. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol. Cell. Biochem. 2014, 397, 195–201. [Google Scholar] [CrossRef]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.L.; Pearsall, A.W., 4th; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martínez-Gomariz, M.; Fernández, M.; Blanco, F.J. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef]
- Gavriilidis, C.; Miwa, S.; von Zglinicki, T.; Taylor, R.W.; Young, D.A. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Rheum. 2013, 65, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Mei, J.; Yuan, K.; Han, X.; Qiao, H.; Tang, T. Isorhamnetin attenuates osteoarthritis by inhibiting osteoclastogenesis and protecting chondrocytes through modulating reactive oxygen species homeostasis. J. Cell. Mol. Med. 2019, 23, 4395–4407. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Ochs, R.L.; Schwarz, H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Pathol. 1995, 146, 75–85. [Google Scholar]
- Clancy, R.M.; Abramson, S.B.; Kohne, C.; Rediske, J. Nitric oxide attenuates cellular hexose monophosphate shunt response to oxidants in articular chondrocytes and acts to promote oxidant injury. J. Cell. Physiol. 1997, 172, 183–191. [Google Scholar] [CrossRef]
- Kühn, K.; Hashimoto, S.; Lotz, M. IL-1 beta protects human chondrocytes from CD95-induced apoptosis. J. Immunol. 2000, 164, 2233–2239. [Google Scholar] [CrossRef]
- Zhuang, C.; Ni, S.; Yang, Z.C.; Liu, R.P. Oxidative Stress Induces Chondrocyte Apoptosis through Caspase-Dependent and Caspase-Independent Mitochondrial Pathways and the Antioxidant Mechanism of Angelica Sinensis Polysaccharide. Oxid. Med. Cell. Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef]
- Wang, B.W.; Jiang, Y.; Yao, Z.L.; Chen, P.S.; Yu, B.; Wang, S.N. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model. Drug Des. Dev. Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef]
- Li, B.; Ji, X.; Tian, F.; Gong, J.; Zhang, J.; Liu, T. Interleukin-37 Attenuates Lipopolysaccharide (LPS)-Induced Neonatal Acute Respiratory Distress Syndrome in Young Mice via Inhibition of Inflammation and Cell Apoptosis. Med. Sci. Monit. 2020, 26, e920365. [Google Scholar] [CrossRef]
- Xiao, S.; Song, X.; Zheng, M.; Cao, X.; Ai, G.; Li, B.; Zhao, G.; Yuan, H. Interleukin-37 ameliorates atherosclerosis by regulating autophagy-mediated endothelial cell apoptosis and inflammation. Int. Immunopharmacol. 2023, 118, 110098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Zhou, Y.; Fei, B. Interleukin 37 (IL-37) Reduces High Glucose-Induced Inflammation, Oxidative Stress, and Apoptosis of Podocytes by Inhibiting the STAT3-Cyclophilin A (CypA) Signaling Pathway. Med. Sci. Monit. 2020, 26, e922979. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Kim, B.; Choe, J.-Y.; Kim, J.-W.; Park, K.-Y. Interleukin-37 Inhibits Interleukin-1β-Induced Articular Chondrocyte Apoptosis by Suppressing Reactive Oxygen Species. Biomedicines 2024, 12, 2025. https://doi.org/10.3390/biomedicines12092025
Kim S-K, Kim B, Choe J-Y, Kim J-W, Park K-Y. Interleukin-37 Inhibits Interleukin-1β-Induced Articular Chondrocyte Apoptosis by Suppressing Reactive Oxygen Species. Biomedicines. 2024; 12(9):2025. https://doi.org/10.3390/biomedicines12092025
Chicago/Turabian StyleKim, Seong-Kyu, Boyoung Kim, Jung-Yoon Choe, Ji-Won Kim, and Ki-Yeun Park. 2024. "Interleukin-37 Inhibits Interleukin-1β-Induced Articular Chondrocyte Apoptosis by Suppressing Reactive Oxygen Species" Biomedicines 12, no. 9: 2025. https://doi.org/10.3390/biomedicines12092025




