Subclinical Left Ventricular Dysfunction over Seven-Year Follow-Up in Type 2 Diabetes Patients without Cardiovascular Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Standard Echocardiography
2.4. Speckle-Tracking Echocardiography
2.5. Statistical Analysis
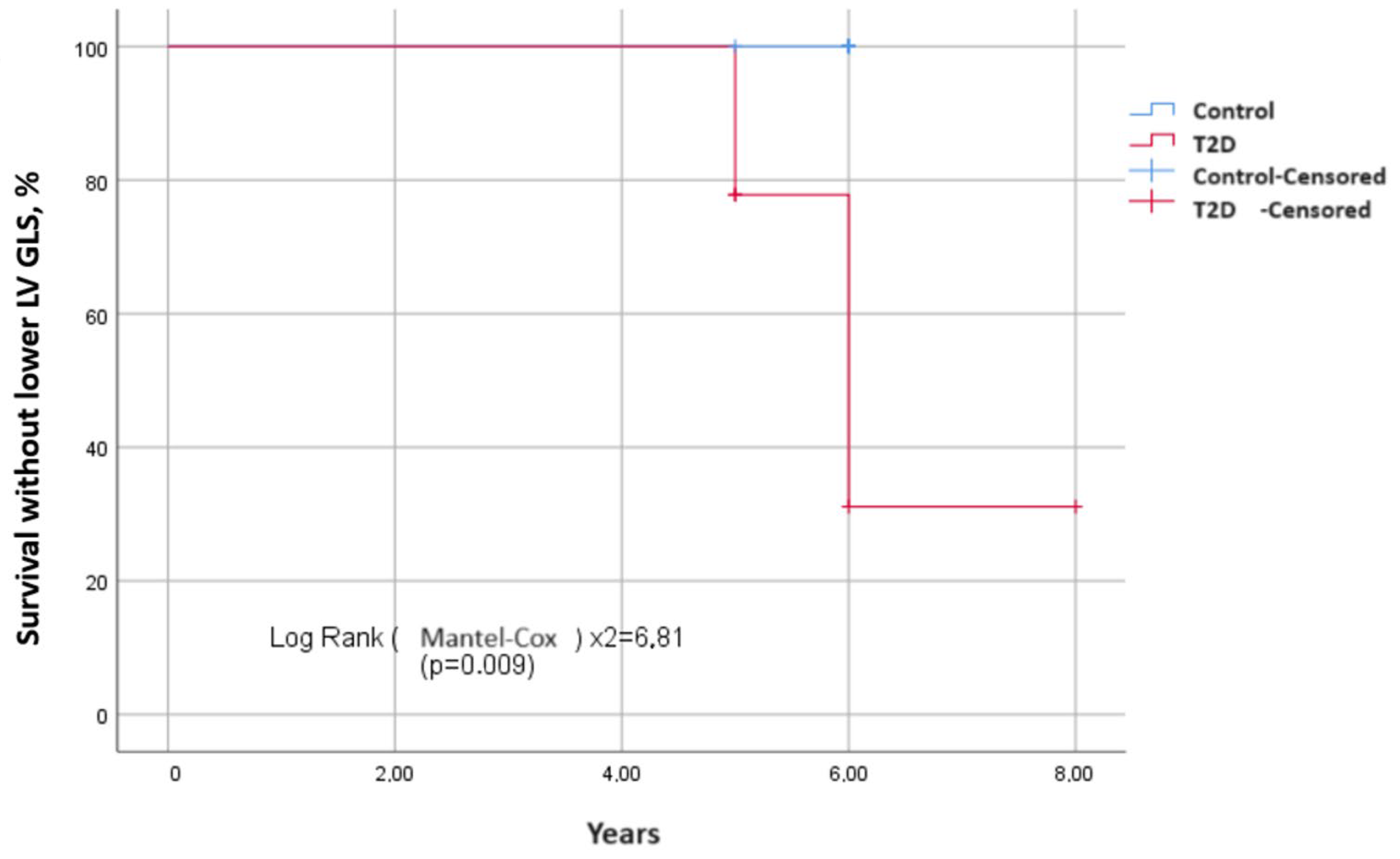
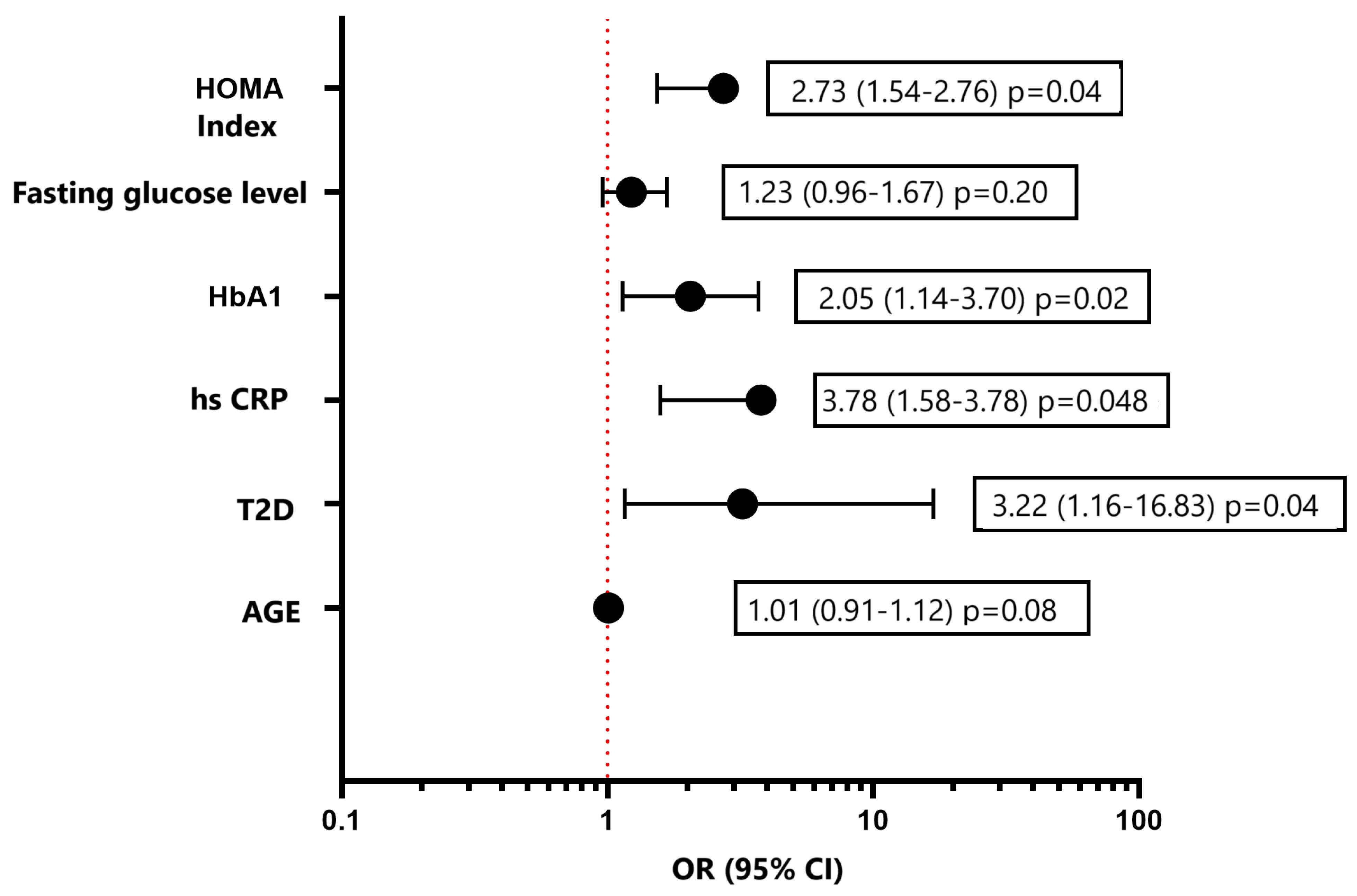
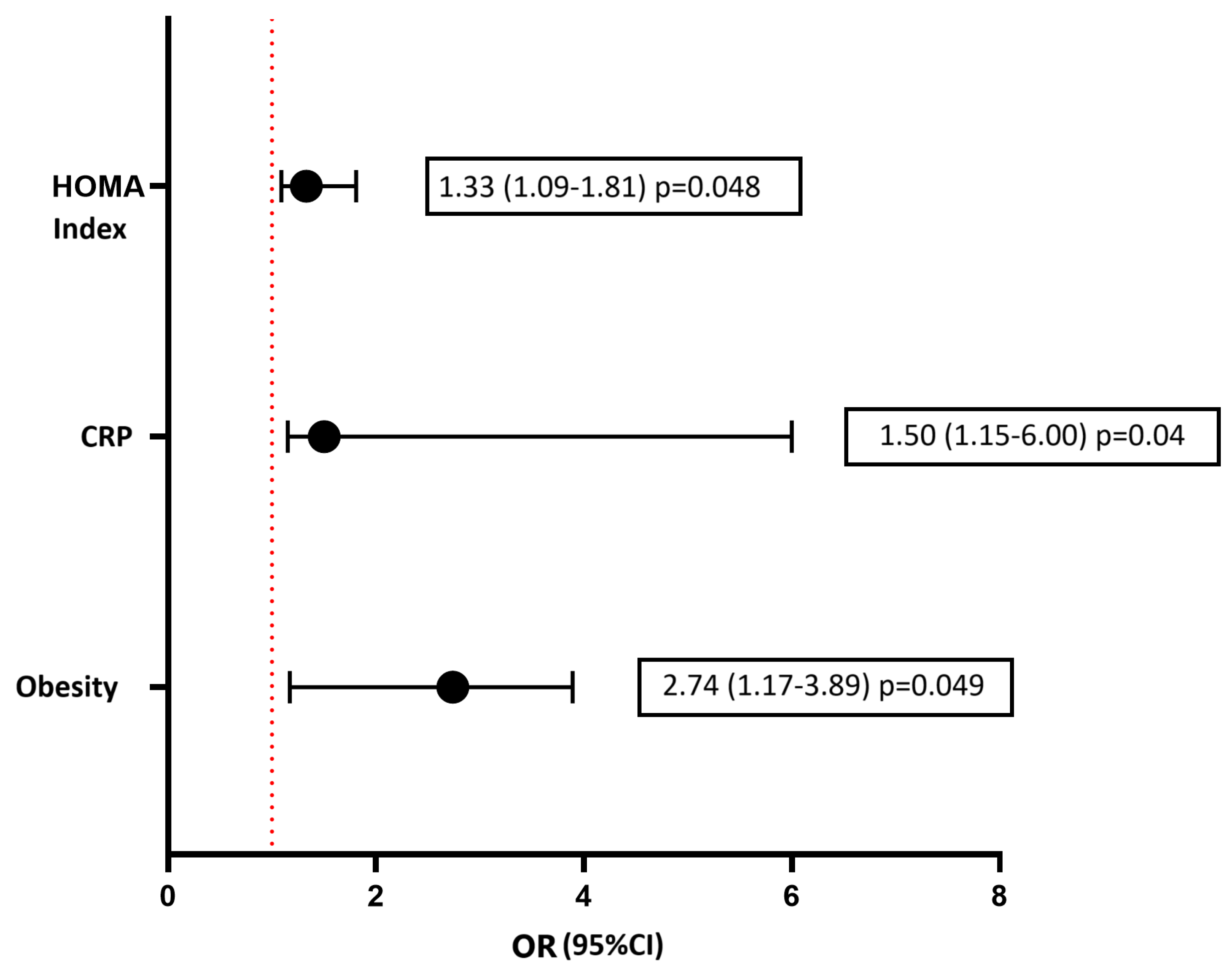
3. Results
3.1. Clinical Characteristics of Patients Included in the Study over Follow-Up
3.2. Echocardiography
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 7 July 2021).
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Wang, X.; Chen, Y.; Pang, P.; Yang, Q.; Lin, J.; Deng, S.; Wu, S.; Fan, G.; et al. Diabetic cardiomyopathy: Clinical phenotype and practice. Front. Endocrinol. 2022, 13, 1032268. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Del Prato, S.; Rosenstock, J.; Butler, J.; Ezekowitz, J.; Ibrahim, N.E.; Lam, C.S.; Marwick, T.; Tang, W.H.W.; Liu, Y.; et al. Characterizing diabetic cardiomyopathy: Baseline results from the ARISE-HF trial. Cardiovasc. Diabetol. 2024, 23, 49. [Google Scholar] [CrossRef]
- Segar, M.W.; Khan, M.S.; Patel, K.V.; Butler, J.; Tang, W.W.; Vaduganathan, M.; Lam, C.S.; Verma, S.; McGuire, D.K.; Pandey, A. Prevalence and Prognostic Implications of Diabetes With Cardiomyopathy in Community-Dwelling Adults. J. Am. Coll. Cardiol. 2021, 78, 1587–1598. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Patel, N.T.; Villarreal-Gonzalez, M.A.; Taub, P.R. Prevalence of diabetic cardiomyopathy in patients with type 2 diabetes in a large academic medical center. BMC Med. 2024, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Marwick, T.H. Asymptomatic Left Ventricular Diastolic Dysfunction. Predicting Progression to Symptomatic Heart Failure. J. Am. Coll. Cardiol. 2020, 13, 215–227. [Google Scholar] [CrossRef]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef]
- Stanton, A.M.; Vaduganathan, M.; Chang, L.S.; Turchin, A.; Januzzi, J.L.; Aroda, V.R. Asymptomatic Diabetic Cardiomyopathy: An Underrecognized Entity in Type 2 Diabetes. Curr. Diabetes Rep. 2021, 21, 41. [Google Scholar] [CrossRef]
- Grigorescu, E.D.; Lacatusu, C.M.; Floria, M.; Mihai, B.-M.; Cretu, I.; Sorodoc, L. Left Ventricular Diastolic Dysfunction in Type 2 Diabetes-Progress and Perspectives. Diagnostics 2019, 9, 121. [Google Scholar] [CrossRef]
- From, A.M.; Scott, C.G.; Chen, H.H. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction: A population-based study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar] [CrossRef]
- Chee, K.H.; Tan, K.L.; Luqman, I.; Saiful, S.S.; Chew, Y.Y.; Chinna, K.; Tan, A.T.B. Prevalence and Predictors of Left Ventricular Diastolic Dysfunction in Malaysian Patients with Type 2 Diabetes Mellitus without Prior Known Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 676862. [Google Scholar] [CrossRef]
- Utina, T.G.; Akasheva, D.U.; Korsunsky, D.V.; Drapkina, O.M. Significance of standard and speckle-tracking echocardiography for early diagnosis of asymptomatic left ventricular dysfunction in type 2 diabetes. Cardiovasc. Ther. Prev. 2023, 22, 47–57. [Google Scholar] [CrossRef]
- Silva, T.R.W.; Silva, R.L.; Martins, A.F.; Marques, J.L.B. Role of Strain in the Early Diagnosis of Diabetic Cardiomyopathy. Arq. Bras. Cardiol. Imagem. Cardiovasc. 2022, 35, eabc293. [Google Scholar] [CrossRef]
- Hodzic, A.; Ribault, V.; Maragnes, P.; Milliez, P.; Saloux, E.; Labombarda, F. Decreased regional left ventricular myocardial strain in type 1 diabetic children: A first sign of diabetic cardiomyopathy? J. Trans. Intern. Med. 2016, 4, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ernande, L.; Audureau, E.; Jellis, C.L.; Bergerot, C.; Henegar, C.; Sawaki, D.; Czibik, G.; Volpi, C.; Canoui-Poitrine, F.; Thibault, H.; et al. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J. Am. Coll. Cardiol. 2017, 70, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.J.; Marwick, T.H.; Haluska, B.A.; Leano, R.; Hordern, M.D.; Hare, J.L.; Fang, Z.Y.; Prins, J.B.; Stanton, T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 2015, 101, 1061–1066. [Google Scholar] [CrossRef]
- Li, Y.; Xian, H.; Xu, Y.; Li, W.; Guo, J.; Wan, K.; Wang, J.; Xu, Z.; Zhang, Q.; Han, Y.; et al. The impact of type 2 diabetes mellitus on the clinical profile, myocardial fibrosis, and prognosis in non-ischemic dilated cardiomyopathy: A prospective cohort study. Cardiovasc. Diabetol. 2024, 23, 48. [Google Scholar] [CrossRef]
- Ling, L.; Sung, S.H. Outcomes of asymptomatic left ventricular dysfunction in the patient with or without type 2 diabetic Mellitus. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.835. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, G.B.; Erqou, S.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Assessing the Risk of Progression from Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure. A Systematic Overview and Meta-Analysis. JACC Heart Fail. 2016, 4, 237–248. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Wang, S.; Tian, C.; Gao, Z.; Zhang, B.; Zhao, L. Research status and trends of the diabetic cardiomyopathy in the past 10 years (2012–2021): A bibliometric analysis. Front. Cardiovasc. Med. 2022, 9, 1018841. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Cameli, M. Echocardiography strain: Why is it used more and more? Eur. Heart J. Suppl. 2022, 24 (Suppl. I), I38–I42. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. J. Am. Coll. Cardiol. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Ritchie, R.; Shaw, J.E.; Kaye, D. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 339. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.-H. The Role of Echocardiography in Evaluating Cardiovascular Diseases in Patients with Diabetes Mellitus. Diabetes Metab. J. 2023, 47, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Bouthoorn, S.; Valstar, G.B.; Gohar, A.; Ruijter, H.M.D.; Reitsma, H.B.; Hoes, A.W.; Rutten, F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diabetes Vasc. Dis. Res. 2018, 15, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.; Anker, S.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Tanaka, H.; Tatsumi, K.; Matsuzoe, H.; Matsumoto, K.; Hirata, K.-I. Impact of diabetes mellitus on left ventricular longitudinal function of patients with non-ischemic dilated cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 84. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Huynh, Q.; Nolan, M.; Negishi, K.; Marwick, T.H. Diagnosis of Nonischemic Stage B Heart Failure in Type 2 Diabetes Mellitus: Optimal Parameters for Prediction of Heart Failure. JACC Cardiovasc. Imaging 2018, 11, 1390–1400. [Google Scholar] [CrossRef]
- Athithan, L.; Gulsin, G.S.; McCann, G.P.; Levelt, E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J. Diabetes 2019, 10, 490–510. [Google Scholar] [CrossRef]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Meijers, W.C.; Hoekstra, T.; Jaarsma, T.; van Veldhuisen, D.J.; de Boer, R.A. Patients with heart failure with preserved ejection fraction and low levels of natriuretic peptides. Neth. Heart J. 2016, 24, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Omote, K.; Reddy, J.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, ehab911. [Google Scholar] [CrossRef] [PubMed]
- Halabi, A.; Potter, E.; Yang, H.; Wright, L.; Sacre, J.W.; Shaw, J.E.; Marwick, T.H. Association of biomarkers and risk scores with subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 278. [Google Scholar] [CrossRef]
- Shah, S.J. BNP: Biomarker Not Perfect in heart failure with preserved ejection fraction. Eur. Heart J. 2022, 43, 1952. [Google Scholar] [CrossRef]
- Utina, T.G.; Akasheva, D.U.; Korsunsky, D.V.; Dzhioeva, O.N.; Drapkina, O.M. Biomarkers and subclinical left ventricular dysfunction in patients with type 2 diabetes without clinical manifestations of cardiovascular diseases. Cardiovasc. Ther. Prev. 2024, 23, 3914. [Google Scholar] [CrossRef]
- Dal Canto, E.; Scheffer, M.; Kortekaas, K.; Driessen-Waaijer, A.; Paulus, W.J.; van Heerebeek, L. Natriuretic Peptide Levels and Stages of Left Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction. Biomedicines 2023, 11, 867. [Google Scholar] [CrossRef]


| Variable | Patients with T2D (n = 29) | Patients without T2D (n = 28) | p Value |
|---|---|---|---|
| Sex m, n (%) | 13 (46.0) | 12 (45.0) | 0.14 |
| Age, years, Me [IQR] | 68 [58–74] | 60 [55–63] | 0.81 |
| Body mass index, kg/m2, Me [IQR] | 28 [27–33] | 27 [21–28] | 0.05 |
| Obesity 1-degree, n (%) | 13 (44.8) | 3 (11.0) | 0.08 |
| Exercise capacity (METs) | 6.0 ± 1.9 | 6.2 ± 2.0 | 0.59 |
| Current smoker, n (%) | 15 (52.0) | 16 (57.0) | 0.35 |
| Creatinine, µmol/L, Me [IQR] | 87 [70–91] | 74 [68–94] | 0.89 |
| Cholesterol, mmol/L, Me [IQR] | 4.4 [4.1–5.4] | 5.6 [5.0–6.1] | 0.003 |
| Low-density lipoprotein-cholesterol, mmol/L, Me [IQR] | 2.6 [1.6–3.3] | 3.6 [2.9–4.1] | 0.009 |
| High-density lipoprotein-cholesterol, mmol/L, Me [IQR] | 1.3 [1.2–1.4] | 1.5 [1.3–1.9] | 0.025 |
| Triglycerides, mmol/L, Me [IQR] | 1.7 [0.9–2.4] | 1.0 [0.7–1.3] | 0.085 |
| Increase in TG, n (%) | 10 (50.0) | 17 (89.5) | 0.017 |
| Presence of dyslipidemia, n (%) | 25 (68.8) | 16 (73.7) | 0.53 |
| C-reactive protein, mL/L, Me [IQR] | 1.7 [1.1–2.57] | 1.22 [0.66–3.01] | 0.75 |
| Increase in CRP, n (%) | 3 (11.0) | 0 | 0.63 |
| Fibrinogen, g/L, Me [IQR] | 4 [3.1–5.8] | 3 [2.6–3.9] | 0.12 |
| Increase in fibrinogen, n (%) | 5 (18.0) | 1 (4.0) | 0.034 |
| NT-proBNP, pg/mL, Me [IQR] | 104 [60–121] | 90 [52–116] | 0.8 |
| Increase in NT-proBNP, n (%) | 5 (18.0) | 6 (21.0) | 0.851 |
| Fasting plasma glucose, mmol/L, Me [IQR] | 8.23 [6.75–10.5] | 5.6 [4.7–6.3] | <0.001 |
| Glycated hemoglobin, %, Me [IQR] | 6.5 [6.0–7.2] | 5.3 [5.1–5.7] | <0.001 |
| Insulin, µU/mL, Me [IQR] | 13.6 [8–22.3] | 8.0 [5.7–11.0] | 0.007 |
| C-peptide, ng/mL, Me [IQR] | 2.74 [2.05–3.75] | 1.66 [1.5–2.3] | 0.013 |
| NOMA Index, Me [IQR] | 6 [3–9] | 2 [1–3] | <0.001 |
| Availability of IR, n (%) | 25 (87.5) | 9 (32.0) | 0.004 |
| Variable | Patients with T2D | p Value | Patients without T2D | p Value | ||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |||
| LVD, n (%) | 27 (53) | 18 (61) | 0.004 * | 28 (39) | 9 (32) | 0.48 |
| LV mass index, g/m2 | 95 [84.1–110] | 90.5 [82.0–110.5] | 0.49 | 84.8 [73–98.3] | 88.0 [84.0–100.0] | 0.84 |
| LA volume index, mL/m2 | 27.0 [24.2–31.1] | 26.5 [23.2–33.0] | 0.63 | 26 [23.9–27.8] | 25.0 [21.0–33.0] | 0.66 |
| IVS in diastole, cm | 1.2 [1.1–1.2] | 1.25 [1.15–1.4] | 0.046 | 1.0 [1.0–1.2] | 1.2 [1.0–1.2] | 0.06 |
| Posterior Wall thickness, cm | 1.0 [1.0–1.0] | 1.1 [1.0–1.15] | 0.007 | 0.93 [0.9–1.0] | 0.9 [0.9–1.0] | 0.53 |
| Relative Wall Thickness | 0.44 [0.41–0.48] | 0.49 [0.45–0.53] | 0.06 | 0.43 [0.41–0.46] | 0.45 [0.43–0.49] | 0.58 |
| Concentric Hypertrophy, n (%) | 41 (22) | 15 (53) | 0.025 | 26 (12) | 5 (16) | 0.15 |
| Concentric Remodeling, n (%) | 28 (15) | 12 (42) | 0.063 | 33 (16) | 6 (20) | 0.65 |
| Transmitral E/A ratio | 0.8 [0.7–0.9] | 0.7 [0.6–0.9] | 0.03 * | 1.1 [0.9–1.2] | 1.2 [0.7–1.3] | 0.15 |
| Isovolumic relaxation time, ms | 91 [87–96] | 83 [72–99] | 0.49 | 77 [70–88] | 74 [67–95] | 0.83 |
| Deceleration time, ms | 218 [187–232] | 226 [191–277] | 0.05 * | 181 [169–203] | 183 [169–229] | 0.53 |
| Average (med/lat) e’, cm/s | 8.7 [7.1–9.6] | 6.7 [5.6–8.7] | 0.034 * | 10.2 [8.3–13.6] | 9.0 [8.0–12.6] | 0.059 |
| E/e’ | 7.2 [6.4–8.5] | 8.7 [7.9–11.1] | 0.017 * | 6.4 [5.4–7.6] | 7.0 [5.6–8.8] | 0.42 |
| Ejection Fraction, % | 60 [59–62] | 65 [58–79] | 0.088 | 64 [61–66] | 67 [60–71] | 0.48 |
| GLS, % | −17.6 ± 1.4 | −17.2 ± 1.24 | 0.008 * | −19.6 ± 1.8 | −19.6 ± 1.8 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasheva, D.U.; Utina, T.G.; Dzhioeva, O.N.; Drapkina, O.M. Subclinical Left Ventricular Dysfunction over Seven-Year Follow-Up in Type 2 Diabetes Patients without Cardiovascular Diseases. Biomedicines 2024, 12, 2031. https://doi.org/10.3390/biomedicines12092031
Akasheva DU, Utina TG, Dzhioeva ON, Drapkina OM. Subclinical Left Ventricular Dysfunction over Seven-Year Follow-Up in Type 2 Diabetes Patients without Cardiovascular Diseases. Biomedicines. 2024; 12(9):2031. https://doi.org/10.3390/biomedicines12092031
Chicago/Turabian StyleAkasheva, Dariga Uaydinichna, Tatyana Gennadyevna Utina, Olga Nikolaevna Dzhioeva, and Oxana Mikhailovna Drapkina. 2024. "Subclinical Left Ventricular Dysfunction over Seven-Year Follow-Up in Type 2 Diabetes Patients without Cardiovascular Diseases" Biomedicines 12, no. 9: 2031. https://doi.org/10.3390/biomedicines12092031






