The Pathomechanism and Current Treatments for Chronic Interstitial Cystitis and Bladder Pain Syndrome
Abstract
1. Introduction
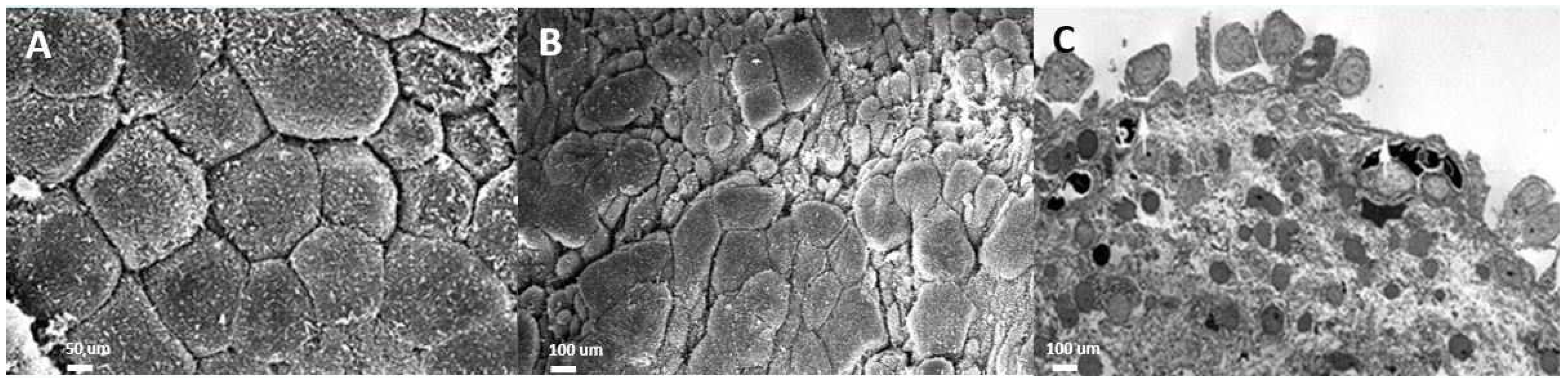
2. The Pathophysiology of NHIC
3. Urothelial Dysfunction and Treatments for NHIC
4. Chronic Inflammation and Intravesical Treatment for NHIC
5. Autoimmune Reactions and Treatment for NHIC
6. Pelvic Floor Dysfunction and Psychological Factors
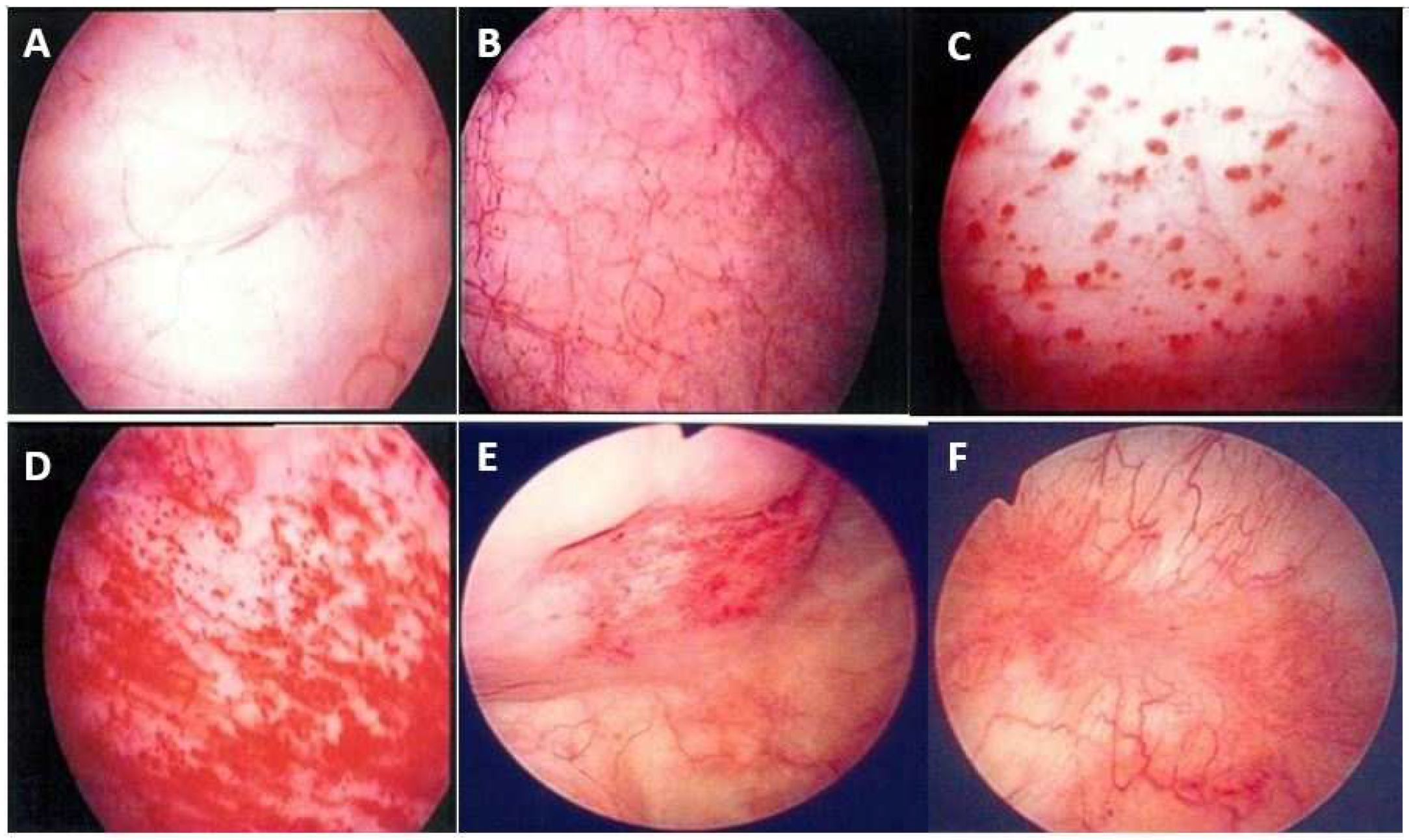
7. Pathophysiology, Clinical Presentations, and Treatment for HIC
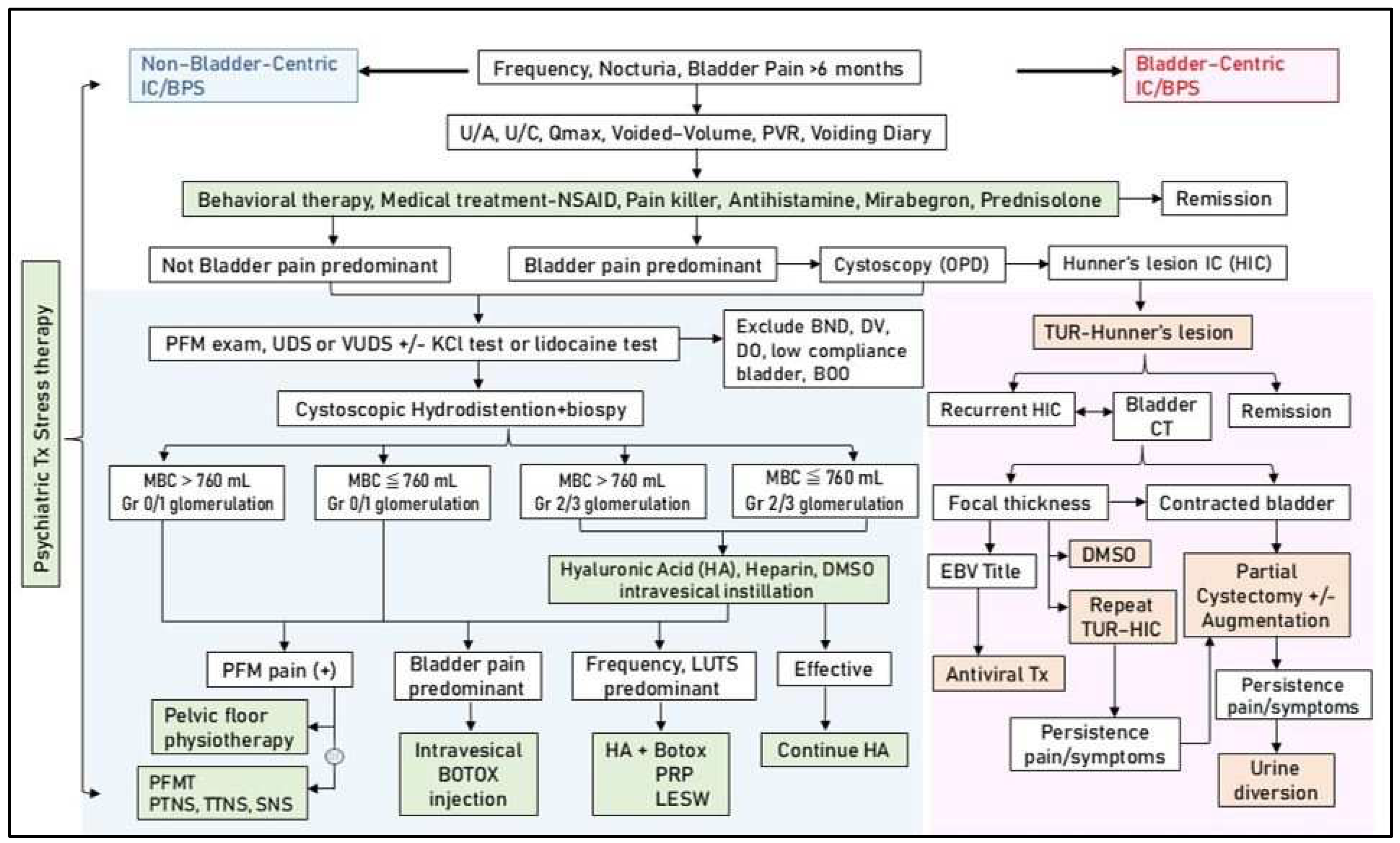
8. Multimodal Active Therapy Based on the Pathophysiology of IC/BPS
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanno, P.M.; Burks, D.A.; Clemens, J.Q.; Dmochowski, R.R.; Erickson, D.; Fitzgerald, M.P.; Forrest, J.B.; Gordon, B.; Gray, M.; Mayer, R.D.; et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2011, 185, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.Y.; Wu, M.P.; Wang, I.T.; Wu, J.C.; Chin, H.Y. Overactive bladder with urodynamic study-induced bladder pain: An overactive bladder subtype with symptoms similar to those of interstitial cystitis/painful bladder syndrome. Medicine 2023, 102, e32790. [Google Scholar] [CrossRef]
- Bernie, J.E.; Hagey, S.; Albo, M.E.; Parsons, C.L. The intravesical potassium sensitivity test and urodynamics: Implications in a large cohort of patients with lower urinary tract symptoms. J. Urol. 2001, 166, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Krieger, J.N.; Pontari, M.A.; Buchwald, D.; Hou, X.; Landis, J.R.; MAPP Research Network. Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J. Urol. 2015, 194, 1634–1641. [Google Scholar] [CrossRef]
- Messing, E.M.; Stamey, T.A. Interstitial cystitis: Early diagnosis, pathology, and treatment. Urology 1978, 12, 381–392. [Google Scholar] [CrossRef]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Lee, J.G.; Kim, D.Y.; Lee, K.S.; Interstitial Cystitis Guideline Committee. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int. J. Urol. 2009, 16, 597–615. [Google Scholar] [CrossRef]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. 2020, 27, 491–503. [Google Scholar] [CrossRef]
- Whitmore, K.E.; Fall, M.; Sengiku, A.; Tomoe, H.; Logadottir, Y.; Kim, Y.H. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2019, 26 (Suppl. 1), 26–34. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Erickson, D.R.; Varela, N.P.; Lai, H.H. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2022, 208, 34–42. [Google Scholar] [CrossRef]
- Masterson, J.M.; Castañeda, P.R.; Kim, J. Pathophysiology and Clinical Biomarkers in Interstitial Cystitis. Urol. Clin. N. Am. 2023, 50, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C. Interstitial cystitis: A chronic pelvic pain syndrome. Med. Clin. N. Am. 2004, 88, 467–481. [Google Scholar] [CrossRef]
- Tornic, J.; Engeler, D. Latest insights into the pathophysiology of bladder pain syndrome/interstitial cystitis. Curr. Opin. Urol. 2024, 34, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Ho, H.C.; Jiang, Y.H.; Lee, C.L.; Hsu, Y.H.; Kuo, H.C. Electron microscopic characteristics of interstitial cystitis/bladder pain syndrome and their association with clinical condition. PLoS ONE 2018, 13, e0198816. [Google Scholar] [CrossRef] [PubMed]
- Anderström, C.R.; Fall, M.; Johansson, S.L. Scanning electron microscopic findings in interstitial cystitis. Br. J. Urol. 1989, 63, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Kuo, H.C. Pathomechanism of Interstitial Cystitis/Bladder Pain Syndrome and Mapping the Heterogeneity of Disease. Int. Neurourol. J. 2016, 20 (Suppl. 2), S95–S104. [Google Scholar] [CrossRef]
- Parsons, C.L. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007, 69 (Suppl. 4), 9–16. [Google Scholar] [CrossRef]
- Homma, Y.; Akiyama, Y.; Tomoe, H.; Furuta, A.; Ueda, T.; Maeda, D.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Oh, S.J.; et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2020, 27, 578–589. [Google Scholar] [CrossRef]
- Gülpınar, Ö.; Esen, B.; Kayış, A.; Gökçe, M.İ.; Süer, E. Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment of bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 2018, 37, 257–262. [Google Scholar] [CrossRef]
- Pyo, J.S.; Cho, W.J. Systematic Review and Meta-Analysis of Intravesical Hyaluronic Acid and Hyaluronic Acid/Chondroitin Sulfate Instillation for Interstitial Cystitis/Painful Bladder Syndrome. Cell. Physiol. Biochem. 2016, 39, 1618–1625. [Google Scholar] [CrossRef]
- Parsons, C.L.; Koziol, J.A.; Proctor, J.G.; Zupkas, P.; Argade, S. Heparin and alkalinized lidocaine versus alkalinized lidocaine for treatment of interstitial cystitis symptoms. Can. J. Urol. 2015, 22, 7739–7744. [Google Scholar]
- Cervigni, M. Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy. Transl. Androl. Urol. 2015, 4, 638–642. [Google Scholar] [PubMed]
- Shie, J.H.; Kuo, H.C. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011, 108 Pt 2, E136–E141. [Google Scholar] [CrossRef]
- Wang, H.J.; Tyagi, P.; Lin, T.K.; Huang, C.C.; Lee, W.C.; Chancellor, M.B.; Chuang, Y.C. Low energy shock wave therapy attenuates mitochondrial dysfunction and improves bladder function in HCl induced cystitis in rats. Biomed. J. 2022, 45, 482–490. [Google Scholar] [CrossRef]
- Dias, D.; Mendes, P.A.; Oliveira, P.D.; Pinto, R.A. What is in the pipeline for new treatments for bladder pain syndrome/interstitial cystitis? Curr. Opin. Urol. 2024, 34, 58–63. [Google Scholar] [CrossRef]
- Chen, P.Y.; Cheng, J.H.; Wu, Z.S.; Chuang, Y.C. New Frontiers of Extracorporeal Shock Wave Medicine in Urology from Bench to Clinical Studies. Biomedicines 2022, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Jiang, Y.H.; Hsu, Y.H.; Ho, H.C.; Birder, L.A.; Lin, T.Y.; Kuo, H.C. Improved Urothelial Cell Proliferation, Cytoskeleton and Barrier Function Protein Expression in the Patients With Interstitial Cystitis/Bladder Pain Syndrome After Intravesical Platelet-Rich Plasma Injection. Int. Neurourol. J. 2022, 26 (Suppl 1), S57–S67. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.H.; Liu, H.T.; Kuo, H.C. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology 2012, 79, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Jiang, Y.H.; Hsu, Y.H.; Ho, H.C.; Kuo, H.C. Decreased urothelial cytoskeleton and cell proliferation protein expression suggest interstitial cystitis/bladder pain syndrome patients with Hunner’s lesion and grade 3 glomerulation might be different from other types of patients. Int. J. Urol. 2021, 28, 823–830. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lu, J.H.; Chuang, S.M.; Chueh, K.S.; Juan, T.J.; Liu, Y.C.; Juan, Y.S. Urinary Biomarkers in Interstitial Cystitis/Bladder Pain Syndrome and Its Impact on Therapeutic Outcome. Diagnostics 2021, 12, 75. [Google Scholar] [CrossRef]
- Sant, G.R.; Kempuraj, D.; Marchand, J.E.; Theoharides, T.C. The mast cell in interstitial cystitis: Role in pathophysiology and pathogenesis. Urology 2007, 69 (Suppl. 4), 34–40. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Peng, C.H.; Liu, H.T.; Kuo, H.C. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS ONE 2013, 8, e76779. [Google Scholar] [CrossRef] [PubMed]
- Overman, E.L.; Rivier, J.E.; Moeser, A.J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS ONE 2012, 7, e39935. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, M.G.; Çakıroğlu, B. Efficacy of Pentosan Polysulfate Treatment in Patients with Interstitial Cystitis/Bladder Pain Syndrome. Bladder 2023, 10, e21200007. [Google Scholar] [CrossRef] [PubMed]
- Brière, R.; Bouchard, F.; Ismail, S.; Gareau Labelle, A.K.; Tu, L.M. A pilot study on oral cyclosporine A in association with fulguration for the treatment of interstitial cystitis with Hunner’s lesions. Neurourol. Urodyn. 2022, 41, 1498–1504. [Google Scholar] [CrossRef]
- Shan, H.; Zhang, E.W.; Zhang, P.; Zhang, X.D.; Zhang, N.; Du, P.; Yang, Y. Differential expression of histamine receptors in the bladder wall tissues of patients with bladder pain syndrome/interstitial cystitis—Significance in the responsiveness to antihistamine treatment and disease symptoms. BMC Urol. 2019, 19, 115. [Google Scholar] [CrossRef]
- Lim, Y.N.; Dwyer, P.; Murray, C.; Karmakar, D.; Rosamilia, A.; Thomas, E. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome. Int. Urogynecol. J. 2017, 28, 1085–1089. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Kuo, H.C. The clinical application of intravesical botulinum toxin A injection in patients with overactive bladder and interstitial cystitis. Tzu Chi Med. J. 2023, 35, 31–37. [Google Scholar] [CrossRef]
- Chen, Y.H.; Man, K.M.; Chen, W.C.; Liu, P.L.; Tsai, K.S.; Tsai, M.Y.; Wu, Y.T.; Chen, H.Y. Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model. Diagnostics 2020, 10, 381. [Google Scholar] [CrossRef]
- Chen, C.L.; Kao, C.C.; Yang, M.H.; Fan, G.Y.; Cherng, J.H.; Tsao, C.W.; Wu, S.T.; Cha, T.L.; Meng, E. A Novel Intravesical Dextrose Injection Improves Lower Urinary Tract Symptoms on Interstitial Cystitis/Bladder Pain Syndrome. Front. Pharmacol. 2021, 12, 755615. [Google Scholar] [CrossRef]
- Kuo, H.C. Repeated intravesical onabotulinumtoxinA injections are effective in treatment of refractory interstitial cystitis/bladder pain syndrome. Int. J. Clin. Pract. 2013, 67, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Jiang, Y.H.; Tsai, Y.C.; Kuo, Y.C. Intravesical botulinum toxin-A injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment—A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourol. Urodyn. 2016, 35, 609–614. [Google Scholar] [CrossRef]
- Shie, J.H.; Liu, H.T.; Wang, Y.S.; Kuo, H.C. Immunohistochemical evidence suggests repeated intravesical application of botulinum toxin A injections may improve treatment efficacy of interstitial cystitis/bladder pain syndrome. BJU Int. 2013, 111, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Di, X.P.; Luo, D.Y.; Jin, X.; Zhao, W.Y.; Li, H.; Wang, K.J. Efficacy and safety comparison of pharmacotherapies for interstitial cystitis and bladder pain syndrome: A systematic review and Bayesian network meta-analysis. Int. Urogynecol. J. 2021, 32, 1129–1141. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Lin, T.Y.; Kuo, H.C. Intravesical injections of platelet-rich plasma is effective and safe in treatment of interstitial cystitis refractory to conventional treatment-A prospective clinical trial. Neurourol. Urodyn. 2019, 38, 703–709. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Kuo, H.C. Changes in the Ultrastructure of the Bladder Urothelium in Patients with Interstitial Cystitis after Intravesical Injections of Platelet-Rich Plasma. Biomedicines 2022, 10, 1182. [Google Scholar] [CrossRef]
- Hung, M.J.; Tsai, C.P.; Ying, T.H.; Chen, G.D.; Su, H.L.; Tseng, C.J. Improved symptoms and signs of refractory interstitial cystitis in women after intravesical Nanofat plus platelet-rich plasma grafting: A pilot study. J. Chin. Med. Assoc. 2022, 85, 730–735. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Sun, H.; Luo, X.; Wang, P.; Fang, S.; Chen, H.; Sun, X. Clinical effects of extracorporeal shock-wave therapy and ultrasound-guided local corticosteroid injections for plantar fasciitis in adults: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e13687. [Google Scholar] [CrossRef]
- Medrano-Sánchez, E.M.; Peña-Cantonero, B.; Candón-Ballester, P.; Blanco-Díaz, M.; Díaz-Mohedo, E. Effectiveness of Low-Intensity Extracorporeal Shock Wave Therapy in Erectile Dysfunction: An Analysis of Sexual Function and Penile Hardness at Erection: An Umbrella Review. J. Pers. Med. 2024, 14, 177. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Meng, E.; Chancellor, M.; Kuo, H.C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome-A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2020, 39, 1505–1514. [Google Scholar] [CrossRef]
- Jhang, L.S.; Hsieh, W.C.; Huang, T.X.; Chou, Y.C.; Lo, T.S.; Liang, C.C.; Lin, Y.H. Use of low-intensity extracorporeal shock wave therapy in the management of interstitial cystitis/bladder pain syndrome patients: A thirty case study in a tertiary medical center. Neurourol. Urodyn. 2023, 42, 65–72. [Google Scholar] [CrossRef]
- Parsons, J.K.; Parsons, C.L. The historical origins of interstitial cystitis. J. Urol. 2004, 171, 20–22. [Google Scholar] [CrossRef]
- Larsen, S.; Thompson, S.A.; Hald, T.; Barnard, R.J.; Gilpin, C.J.; Dixon, J.S.; Gosling, J.A. Mast cells in interstitial cystitis. Br. J. Urol. 1982, 54, 283–286. [Google Scholar] [CrossRef]
- Lynes, W.L.; Flynn, S.D.; Shortliffe, L.D.; Lemmers, M.; Zipser, R.; Roberts, L.J., 2nd; Stamey, T.A. Mast cell involvement in interstitial cystitis. J. Urol. 1987, 138, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Hald, T.; Larsen, S.; Nielsen, V.G. Histamine content and mast cell count of detrusor muscle in patients with interstitial cystitis and other types of chronic cystitis. Br. J. Urol. 1983, 55, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Lamale, L.M.; Lutgendorf, S.K.; Zimmerman, M.B.; Kreder, K.J. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology 2006, 68, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, E.J.; Alfthan, O.S.; Oravisto, K.J. Antitissue antibodies in interstitial cystitis. Clin. Exp. Immunol. 1972, 11, 333–339. [Google Scholar]
- Santacruz, J.C.; Pulido, S.; Arzuaga, A.; Mantilla, M.J.; Londono, J. Lupus Cystitis, From Myth to Reality: A Narrative Review. Cureus 2021, 13, e20409. [Google Scholar] [CrossRef]
- Logadottir, Y.; Delbro, D.; Fall, M.; Gjertsson, I.; Jirholt, P.; Lindholm, C.; Peeker, R. Cytokine expression in patients with bladder pain syndrome/interstitial cystitis ESSIC type 3C. J. Urol. 2014, 192, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Sonehara, K.; Maeda, D.; Katoh, H.; Naito, T.; Yamamoto, K.; Biobank Japan Project; Morisaki, T.; Ishikawa, S.; Ushiku, T.; et al. Genome-wide association study identifies risk loci within the major histocompatibility complex region for Hunner-type interstitial cystitis. Cell Rep. Med. 2023, 4, 101114. [Google Scholar] [CrossRef]
- Akiyama, Y.; Harada, K.; Miyakawa, J.; Kreder, K.J.; O’Donnell, M.A.; Daichi, M.; Katoh, H.; Hori, M.; Owari, K.; Futami, K.; et al. Th1/17 polarization and potential treatment by an anti-interferon-γ DNA aptamer in Hunner-type interstitial cystitis. iScience 2023, 26, 108262. [Google Scholar] [CrossRef]
- el-Mansoury, M.; Boucher, W.; Sant, G.R.; Theoharides, T.C. Increased urine histamine and methylhistamine in interstitial cystitis. J. Urol. 1994, 152 Pt 1, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Sant, G.R. Hydroxyzine therapy for interstitial cystitis. Urology 1997, 49 (Suppl. 5A), 108–110. [Google Scholar] [CrossRef] [PubMed]
- Sant, G.R.; Propert, K.J.; Hanno, P.M.; Burks, D.; Culkin, D.; Diokno, A.C.; Hardy, C.; Landis, J.R.; Mayer, R.; Madigan, R.; et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J. Urol. 2003, 170, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Minogiannis, P.; El-Mansoury, M.; Betances, J.A.; Sant, G.R.; Theoharides, T.C. Hydroxyzine inhibits neurogenic bladder mast cell activation. Int. J. Immunopharmacol. 1998, 20, 553–563. [Google Scholar] [CrossRef]
- Forsell, T.; Ruutu, M.; Isoniemi, H.; Ahonen, J.; Alfthan, O. Cyclosporine in severe interstitial cystitis. J. Urol. 1996, 155, 1591–1593. [Google Scholar] [CrossRef]
- Sairanen, J.; Tammela, T.L.; Leppilahti, M.; Multanen, M.; Paananen, I.; Lehtoranta, K.; Ruutu, M. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: A randomized comparative study. J. Urol. 2005, 174, 2235–2238. [Google Scholar] [CrossRef]
- Sairanen, J.; Hotakainen, K.; Tammela, T.L.; Stenman, U.H.; Ruutu, M. Urinary epidermal growth factor and interleukin-6 levels in patients with painful bladder syndrome/interstitial cystitis treated with cyclosporine or pentosan polysulfate sodium. Urology 2008, 71, 630–633. [Google Scholar] [CrossRef]
- Mishra, N.N.; Riedl, C.; Shah, S.; Pathak, N. Intravesical tacrolimus in treatment of intractable interstitial cystitis/bladder pain syndrome—A pilot study. Int. J. Urol. 2019, 26 (Suppl. 1), 68–72. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.C. A randomized, double-blind, placebo controlled trial of adalimumab for interstitial cystitis/bladder pain syndrome. J. Urol. 2014, 191, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.C. A Randomized, Double-blind, Placebo-controlled Trial of Certolizumab Pegol in Women with Refractory Interstitial Cystitis/Bladder Pain Syndrome. Eur. Urol. 2018, 74, 623–630. [Google Scholar] [CrossRef]
- Segawa, C.; Wada, T.; Furuichi, K.; Takasawa, K.; Yokoyama, H.; Kobayashi, K. Steroid pulse therapy in lupus cystitis. Intern. Med. 1996, 35, 155–158. [Google Scholar] [CrossRef]
- Meulders, Q.; Michel, C.; Marteau, P.; Grange, J.D.; Mougenot, B.; Ronco, P.; Mignon, F. Association of chronic interstitial cystitis, protein-losing enteropathy and paralytic ileus with seronegative systemic lupus erythematosus: Case report and review of the literature. Clin. Nephrol. 1992, 37, 239–244. [Google Scholar]
- Jeong, H.J. Effects of a Short Course of Oral Prednisolone in Patients with Bladder Pain Syndrome with Fluctuating, Worsening Pain despite Low-Dose Triple Therapy. Int. Neurourol. J. 2012, 16, 175–180. [Google Scholar] [CrossRef][Green Version]
- Akiyama, Y.; Niimi, A.; Nomiya, A.; Taguchi, S.; Yamada, Y.; Sato, Y.; Yamada, D.; Maeda, D.; Ushiku, T.; Kume, H.; et al. Efficacy and Safety of Low-dose Oral Prednisolone for Patients with Refractory Hunner-type Interstitial Cystitis. Eur. Urol. Open Sci. 2023, 56, 1–8. [Google Scholar] [CrossRef]
- Greer, J.A.; Heye, K.R.; McGlynn, A.; Johansson, S.; Vaccaro, C.M. Association of Pelvic Floor Disorders, Perceived Psychological Stress, and Military Service in U.S. Navy Servicewomen: A Cross-Sectional Survey. Urogynecology 2023, 29, 966–973. [Google Scholar] [CrossRef]
- Gupta, P.; Gallop, R.; Spitznagle, T.; Lai, H.; Tu, F.; Krieger, J.N.; Clemens, J.Q.; Bradley, C.S.; Yang, C.; Sutcliffe, S.; et al. Is Pelvic Floor Muscle Tenderness a Distinct Urologic Chronic Pelvic Pain Syndrome Phenotype? Findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Symptom Pattern Study. J. Urol. 2022, 208, 341–349. [Google Scholar] [CrossRef]
- Lai, H.H.; Thu, J.H.L.; Moh, F.V.; Paradis, A.; Vetter, J. Clustering of Patients with Interstitial Cystitis/Bladder Pain Syndrome and Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J. Urol. 2019, 202, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.R.; Chuang, F.C.; Chang, W.C.; Kuo, H.C. Pelvic Floor Myofascial Pain Might Influence Treatment Outcome of Interstitial Cystitis/Bladder Pain Syndrome: A Prospective Study. Pain Physician 2022, 25, E1315–E1322. [Google Scholar] [CrossRef]
- Yunus, M.B. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin. Arthritis Rheum. 2007, 36, 339–356. [Google Scholar] [CrossRef] [PubMed]
- McKernan, L.C.; Walsh, C.G.; Reynolds, W.S.; Crofford, L.J.; Dmochowski, R.R.; Williams, D.A. Psychosocial co-morbidities in Interstitial Cystitis/Bladder Pain syndrome (IC/BPS): A systematic review. Neurourol. Urodyn. 2018, 37, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Yu, W.R.; Jhang, J.F.; Chen, B.Y.; Ou, S.R.; Li, H.M.; Kuo, H.C. Multimodal Treatment with Cognitive Behavioral Therapeutic Intervention Plus Bladder Treatment Is More Effective than Monotherapy for Patients with Interstitial Cystitis/Bladder Pain Syndrome-A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 6221. [Google Scholar] [CrossRef]
- Zwaans, B.M.; Mota, S.; Bartolone, S.N.; Ward, E.P.; Peters, K.M.; Chancellor, M.B. Evaluating symptom severity and urinary cytokine levels in interstitial cystitis/bladder pain syndrome patients, with and without Hunner’s lesions. Am. J. Clin. Exp. Urol. 2024, 12, 110–118. [Google Scholar] [CrossRef]
- Yu, W.R.; Jiang, Y.H.; Jhang, J.F.; Kuo, H.C. Cystoscopic characteristic findings of interstitial cystitis and clinical implications. Tzu Chi Med. J. 2023, 36, 30–37. [Google Scholar] [CrossRef]
- Tyagi, P.; Janicki, J.; Moon, C.H.; Kaufman, J.; Chermansky, C. Novel contrast mixture achieves contrast resolution of human bladder wall suitable for T1 mapping: Applications in interstitial cystitis and beyond. Int. Urol. Nephrol. 2018, 50, 401–409. [Google Scholar] [CrossRef]
- Torimoto, K.; Ueda, T.; Kasahara, M.; Hirayama, A.; Matsushita, C.; Matsumoto, Y.; Gotoh, D.; Nakai, Y.; Miyake, M.; Aoki, K.; et al. Identification of diagnostic serum biomarkers for Hunner-type interstitial cystitis. Low. Urin. Tract Symptoms 2022, 14, 334–340. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Kuo, H.C. Can We Use Urinary Cytokine/Chemokine Analysis in Discriminating Ulcer-Type Interstitial Cystitis/Bladder Pain Syndrome? Diagnostics 2022, 12, 1093. [Google Scholar] [CrossRef]
- Akiyama, Y.; Homma, Y.; Maeda, D. Pathology and terminology of interstitial cystitis/bladder pain syndrome: A review. Histol. Histopathol. 2019, 34, 25–32. [Google Scholar] [PubMed]
- Maeda, D.; Akiyama, Y.; Morikawa, T.; Kunita, A.; Ota, Y.; Katoh, H.; Niimi, A.; Nomiya, A.; Ishikawa, S.; Goto, A.; et al. Hunner-Type (Classic) Interstitial Cystitis: A Distinct Inflammatory Disorder Characterized by Pancystitis, with Frequent Expansion of Clonal B-Cells and Epithelial Denudation. PLoS ONE 2015, 10, e0143316. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.F.; Hsu, Y.H.; Peng, C.W.; Jiang, Y.H.; Ho, H.C.; Kuo, H.C. Epstein-Barr Virus as a Potential Etiology of Persistent Bladder Inflammation in Human Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2018, 200, 590–596. [Google Scholar] [CrossRef]
- van Ophoven, A.; Oberpenning, F.; Hertle, L. Long-term results of trigone-preserving orthotopic substitution enterocystoplasty for interstitial cystitis. J. Urol. 2002, 167 Pt 1, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Peng, C.W.; Jiang, Y.H.; Jhang, J.F. Urinary Viral Spectrum in Patients with Interstitial Cystitis/Bladder Pain Syndrome and the Clinical Efficacy of Valacyclovir Treatment. Biomedicines 2024, 12, 522. [Google Scholar] [CrossRef]
- Shen, S.H.; Peng, L.; Zeng, X.; Zhang, J.; Shen, H.; Luo, D.Y. Intravesical Interferon Therapy vs. Hyaluronic Acid for Pain among Female Individuals with Interstitial Cystitis: A Randomized Clinical Trial. AMA Netw. Open 2024, 7, e244880. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, Y.C.; Lee, W.C.; Chuang, Y.C. New Frontiers or the Treatment of Interstitial Cystitis/Bladder Pain Syndrome—Focused on Stem Cells, Platelet-Rich Plasma, and Low-Energy Shock Wave. Int. Neurourol. J. 2020, 24, 211–221. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, K.T.; Lee, E.J.; Chun, J.; Lee, S.; Shim, S.R.; Kim, J.H. Current updates relating totreatment for interstitial cystitis/bladder pain syndrome: Systematic review and network meta-analysis. BMC Urol. 2024, 24, 95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.-R.; Jhang, J.-F.; Jiang, Y.-H.; Kuo, H.-C. The Pathomechanism and Current Treatments for Chronic Interstitial Cystitis and Bladder Pain Syndrome. Biomedicines 2024, 12, 2051. https://doi.org/10.3390/biomedicines12092051
Yu W-R, Jhang J-F, Jiang Y-H, Kuo H-C. The Pathomechanism and Current Treatments for Chronic Interstitial Cystitis and Bladder Pain Syndrome. Biomedicines. 2024; 12(9):2051. https://doi.org/10.3390/biomedicines12092051
Chicago/Turabian StyleYu, Wan-Ru, Jia-Fong Jhang, Yuan-Hong Jiang, and Hann-Chorng Kuo. 2024. "The Pathomechanism and Current Treatments for Chronic Interstitial Cystitis and Bladder Pain Syndrome" Biomedicines 12, no. 9: 2051. https://doi.org/10.3390/biomedicines12092051
APA StyleYu, W.-R., Jhang, J.-F., Jiang, Y.-H., & Kuo, H.-C. (2024). The Pathomechanism and Current Treatments for Chronic Interstitial Cystitis and Bladder Pain Syndrome. Biomedicines, 12(9), 2051. https://doi.org/10.3390/biomedicines12092051







