Is Arterial Stiffness Interconnected with Cardiovascular Drug Prescription Patterns, Body Composition Parameters, and the Quality of Blood Pressure Regulation in Hypertensive Patients?
Abstract
:1. Introduction
2. Materials and Methods
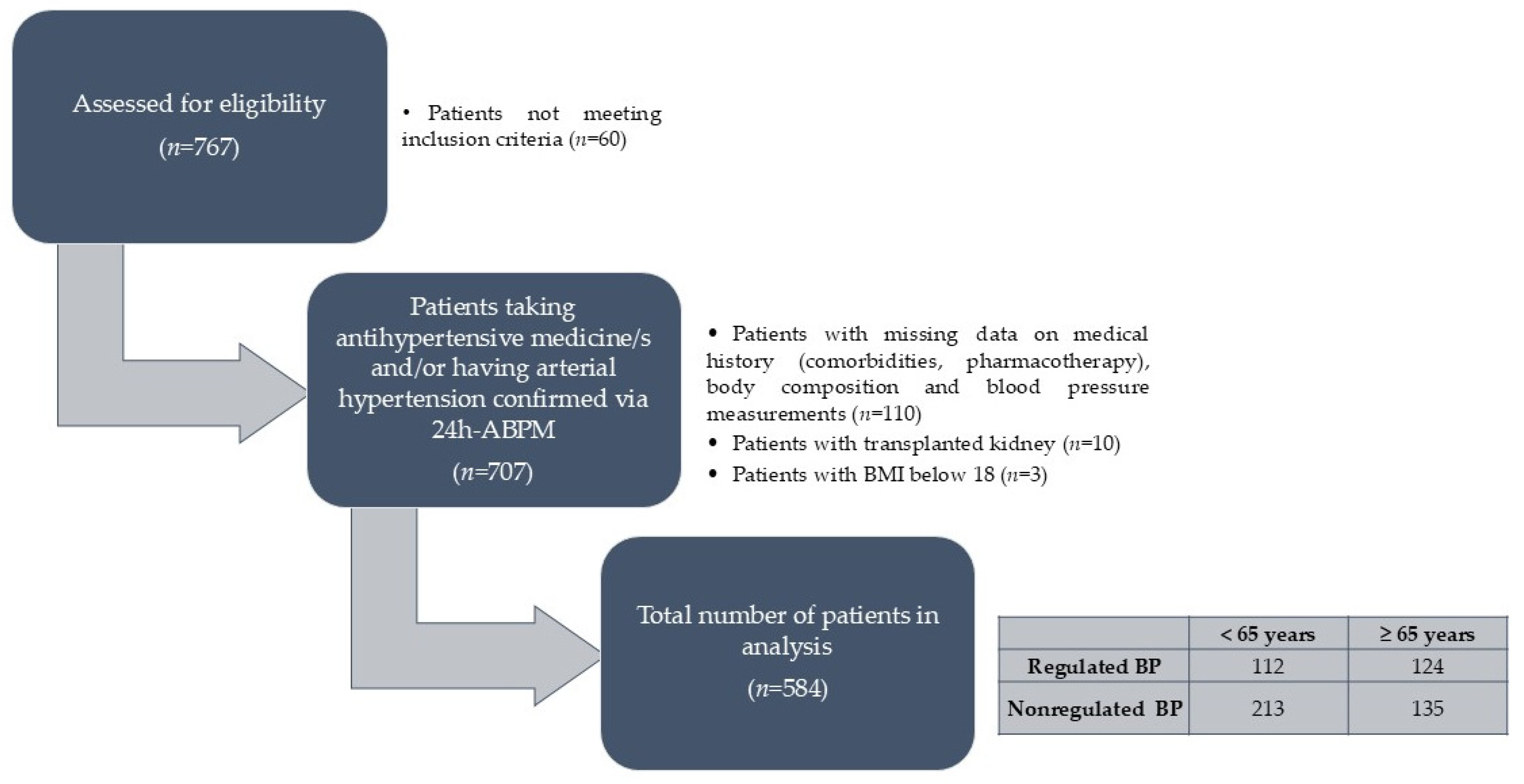
2.1. Study Population and Design
2.2. Blood Pressure Measurement
2.3. Body Composition Measurements
2.4. Medical History and Current Medication Treatment
2.5. Statistical Analysis
3. Results
4. Discussion
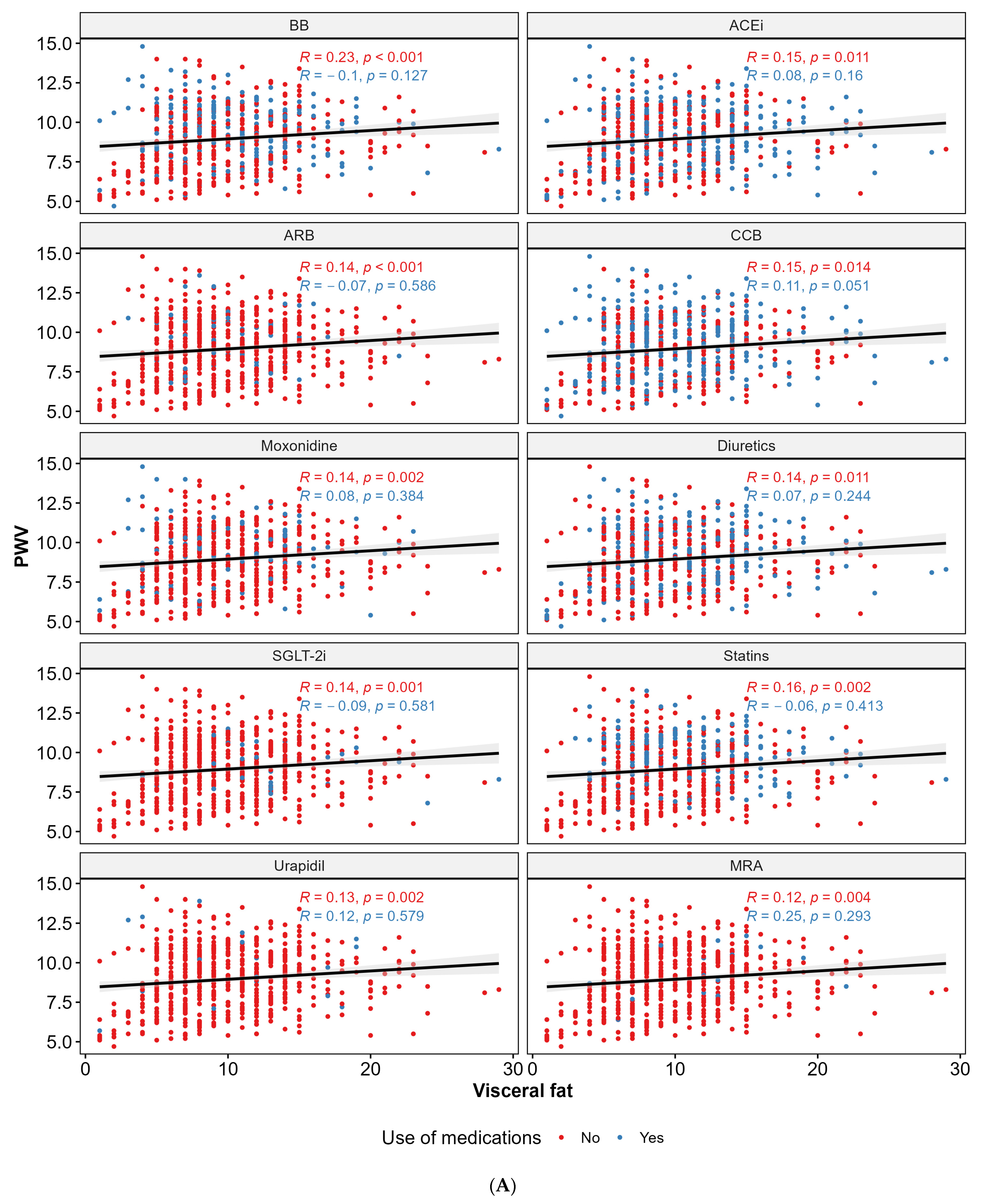
4.1. Pharmacological Therapy and PWV
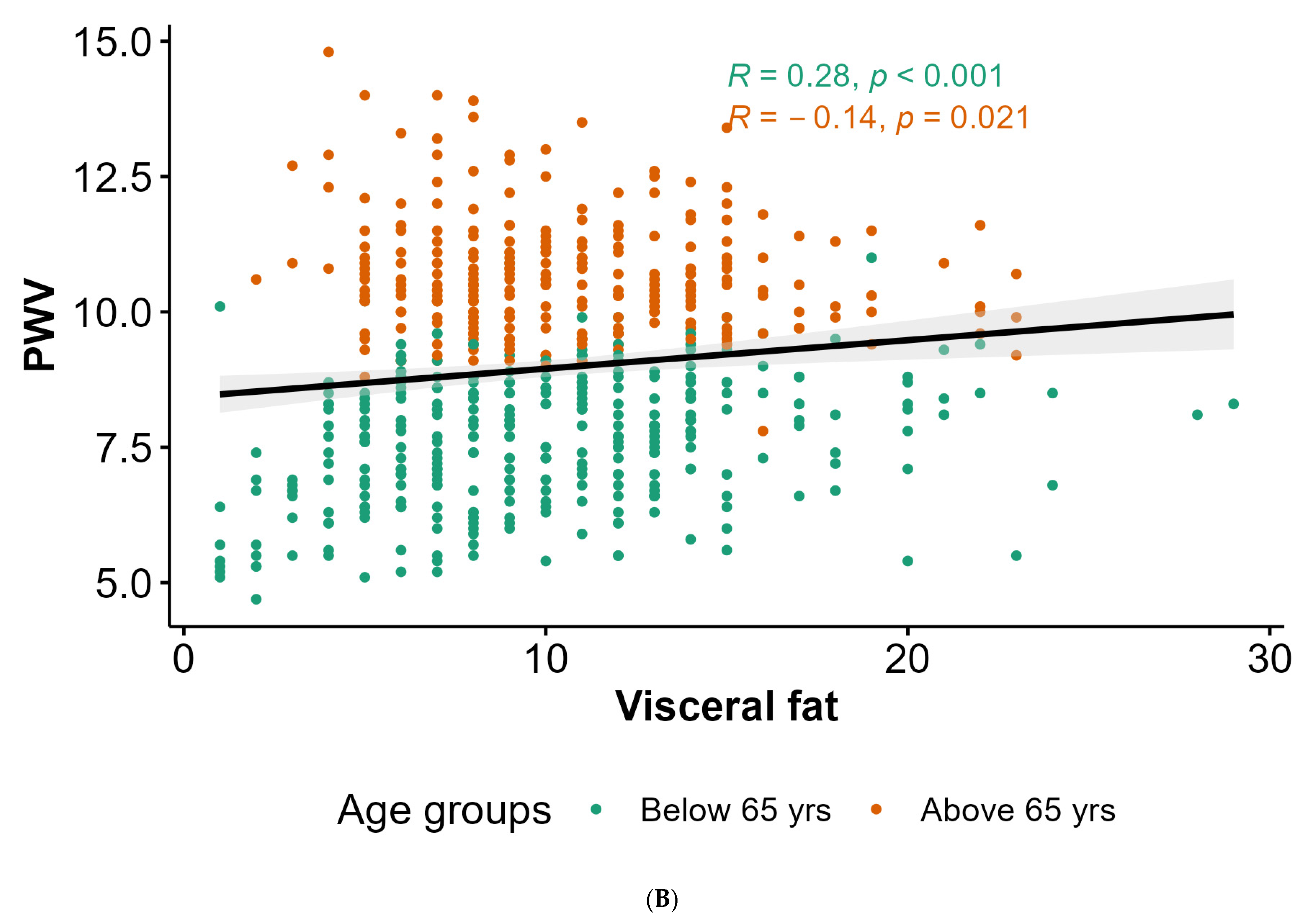
4.2. Anthropometric Parameters, Body Composition, and PWV
4.3. Blood Pressure, Hemodynamic Parameters, and PWV
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, R.S.; Kaufman, J.S.; Bovet, P. Global Burden of Disease Attributable to Hypertension. JAMA 2017, 317, 2017–2018. [Google Scholar] [CrossRef]
- Laurent, S.; Marais, L.; Boutouyrie, P. The Noninvasive Assessment of Vascular Aging. Can. J. Cardiol. 2016, 32, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Laurent, S.; Cunha, P.G.; Olsen, M.H.; Rietzschel, E.; Franco, O.H.; Ryliškyte, L.; Strazhesko, I.; Vlachopoulos, C.; Chen, C.H.; et al. Characteristics of Healthy Vascular Ageing in Pooled Population-Based Cohort Studies: The Global Metabolic Syndrome and Artery Research Consortium. J. Hypertens. 2018, 36, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huo, Y.; Wang, X.; Xu, X.; Qin, X.; Tang, G.; Xing, H.; Fan, F.; Li, J.; Zhang, Y.; et al. A Prospective Study on Pulse Wave Velocity (PWV) and Response to Anti-Hypertensive Treatments: PWV Determines BP Control. Int. J. Cardiol. 2015, 178, 226–231. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Saz-Lara, A.; Lugones-Sánchez, C.; Pozuelo-Carrascosa, D.P.; Gómez-Sánchez, L.; López-Gil, J.F.; García-Ortiz, L.; Bruno, R.M.; Gómez-Marcos, M.Á. Comparative Effect of Antihypertensive Drugs in Improving Arterial Stiffness in Adults with Hypertension (RIGIPREV Study). A Network Meta-Analysis. Front. Pharmacol. 2023, 14, 1225795. [Google Scholar] [CrossRef]
- Antonio-Villa, N.E.; Bello-Chavolla, O.Y.; Vargas-Vázquez, A.; Mehta, R.; Fermín-Martínez, C.A.; Martagón-Rosado, A.J.; Barquera-Guevara, D.A.; Aguilar-Salinas, C.A.; Arellano-Campos, O.; Gómez-Velasco, D.V.; et al. Increased Visceral Fat Accumulation Modifies the Effect of Insulin Resistance on Arterial Stiffness and Hypertension Risk. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 506–517. [Google Scholar] [CrossRef]
- Batzias, K.; Antonopoulos, A.S.; Oikonomou, E.; Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Mistakidi, C.V.; Noutsou, M.; Katsiki, N.; Karopoulos, P.; et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2018, 2018, 1232583. [Google Scholar] [CrossRef]
- Hidalgo Santiago, J.C.; Maraver Delgado, J.; Cayón Blanco, M.; López Saez, J.B.; Gómez-Fernández, P. Effect of Dapagliflozin on Arterial Stiffness in Patients with Type 2 Diabetes Mellitus. Med. Clin. 2020, 154, 171–174. [Google Scholar] [CrossRef]
- Sjöström, C.D.; Johansson, P.; Ptaszynska, A.; List, J.; Johnsson, E. Dapagliflozin Lowers Blood Pressure in Hypertensive and Non-Hypertensive Patients with Type 2 Diabetes. Diab. Vasc. Dis. Res. 2015, 12, 352–358. [Google Scholar] [CrossRef]
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin Decreases Small Dense Low-Density Lipoprotein-Cholesterol and Increases High-Density Lipoprotein 2-Cholesterol in Patients with Type 2 Diabetes: Comparison with Sitagliptin. Cardiovasc. Diabetol. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Rizos, E.C.; Tagkas, C.F.; Asimakopoulos, A.-G.I.; Tsimihodimos, V.; Anastasiou, G.; Rizzo, M.; Agouridis, A.P.; Ntzani, E.E. The Effect of SGLT2 Inhibitors and GLP1 Receptor Agonists on Arterial Stiffness: A Meta-Analysis of Randomized Controlled Trials. J. Diabetes Complicat. 2024, 38, 108781. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, A.O.; Barker, A.R.; Tuomainen, T.P. Cardiorespiratory Fitness, Fat Mass, and Cardiometabolic Health with Endothelial Function, Arterial Elasticity, and Stiffness. Med. Sci. Sports Exerc. 2022, 54, 141–152. [Google Scholar] [CrossRef] [PubMed]
- IEM Mobil-O-Graph User Manual. Available online: https://medaval.ie/docs/manuals/IEM-Mobil-O-Graph-Manual.pdf (accessed on 30 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Dudenbostel, T.; Glasser, S.P. Effects of Antihypertensive Drugs on Arterial Stiffness. Cardiol. Rev. 2012, 20, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Klug, E.; Rayner, B.; Wasserfall, M.; Kok, A.; Mpe, M.; Ruder, S.; Mohamed, N.; Webb, D. Cardiorenal Effects of SGLT2 Inhibitors: Who Might Benefit? J. Endocrinol. Metab. Diabetes South Afr. 2024, 29, 8–17. [Google Scholar] [CrossRef]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium–Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging—What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Liu, L.; Ni, Y.Q.; Zhan, J.K.; Liu, Y.S. The Role of Sglt2 Inhibitors in Vascular Aging. Aging Dis. 2021, 12, 1323–1336. [Google Scholar] [CrossRef]
- Alidadi, M.; Montecucco, F.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Beneficial Effect of Statin Therapy on Arterial Stiffness. Biomed. Res. Int. 2021, 2021, 5548310. [Google Scholar] [CrossRef]
- Upala, S.; Wirunsawanya, K.; Jaruvongvanich, V.; Sanguankeo, A. Effects of Statin Therapy on Arterial Stiffness: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Int. J. Cardiol. 2017, 227, 338–341. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Wang, Y.; Wang, G.; Zhou, Z.; Chen, S.; Geng, T.; Zhang, Y.B.; Wang, Y.; Chen, J.X.; Pan, A.; et al. Association between Statin Use and Progression of Arterial Stiffness among Adults with High Atherosclerotic Risk. JAMA Netw. Open 2022, 5, e2218323. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Meng, X.; Li, Y.; Liu, X.; Teng, F.; Wang, Y.; Zang, X.; Wang, Y.; Liang, J. Evaluation of the Associations of Body Height with Blood Pressure and Early-Stage Atherosclerosis in Chinese Adults. J. Clin. Hypertens. 2020, 22, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, Y.; Bao, B. Relationship of Body Mass Index and Visceral Fat Area Combination with Arterial Stiffness and Cardiovascular Risk in Cardiovascular Disease-Free People: NHANES (2011–2018). Endocr. Connect. 2023, 12, e230291. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.; Filippatos, T.; Joshi, J.; Mikhailidis, D. Obesity and Arterial Compliance Alterations. Curr. Vasc. Pharmacol. 2010, 8, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Taira, H.; Shinzato, T.; Ohya, Y. Association between Visceral Fat Mass and Arterial Stiffness among Community-Based Screening Participants. Hypertens. Res. 2023, 46, 2488–2496. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Karim, M.N.; Srikanth, V.; Ebeling, P.R.; Scott, D. Lower Muscle Tissue Is Associated with Higher Pulse Wave Velocity: A Systematic Review and Meta-Analysis of Observational Study Data. Clin. Exp. Pharmacol. Physiol. 2017, 44, 980–992. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 715. [Google Scholar] [CrossRef]
- Aminuddin, A.; Noor Hashim, M.F.; Mohd Zaberi, N.A.S.; Zheng Wei, L.; Ching Chu, B.; Jamaludin, N.A.; Salamt, N.; Che Roos, N.A.; Ugusman, A. The Association between Arterial Stiffness and Muscle Indices among Healthy Subjects and Subjects With Cardiovascular Risk Factors: An Evidence-Based Review. Front. Physiol. 2021, 12, 742338. [Google Scholar] [CrossRef]
- Ohara, M.; Kohara, K.; Tabara, Y.; Ochi, M.; Nagai, T.; Igase, M.; Miki, T. Sarcopenic Obesity and Arterial Stiffness, Pressure Wave Reflection and Central Pulse Pressure: The J-SHIPP Study. Int. J. Cardiol. 2014, 174, 214–217. [Google Scholar] [CrossRef]
- Kim, H.L. Arterial Stiffness and Hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Hashimoto, J. Mechanical Factors in Arterial Aging. A Clinical Perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Oikonomou, E.; Lazaros, G.; Christoforatou, E.; Vogiatzi, G.; Tsalamandris, S.; Chasikidis, C.; Kalambogias, A.; Mystakidi, V.X.; Galiatsatos, N.; et al. The Influence of Resting Heart Rate on Pulse Wave Velocity Measurement Is Mediated by Blood Pressure and Depends on Aortic Stiffness Levels: Insights from the Corinthia Study. Physiol. Meas. 2019, 40, 055005. [Google Scholar] [CrossRef] [PubMed]


| Variable *,**,*** | All (n = 584) | |
|---|---|---|
| Sex, n (%) | Male | 300 (51.37) |
| Female | 284 (48.63) | |
| Age (years), median (IQR) | 63 (19) | |
| Height (cm), mean (SD) | 173.61 (9.75) | |
| Weight (kg), median (IQR) | 86 (23.43) | |
| BMI (kg/m2), median (IQR) | 28.75 (6.52) | |
| FM (%), mean (SD) | 29.03 (8.69) | |
| FM (kg), median (IQR) | 24.9 (13.6) | |
| VF level, median (IQR) | 10 (6) | |
| FFM (kg), median (IQR) | 61.35 (16.85) | |
| PMM (%), median (IQR) | 58.25 (16.05) | |
| SMI, median (IQR) | 8.42 (2.05) | |
| PhA (°), median (IQR) | 5.4 (1) | |
| Smoking, n (%) | Yes | 127 (21.75) |
| No | 457 (78.25) | |
| CKD, n (%) | Yes | 243 (41.61) |
| No | 341 (58.39) | |
| DM, n (%) | Yes | 188 (32.19) |
| No | 396 (67.81) | |
| Malignancies, n (%) | Yes | 66 (11.3) |
| No | 518 (88.7) | |
| CVD, n (%) | Yes | 120 (20.55) |
| No | 464 (79.45) | |
| CBD, n (%) | Yes | 16 (2.74) |
| No | 568 (97.26) | |
| pSBP (mmHg), median (IQR) | 129 (17) | |
| pDBP (mmHg), median (IQR) | 78 (13) | |
| HR (bpm), median (IQR) | 70 (12) | |
| pMAP (mmHg), median (IQR) | 101 (14) | |
| pPP (mmHg), median (IQR) | 50 (13) | |
| cSBP (mmHg), median (IQR) | 118 (15) | |
| cDBP (mmHg), median (IQR) | 79.5 (14) | |
| AIx@75, median (IQR) | 25 (10) | |
| PWV (m/s), median (IQR) | 9 (2.9) | |
| SV (mL/beat), median (IQR) | 70 (9) | |
| Dipping systole, n (%) | No | 423 (72.43) |
| Yes | 161 (27.57) | |
| Dipping diastole, n (%) | No | 346 (59.25) |
| Yes | 238 (40.75) | |
| Variable *,**,*** | Regulated BP < 65 Years (n = 112) | Nonregulated BP < 65 Years (n = 213) | p | Regulated BP ≥ 65 Years (n = 124) | Nonregulated BP ≥ 65 Years (n = 135) | p | |
|---|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 55.5 (12.25) | 52 (13) | 0.001 | 72 (7) | 71 (8) | 0.591 | |
| Height (cm), mean (SD) | 173 (12.4) | 176 (13) | 0.013 | 170.21 (9.15) | 171.25 (9.32) | 0.367 | |
| FFM (kg), median (IQR) | 62.25 (17.2) | 64.1 (17) | 0.025 | 58.2 (13.52) | 61.3 (16.5) | 0.039 | |
| PMM (%), median (IQR) | 59.15 (16.4) | 60.9 (16.2) | 0.025 | 55.3 (12.9) | 58.2 (15.7) | 0.039 | |
| CVD, n (%) | Yes | 21 (18.75) | 21 (9.86) | 0.036 | 37 (29.84) | 41 (30.37) | 1.000 |
| No | 91 (81.25) | 192 (90.14) | 0.036 | 87 (70.16) | 94 (69.63) | 1.000 | |
| pSBP (mmHg), median (IQR) | 120 (10) | 134 (13) | 0.000 | 121 (10) | 138 (15) | <0.001 | |
| pDBP (mmHg), median (IQR) | 73 (8) | 86 (10) | 0.000 | 69.52 (5.55) | 80.23 (7.43) | <0.001 | |
| HR (bpm), median (IQR) | 70.26 (8.57) | 73.95 (9.38) | 0.001 | 66.4 (8.92) | 67.91 (9.31) | 0.183 | |
| pMAP (mmHg), median (IQR) | 94 (8) | 107 (11) | <0.001 | 93.5 (8) | 107 (9) | <0.001 | |
| pPP (mmHg), median (IQR) | 47 (9.25) | 48 (15) | 0.008 | 50 (7) | 58 (16) | <0.001 | |
| cSBP (mmHg), median (IQR) | 110 (7.25) | 123 (13) | <0.001 | 110 (10) | 125 (11.5) | <0.001 | |
| cDBP (mmHg), median (IQR) | 75 (8.25) | 87 (9) | <0.001 | 71.5 (8) | 82 (10.5) | <0.001 | |
| AIx@75, median (IQR) | 22.05 (7.55) | 23.39 (7.59) | 0.132 | 26 (10) | 28 (8) | 0.001 | |
| PWV (m/s), median (IQR) | 7.7 (1.63) | 7.8 (1.9) | 0.198 | 10.2 (1.1) | 10.9 (1.35) | <0.001 | |
| ACEi, n (%) | Yes | 73 (65.18) | 85 (39.91) | <0.001 | 71 (57.26) | 67 (49.63) | 0.269 |
| No | 39 (34.82) | 128 (60.09) | <0.001 | 53 (42.74) | 68 (50.37) | 0.269 | |
| Statins, n (%) | Yes | 35 (31.25) | 41 (19.25) | 0.022 | 66 (53.23) | 61 (45.19) | 0.243 |
| No | 77 (68.75) | 172 (80.75) | 0.022 | 58 (46.77) | 74 (54.81) | 0.243 | |
| Fixed antihypertensive drugs combinations use, n (%) | Yes | 60 (53.57) | 61 (28.64) | <0.001 | 63 (50.81) | 54 (40) | 0.105 |
| No | 52 (46.43) | 152 (71.36) | <0.001 | 61 (49.19) | 81 (60) | 0.105 | |
| Variable * | Beta | SE | p |
|---|---|---|---|
| Height | −0.01237 | 0.00407 | 0.002 |
| Weight | −0.0031 | 0.00151 | 0.04 |
| VF level | −0.02303 | 0.00668 | <0.001 |
| FFM | −0.00811 | 0.00316 | 0.011 |
| PMM | −0.00851 | 0.00331 | 0.011 |
| pSBP | 0.03512 | 0.00193 | <0.001 |
| HR | −0.00856 | 0.0029 | 0.003 |
| pMAP | 0.03299 | 0.00313 | <0.001 |
| pPP | 0.04143 | 0.00202 | <0.001 |
| Csbp | 0.03213 | 0.00232 | <0.001 |
| AIx@75 | 0.02005 | 0.00488 | <0.001 |
| CCB use | 0.11212 | 0.05252 | 0.033 |
| Moxonidine use | 0.1839 | 0.06752 | 0.007 |
| SGLT-2i use | −0.23252 | 0.10858 | 0.033 |
| Statin use | −0.12192 | 0.05876 | 0.038 |
| Urapidil use | 0.3763 | 0.12964 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, J.; Vučković, M.; Đogaš, H.; Gelemanović, A.; Belančić, A.; Radić, M. Is Arterial Stiffness Interconnected with Cardiovascular Drug Prescription Patterns, Body Composition Parameters, and the Quality of Blood Pressure Regulation in Hypertensive Patients? Biomedicines 2024, 12, 2062. https://doi.org/10.3390/biomedicines12092062
Radić J, Vučković M, Đogaš H, Gelemanović A, Belančić A, Radić M. Is Arterial Stiffness Interconnected with Cardiovascular Drug Prescription Patterns, Body Composition Parameters, and the Quality of Blood Pressure Regulation in Hypertensive Patients? Biomedicines. 2024; 12(9):2062. https://doi.org/10.3390/biomedicines12092062
Chicago/Turabian StyleRadić, Josipa, Marijana Vučković, Hana Đogaš, Andrea Gelemanović, Andrej Belančić, and Mislav Radić. 2024. "Is Arterial Stiffness Interconnected with Cardiovascular Drug Prescription Patterns, Body Composition Parameters, and the Quality of Blood Pressure Regulation in Hypertensive Patients?" Biomedicines 12, no. 9: 2062. https://doi.org/10.3390/biomedicines12092062








