Unraveling the Liver–Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Predator Stress Paradigm
2.3. Fox Urine Collection and Application
2.4. Resveratrol Administration
2.5. Experimental Groups
- Control: Rats treated with vehicle only for 10 days (n = 12).
- PS: Rats previously exposed to chronic predator stress (n = 12).
- RES + PS: Rats administered an effective dose of resveratrol via intraperitoneal injection one hour before the onset of predatory stress (n = 22).
2.6. Behavioral Testing
- Number of entries into the open and closed arms of the EPM;
- Time spent in the open and closed arms.
2.7. Blood and Tissue Collection and Storage
2.8. Quantification of RES and RES-O-Glucuronide in Rat Plasma
2.9. Plasma Corticosterone Measurement
2.10. Hepatic 11-HSD-1 Activity
2.11. Monoamine Oxidase Activity Measurement
2.12. Content of Lipid Peroxidation Products in Liver and Hepatic Mitochondria
2.13. Histology
2.14. Statistical Analysis
3. Results
3.1. Timeline of PS Exposures, RES Treatment, and Blood RES Concentration Evaluation
3.2. Evaluation of Behavioral Effects of RES Treatment on PS Rats
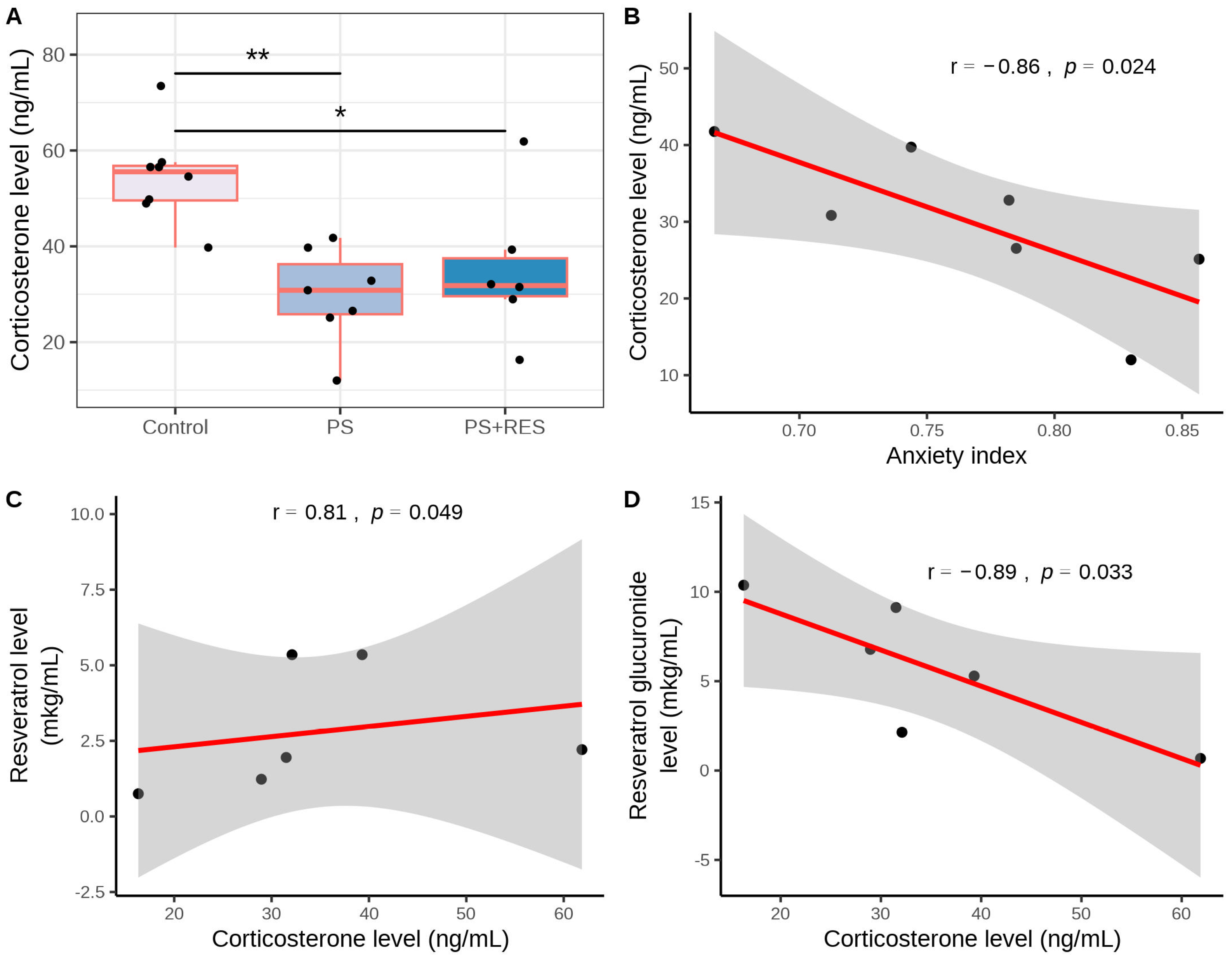
3.3. Effects of RES Treatment on Plasma CORT Concentration in PS Rats
3.4. Effects of RES Treatment on Hepatic 11-HSD-1 Activity and Expression in PS Rats
3.5. Effects of RES Treatment on MAO-A and MAO-B Activities in PS Rats
3.6. Effects of RES Treatment on Brain LPO Levels in PS Rats
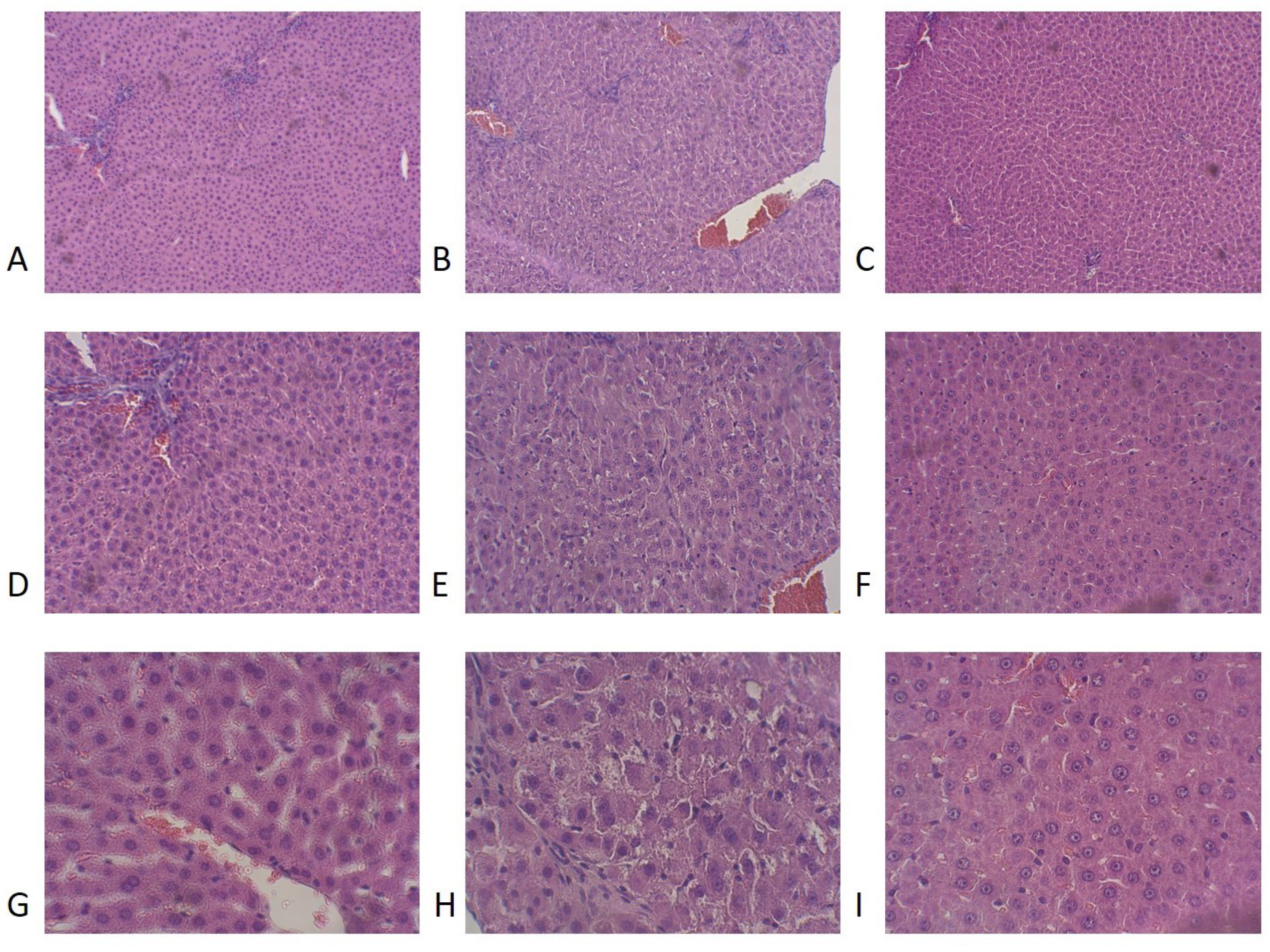
3.7. Effects of RES Treatment on Liver Histology and LPO Levels in PS Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saviola, F.; Pappaianni, E.; Monti, A.; Grecucci, A.; Jovicich, J.; De Pisapia, N. Trait and state anxiety are mapped differently in the human brain. Sci. Rep. 2020, 10, 11112. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction; StatPearls Publishing: Treasure Islan, FL, USA, 2024. [Google Scholar]
- Ramsay, D.S.; Woods, S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014, 121, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M.; Koolhaas, J.M.; Wingfield, J.C.; McEwen, B.S. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005, 29, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Krupnik, V. Depression as a failed anxiety: The continuum of precision-weighting dysregulation in affective disorders. Front. Psychol. 2021, 12, 657738. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.; Komelkova, M.; Lapshin, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Kondashevskaya, M.; et al. High and low anxiety phenotypes in a rat model of complex post-traumatic stress disorder are associated with different alterations in regional brain monoamine neurotransmission. Psychoneuroendocrinology 2020, 117, 104691. [Google Scholar] [CrossRef]
- Farach, F.J.; Pruitt, L.D.; Jun, J.J.; Jerud, A.B.; Zoellner, L.A.; Roy-Byrne, P.P. Pharmacological treatment of anxiety disorders: Current treatments and future directions. J. Anxiety Disord. 2012, 26, 833–843. [Google Scholar] [CrossRef]
- Wilson, C.B.; McLaughlin, L.D.; Ebenezer, P.J.; Nair, A.R.; Dange, R.; Harre, J.G.; Shaak, T.L.; Diamond, D.M.; Francis, J. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Front. Behav. Neurosci. 2014, 8, 256. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhen, L.; Li, Z.; Xu, W.; Leng, H.; Xu, W.; Zheng, V.; Luria, V.; Pan, J.; Tao, Y.; et al. trans-Resveratrol ameliorates anxiety-like behaviors and neuropathic pain in mouse model of post-traumatic stress disorder. J. Psychopharmacol. 2020, 34, 726–736. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol:In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Shayganfard, M. Molecular and biological functions of resveratrol in psychiatric disorders: A review of recent evidence. Cell Biosci. 2020, 10, 128. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a promising nutraceutical: Implications in gut microbiota modulation, inflammatory disorders, and colorectal cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2017, 19, 121–135. [Google Scholar] [CrossRef]
- Price, N.; Gomes, A.; Ling, A.; Duarte, F.; Martin-Montalvo, A.; North, B.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol promotes neurotrophic factor release from astroglia. Exp. Biol. Med. 2012, 237, 943–948. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.J.; Zhang, H.B.; Liang, J.X.; Cao, W.D.; Wu, Q.; He, C.P.; Chen, C. The function of PPARγ/AMPK/SIRT-1 pathway in inflammatory response of human articular chondrocytes stimulated by advanced glycation end products. Biol. Pharm. Bull. 2019, 42, 1303–1309. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, P.; Cheng, P.; Zhang, Z.; Yang, S.; Wang, J.; Wang, X.; Zhu, G. Exploring the effect of Anshen Dingzhi prescription on hippocampal mitochondrial signals in single prolonged stress mouse model. J. Ethnopharmacol. 2024, 323, 117713. [Google Scholar] [CrossRef]
- Novak, J.; Tseilikman, V.E.; Tseilikman, O.B.; Lazuko, S.S.; Belyeva, L.E.; Rahmani, A.; Fedotova, J. Can resveratrol influence the activity of 11β-hydroxysteroid dehydrogenase type 1? A combined in silico and in vivo study. Pharmaceuticals 2023, 16, 251. [Google Scholar] [CrossRef]
- Wheelan, N.; Seckl, J.R.; Yau, J.L. 11β-Hydroxysteroid dehydrogenase 1 deficiency prevents PTSD-like memory in young adult mice. Psychoneuroendocrinology 2022, 146, 105945. [Google Scholar] [CrossRef]
- Tseilikman, V.; Dremencov, E.; Tseilikman, O.; Pavlovicova, M.; Lacinova, L.; Jezova, D. Role of glucocorticoid- and monoamine-metabolizing enzymes in stress-related psychopathological processes. Stress 2019, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.B.; Jaffee, M.S.; Jorge, R.E. Posttraumatic stress disorder and anxiety-related conditions. Contin. Lifelong Learn. Neurol. 2021, 27, 1738–1763. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Eiland, L.; Hunter, R.G.; Miller, M.M. Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012, 62, 3–12. [Google Scholar] [CrossRef]
- Resick, P.A.; Bovin, M.J.; Calloway, A.L.; Dick, A.M.; King, M.W.; Mitchell, K.S.; Suvak, M.K.; Wells, S.Y.; Stirman, S.W.; Wolf, E.J. A critical evaluation of the complex PTSD literature: Implications for DSM-5. J. Trauma. Stress 2012, 25, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.E.; Fedotova, J.O.; Tseilikman, O.B.; Novak, J.; Karpenko, M.N.; Maistrenko, V.A.; Lazuko, S.S.; Belyeva, L.E.; Kamel, M.; Buhler, A.V.; et al. Resistance to resveratrol treatment in experimental PTSD is associated with abnormalities in hepatic metabolism of glucocorticoids. Int. J. Mol. Sci. 2023, 24, 9333. [Google Scholar] [CrossRef]
- Tseilikman, V.; Lapshin, M.; Klebanov, I.; Chrousos, G.; Vasilieva, M.; Pashkov, A.; Fedotova, J.; Tseilikman, D.; Shatilov, V.; Manukhina, E.; et al. The link between activities of hepatic 11β-hydroxysteroid dehydrogenase-1 and monoamine oxidase-A in the brain following repeated predator stress: Focus on heightened anxiety. Int. J. Mol. Sci. 2022, 23, 4881. [Google Scholar] [CrossRef]
- Lazuko, S.S.; Kuzhel, O.P.; Belyaeva, L.E.; Manukhina, E.B.; Fred Downey, H.; Tseilikman, O.B.; Komelkova, M.V.; Tseilikman, V.E. Posttraumatic stress disorder disturbs coronary tone and its regulatory mechanisms. Cell. Mol. Neurobiol. 2017, 38, 209–217. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Kozochkin, D.A.; Sinitskii, A.I.; Tseylikman, O.B.; Lapshin, M.S.; Kuzina, O.V.; Komel’kova, M.V.; Telesheva, I.B. Effect of repeated 1-h episodes of immobilization stress on activity of glucocorticoid metabolism enzymes in the liver. Bull. Exp. Biol. Med. 2016, 160, 614–616. [Google Scholar] [CrossRef]
- Satav, J.G.; Katyare, S.S. Effect of experimental thyrotoxicosis on oxidative phosphorylation in rat liver, kidney and brain mitochondria. Mol. Cell. Endocrinol. 1982, 28, 173–189. [Google Scholar] [CrossRef]
- Tipton, K.F.; Davey, G.; Motherway, M. Monoamine oxidase assays. Curr. Protoc. Pharmacol. 2000, 9, 3.6.1–3.6.42. [Google Scholar] [CrossRef]
- Volchegorskii, I.A.; Rassokhina, L.M.; Miroshnichenko, I.Y. Dynamics of lipid peroxidation—Antioxidant defense system during alloxan diabetes in rats. Bull. Exp. Biol. Med. 2013, 155, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Korourian, S.; Hakkak, R.; Ronis, M.J.; Shelnutt, S.R.; Waldron, J.; Ingelman-Sundberg, M.; Badger, T.M. Diet and risk of ethanol-induced hepatotoxicity: Carbohydrate-fat relationships in rats. Toxicol. Sci. 1999, 47, 110–117. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Shatilov, V.A.; Zhukov, M.S.; Buksha, I.A.; Epitashvily, A.E.; Lipatov, I.A.; Aristov, M.R.; Koshelev, A.G.; Karpenko, M.N.; Traktirov, D.S.; et al. Limited cheese intake paradigm replaces patterns of behavioral disorders in experimental PTSD: Focus on resveratrol supplementation. Int. J. Mol. Sci. 2023, 24, 14343. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Holschneider, D.P.; Wu, W.; Rebrin, I.; Shih, J.C. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J. Biol. Chem. 2004, 279, 39645–39652. [Google Scholar] [CrossRef]
- Glover, V. Function of endogenous monoamine oxidase inhibitors (tribulin). In MAO—The Mother of All Amine Oxidases; Journal of Neural Transmission. Supplement; Springer: Vienna, Austria, 1998; pp. 307–313. [Google Scholar] [CrossRef]
- Tseilikman, O.B.; Kozochkin, D.A.; Manukhina, E.B.; Downey, H.F.; Misharina, M.E.; Komelkova, M.V.; Nikitina, A.A.; Golodnii, S.V.; Dodohova, M.A.; Tseilikman, V.E. Predicting anxiety responses to halogenated glucocorticoid drugs using the hexobarbital sleep time test. Stress 2016, 19, 390–394. [Google Scholar] [CrossRef]
- Lee, B.; Choi, G.M.; Sur, B. Antidepressant-like effects of hesperidin in animal model of post-traumatic stress disorder. Chin. J. Integr. Med. 2020, 27, 39–46. [Google Scholar] [CrossRef]
- Higuchi, Y.; Soga, T.; Parhar, I.S. Potential roles of microRNAs in the regulation of monoamine oxidase A in the brain. Front. Mol. Neurosci. 2018, 11, 339. [Google Scholar] [CrossRef]
- Libert, S.; Pointer, K.; Bell, E.; Das, A.; Cohen, D.; Asara, J.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef]
- Ooi, J.; Hayden, M.R.; Pouladi, M.A. Inhibition of excessive monoamine oxidase A/B activity protects against stress-induced neuronal death in Huntington disease. Mol. Neurobiol. 2014, 52, 1850–1861. [Google Scholar] [CrossRef]
- Pizzinat, N.; Copin, N.; Vindis, C.; Parini, A.; Cambon, C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn-Schmiedeberg Arch. Pharmacol. 1999, 359, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Carpi, A.; Nagayama, T.; Sivakumaran, V.; Zhu, G.; Lai, E.W.; Bedja, D.; De Mario, A.; Chen, K.; Gabrielson, K.L.; et al. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxidants Redox Signal. 2014, 20, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Rajgorodskaya, D.I.; Gorkin, V.Z.; Fedotova, I.B.; Semiokhina, A.F. The role of lipid peroxidation in the possible involvement of membrane-Bound monoamine oxidases in gamma-aminobutyric acid and glucosamine deamination in rat brain: Focus on chemical pathogenesis of experimental audiogenic epilepsy. Mol. Chem. Neuropathol. 1992, 16, 187–201. [Google Scholar] [CrossRef]
- Dean, R.T.; Thomas, S.M.; Garner, A. Free-radical-mediated fragmentation of monoamine oxidase in the mitochondrial membrane Roles for lipid radicals. Biochem. J. 1986, 240, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Komelkova, M.; Manukhina, E.; Downey, H.F.; Sarapultsev, A.; Cherkasova, O.; Kotomtsev, V.; Platkovskiy, P.; Fedorov, S.; Sarapultsev, P.; Tseilikman, O.; et al. Hexobarbital sleep test for predicting the susceptibility or resistance to experimental posttraumatic stress disorder. Int. J. Mol. Sci. 2020, 21, 5900. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.; Dremencov, E.; Maslennikova, E.; Ishmatova, A.; Manukhina, E.; Downey, H.F.; Klebanov, I.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; et al. Post-traumatic stress disorder chronification via monoaminooxidase and cortisol metabolism. Horm. Metab. Res. 2019, 51, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, K.; Yang, X.; Gu, J.; Ge, L.; Wang, X.; Wang, Z. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav. Brain Res. 2014, 264, 9–16. [Google Scholar] [CrossRef]
- Rasouri, S.; Lagouge, M.; Auwerx, J. SIRT1/PGC-1: Un axe neuroprotecteur? Médecine/Sci. 2007, 23, 840–844. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, X.Y.; Meng, Q.P.; Teng, D.; Deng, K.; Lin, N. Resveratrol activates the SIRT1/PGC-1 pathway in mice to improve synaptic-related cognitive impairment after TBI. Brain Res. 2022, 1796, 148109. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Li, W.; Guo, B.; Tao, K.; Li, F.; Liu, Z.; Yao, H.; Feng, D.; Liu, X. Inhibition of SIRT1 in hippocampal CA1 ameliorates PTSD-like behaviors in mice by protections of neuronal plasticity and serotonin homeostasis via NHLH2/MAO-A pathway. Biochem. Biophys. Res. Commun. 2019, 518, 344–350. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Issa, M.Y.; Ebrahim, H.S.; Alaaeldin, R.; Elrehany, M.A.; Abd El-Kadder, E.M.; Abdelmohsen, U.R. Anti-Alzheimer chemical constituents of Morus macroura Miq.: Chemical profiling, in silico and in vitro investigations. Food Funct. 2021, 12, 8078–8089. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Basheer, L.; Schultz, K.; Guttman, Y.; Kerem, Z. In silico and in vitro inhibition of cytochrome P450 3A by synthetic stilbenoids. Food Chem. 2017, 237, 895–903. [Google Scholar] [CrossRef]
- Tseilikman, V.; Kozochkin, D.; Synitsky, A.; Sibiriak, S.; Tseilikman, O.; Katashinsky, E.; Nikitina, A.; Vinogradov, D.; Simbirtsev, A. Does stress-induced release of interleukin-1 cause liver injury? Cell. Mol. Neurobiol. 2012, 32, 1069–1078. [Google Scholar] [CrossRef]
- Xie, H.; Xie, D.; Zhang, J.; Jin, W.; Li, Y.; Yao, J.; Pan, Z.; Xie, D. ROS/NF-κB signaling pathwaymMediated transcriptional activation of TRIM37 promotes HBV-associated hepatic fibrosis. Mol. Ther. Nucleic Acids 2020, 22, 114–123. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhang, M.; Yang, W.; Qin, W.; Zheng, Q.; Chu, Y.; Wu, Y.; Wu, D.; Yuan, X. 11β-HSD1 inhibitor alleviates non-alcoholic fatty liver disease by activating the AMPK/SIRT1 signaling pathway. Nutrients 2022, 14, 2358. [Google Scholar] [CrossRef]
- Pshenichnyuk, S.A.; Komolov, A.S. Dissociative electron attachment to resveratrol as a likely pathway for generation of the H2 antioxidant species inside mitochondria. J. Phys. Chem. Lett. 2015, 6, 1104–1110. [Google Scholar] [CrossRef]
- Zeqiraj, E.; Filippi, B.M.; Deak, M.; Alessi, D.R.; van Aalten, D.M.F. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 2009, 326, 1707–1711. [Google Scholar] [CrossRef]
- He, J.; Han, J.; Lin, K.; Wang, J.; Li, G.; Li, X.; Gao, Y. PTEN/AKT and Wnt/β-catenin signaling pathways regulate the proliferation of Lgr5+ cells in liver cancer. Biochem. Biophys. Res. Commun. 2023, 683, 149117. [Google Scholar] [CrossRef]
- Snyder, J.C.; Pack, T.F.; Rochelle, L.K.; Chakraborty, S.K.; Zhang, M.; Eaton, A.W.; Bai, Y.; Ernst, L.A.; Barak, L.S.; Waggoner, A.S.; et al. A rapid and affordable screening platform for membrane protein trafficking. BMC Biol. 2015, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mao, Z.; He, S.; Zhan, Y.; Ning, R.; Liu, W.; Yan, B.; Yang, J. Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling pathway. Biochem. Pharmacol. 2017, 136, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the bioavailability of resveratrol: Combine it, derivatize it, or encapsulate it? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.F.; Abbas, A.M.; Elsamanoudy, A.Z.; Ghoneim, F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiol. Pharmacol. 2015, 66, 515–527. [Google Scholar] [PubMed]
- Pivac, N.; Knezevic, J.; Kozaric-Kovacic, D.; Dezeljin, M.; Mustapic, M.; Rak, D.; Matijevic, T.; Pavelic, J.; Muck-Seler, D. Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. J. Affect. Disord. 2007, 103, 131–138. [Google Scholar] [CrossRef]
- Yehuda, R.; Seckl, J. Minireview: Stress-related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology 2011, 152, 4496–4503. [Google Scholar] [CrossRef]









| Control (n = 8) | PS a (n = 7) | PS + RES b (n = 7) | |
|---|---|---|---|
| Time spent in open arms (s) | 193.37 ± 41.44 | 83.42 ± 27.64 ** | 176.71 ± 48.35 ## |
| Time spent in closed arms (s) | 106.62 ± 41.44 | 216.57 ± 27.6 ** | 137.57 ± 48.35 ## |
| Entries in open arms | 1.87 ± 0.96 | 1.71 ± 1.01 | 3.14 ± 0.68 |
| Entries in closed arms | 2.0 ± 0.89 | 5.14 ± 1.51 ** | 3.14 ± 0.35 ## |
| AI c | 0.38 ± 0.09 | 0.76 ± 0.8 ** | 0.45 ± 0.15 ## |
| Freezing | 0.36 ± 0.09 | 2.42 ± 0.3 *** | 0 ± 0 ### |
| Group | Necrosis a | Macrophages a | Lymphocytes a |
|---|---|---|---|
| Control | 0 ± 0 | 1.05 ± 0.15 | 0.7 ± 0 |
| PS | 2.98 ± 0.43 *** | 2.25 ± 0.05 ** | 3.1 ± 0.29 ** |
| PS + RES | 0.3 ± 0.07 ### | 0.49 ± 0.11 ### | 0.88 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseilikman, V.E.; Tseilikman, O.B.; Shevyrin, V.A.; Yegorov, O.N.; Epitashvili, A.A.; Aristov, M.R.; Karpenko, M.N.; Lipatov, I.A.; Pashkov, A.A.; Shamshurin, M.V.; et al. Unraveling the Liver–Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety. Biomedicines 2024, 12, 2063. https://doi.org/10.3390/biomedicines12092063
Tseilikman VE, Tseilikman OB, Shevyrin VA, Yegorov ON, Epitashvili AA, Aristov MR, Karpenko MN, Lipatov IA, Pashkov AA, Shamshurin MV, et al. Unraveling the Liver–Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety. Biomedicines. 2024; 12(9):2063. https://doi.org/10.3390/biomedicines12092063
Chicago/Turabian StyleTseilikman, Vadim E., Olga B. Tseilikman, Vadim A. Shevyrin, Oleg N. Yegorov, Alexandr A. Epitashvili, Maxim R. Aristov, Marina N. Karpenko, Ilya A. Lipatov, Anton A. Pashkov, Maxim V. Shamshurin, and et al. 2024. "Unraveling the Liver–Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety" Biomedicines 12, no. 9: 2063. https://doi.org/10.3390/biomedicines12092063
APA StyleTseilikman, V. E., Tseilikman, O. B., Shevyrin, V. A., Yegorov, O. N., Epitashvili, A. A., Aristov, M. R., Karpenko, M. N., Lipatov, I. A., Pashkov, A. A., Shamshurin, M. V., Buksha, I. A., Shonina, A. K., Kolesnikova, A., Shatilov, V. A., Zhukov, M. S., & Novak, J. (2024). Unraveling the Liver–Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety. Biomedicines, 12(9), 2063. https://doi.org/10.3390/biomedicines12092063






