Features of Metabolites and Biomarkers in Inflammatory and Infectious Complications of Childhood Cancers
Abstract
:1. Introduction
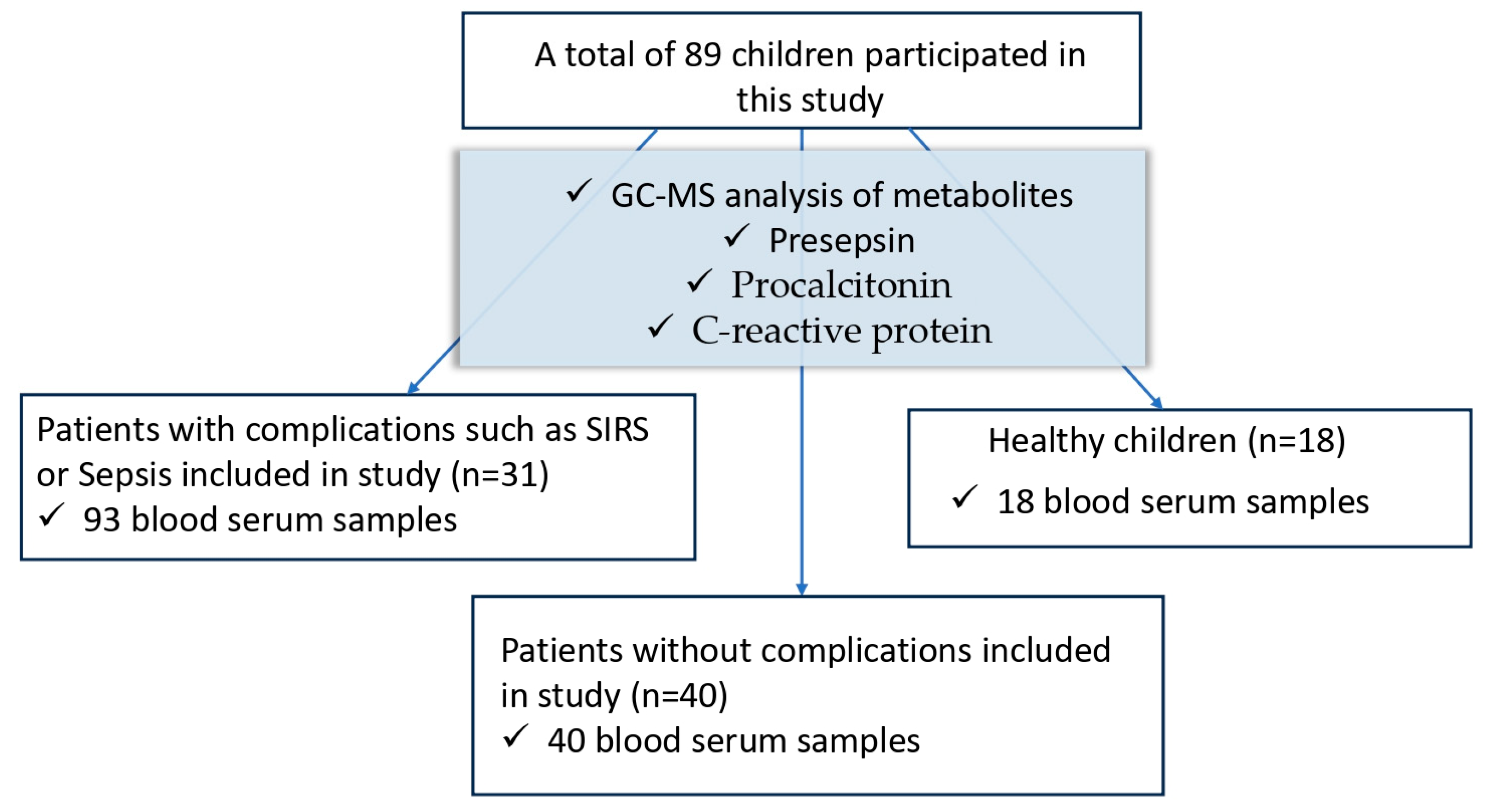
2. Materials and Methods
2.1. Study Design
- Patients less than 18 years old.
- Pediatric patients with cancer.
- Risk of infectious complications (immunosuppression, neutropenia, surgical interventions).
- Patients over 18 years old.
- No risk of infectious complications according to clinical and laboratory data.
- Terminal stage.
2.2. Patients and Samples
2.3. Methods
2.3.1. Reagents for GC–MS Analysis
2.3.2. GC–MS Analysis
2.3.3. Biomarker Analysis
2.3.4. Statistical Analysis
3. Results
3.1. Metabolic Changes
3.1.1. Metabolites and Biomarkers in Healthy Children, Patients without Complications, and Patients with Complications (SIRS, Sepsis, Septic Shock)
3.1.2. Monitoring Metabolites and Biomarkers in Patients with Complications
3.1.3. Changes in the Metabolomic Profile in Patients with Complications
- Body temperature ≥ 38 °C (febrile) or ≤36 °C (hypothermia).
- Heart rate ≥ 90/min (tachycardia).
- Tachypnea: respiratory rate ≥ 20/min or hyperventilation with blood carbon dioxide ≤ 32 mmHg.
- Leukocytosis (≥12,000/μL) or leukopenia (≤4000/μL) or shift in the leukocyte formula to the left.
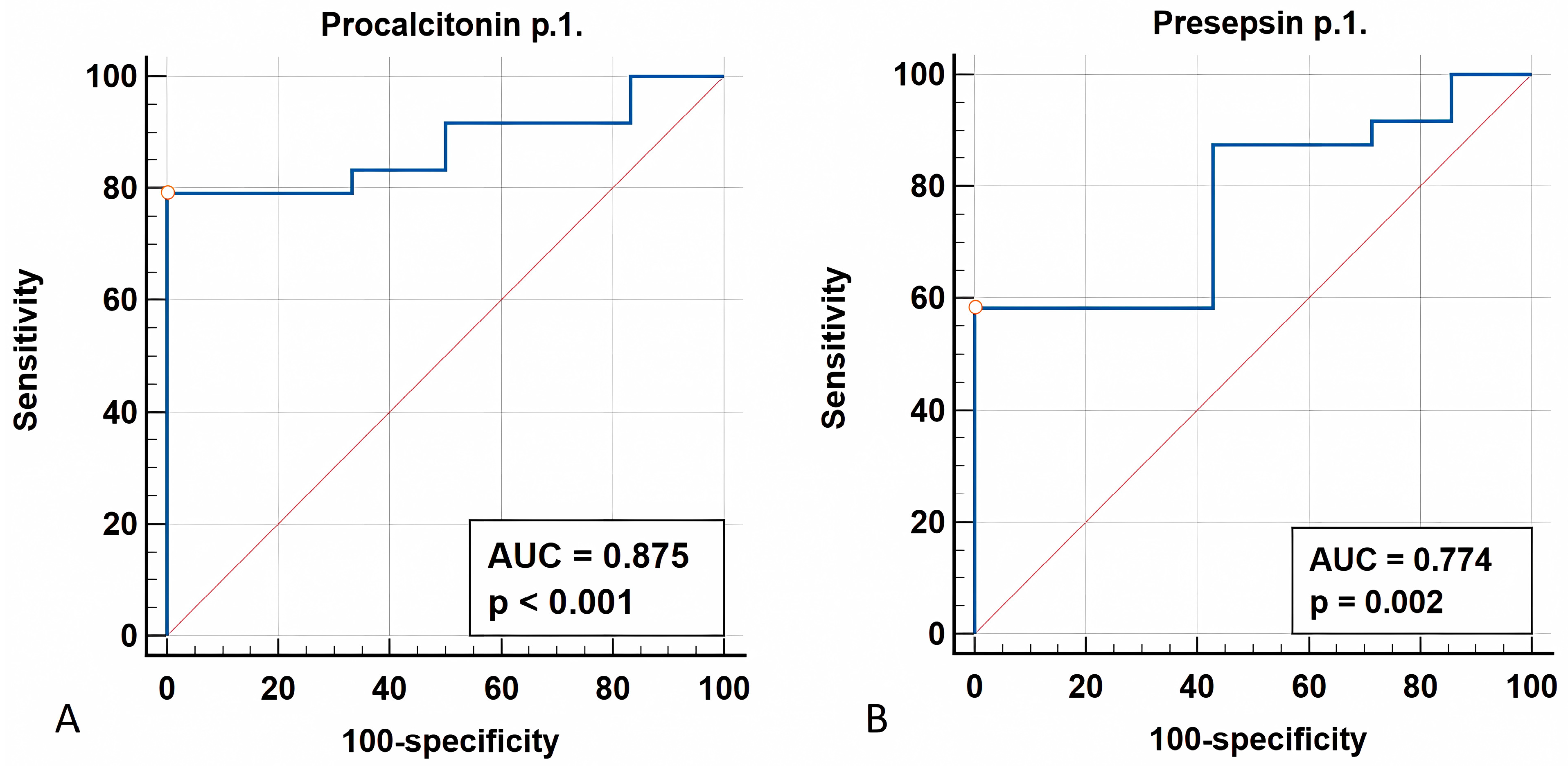
3.1.4. ROC Analysis
3.1.5. Metabolomic Profile Changes in Survivors vs. Non-Survivors
4. Discussion
4.1. Metabolites
4.2. Biomarkers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.M.; Abrahão, R.; Alvarez, E.M. Survival Trends Among Adolescents and Young Adults Diagnosed With Cancer in the United States: Comparisons with Children and Older Adults. J. Clin. Oncol. 2024, 42, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Barchuk, A.; Belyaev, A.; Gretsova, O.; Tursun-Zade, R.; Moshina, N.; Znaor, A. History and current status of cancer registration in Russia. Cancer Epidemiol. 2021, 73, 101963. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, V.M.; Shakhzadova, A.O.; Kulyova, S.A.; Perelygin, V.V. The state of cancer care in Russia: Age and cancer. Features of the localization structure of the quality of accounting and survival of patients with malignant tumors among the child population, adolescents and young adults in Russia (clinical and population study). Part 1. Pharm. Formulas 2023, 5, 20–32. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.E.; Schilberg, K.; Siegelin, M.D.; et al. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. An Official Website of the United States Government. Available online: https://www.cancer.gov/types/childhood-cancers (accessed on 9 August 2024).
- Pineros, M.; Mery, L.; Soerjomataram, I.; Bray, F.; Steliarova-Foucher, E. Scaling Up the Surveillance of Childhood Cancer: A Global Roadmap. JNCI J. Natl. Cancer Inst. 2021, 113, djaa069. [Google Scholar] [CrossRef]
- Bo, L.; Wang, Y.; Li, Y.; Wurpel, J.N.D.; Huang, Z.; Chen, Z.S. The Battlefield of Chemotherapy in Pediatric Cancers. Cancers 2023, 15, 1963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, H.; Wang, P.; Yang, F.; Cheng, W.; Chen, C.; Zhai, B.; Zhou, Y. Anticancer therapy-induced adverse drug reactions in children and preventive and control measures. Front. Pharmacol. 2024, 15, 1329220. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, A.R.; Shaw, P.J.; Rowell, G.; O’Connell, A.; Schell, D.; Gillis, J. Improved Outcomes of Children with Malignancy Admitted to a Pediatric Intensive Care Unit. Crit. Care Med. 2000, 28, 3718–3721. [Google Scholar] [CrossRef]
- Al Haj Moussa, A.; Maaz, A.U.R.; Faqih, N.; Sundaram, M. The Critically-Ill Pediatric Oncology Patients: What the Intensivist Needs to Know? Pediatric Critical Care Medicine. Indian J. Crit. Care Med. 2020, 24, 1256–1263. [Google Scholar] [CrossRef]
- Agulnik, A. Management of septic shock in children with cancer-Common challenges and research priorities. J. Pediatr. 2023, 99, 101–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wösten-van Asperen, R.M.; van Gestel, J.P.J.; van Grotel, M.; Tschiedel, E.; Dohna-Schwake, C.; Valla, F.V.; Willems, J.; Angaard Nielsen, J.S.; Krause, M.F.; Potratz, J.; et al. PICU mortality of children with cancer admitted to pediatric intensive care unit a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 142, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.M.; Johnston, D.L.; Armstrong, R.; Gaboury, I.; Menon, K. The Morbidity and Mortality of Pediatric Oncology Patients Presenting to the Intensive Care Unit with Septic Shock. Pediatr. Blood Cancer 2008, 51, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Hilarius, K.W.E.; Skippen, P.W.; Kissoon, N. Early Recognition and Emergency Treatment of Sepsis and Septic Shock in Children. Pediatr. Emerg. Care 2020, 36, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Souza, D.C.; Brandão, M.B.; Piva, J.P. From the International Pediatric Sepsis Conference 2005 to the Sepsis-3 Consensus. Rev. Bras. Ter. Intensiva 2018, 30, 1–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierrakos, C.; Vincent, J.L. Sepsis Biomarkers: A Review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Pautova, A.K. Metabolic Profiling of Aromatic Compounds. Metabolites 2024, 14, 107. [Google Scholar] [CrossRef]
- Trembach, N.V.; Magomedov, M.A.; Krasnov, V.G.; Chernienko, L.Y.; Shevyrev, S.N.; Popov, A.S.; Tyutyunova, E.V.; Vatutin, S.N.; Dmitriev, A.A.; Fisher, V.V.; et al. The Effect of ACE Inhibitors/ARBs Withdrawal on the Risk of Postoperative Complications in Abdominal Surgery. Obs. Reanimatol. Gen. Reanimatol. 2023, 19, 21–30, (In Russian and English). [Google Scholar] [CrossRef]
- Sokolov, D.A.; Kozlov, I.A. Informativeness of various predictors of perioperative cardiovascular complications in non-cardiac surgery. Bull. Anesthesiol. Reanimatol. 2023, 20, 6–16. (In Russian) [Google Scholar] [CrossRef]
- Hussain, H.; Vutipongsatorn, K.; Jiménez, B.; Antcliffe, D.B. Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites 2022, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhou, J.; Yang, T.; Li, J.; Jiang, X.; Zhang, W.; Gu, S.; Wang, J. Landscape of Metabolic Fingerprinting for Diagnosis and Risk Stratification of Sepsis. Front. Immunol. 2022, 8, 883628. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, S.H.; Chung, K.S.; Ku, N.S.; Hyun, Y.M.; Chun, S.; Park, M.S.; Lee, S.G. Development and validation of a novel sepsis biomarker based on amino acid profiling. Clin. Nutr. 2021, 6, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Pautova, A.K.; Burnakova, N.A.; Beloborodova, N.B.; Revelsky, A.I. Simultaneous determination of aromatic, short-chain fatty and dicarboxylic acids in blood serum and cerebrospinal fluid by gas chromatography-mass spectrometry. J. Anal. Chem. 2023, 79, 1–13. [Google Scholar] [CrossRef]
- Han, Q.; Phillips, R.S.; Li, J. Editorial: Aromatic Amino Acid Metabolism. Front. Mol. Biosci. 2019, 6, 22. [Google Scholar] [CrossRef]
- Chernevskaya, E.A.; Getsina, M.L.; Cherpakov, R.A.; Sorokina, E.A.; Shabanov, A.K.; Moroz, V.V.; Belobo-rodova, N.V. Sepsis-associated metabolites and their biotransformation by intestinal microbiota. Gen. Reanimatol. 2023, 19, 4–12, (In Russian and English). [Google Scholar] [CrossRef]
- Sun, S.; Wang, D.; Dong, D.; Xu, L.; Xie, M.; Wang, Y.; Ni, T.; Jiang, W.; Zhu, X.; Ning, N.; et al. Altered intestinal microbiome and metabolome correspond to the clinical outcome of sepsis. Crit. Care 2023, 27, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, F.; Dai, X.; Zhou, C.C.; Li, K.X.; Zhang, Y.J.; Lou, X.Y.; Zhu, Y.M.; Sun, Y.L.; Peng, B.X.; Cui, W. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut 2022, 71, 1315–1325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Getsina, M.; Tsyba, N.; Polyakov, P.; Beloborodova, N.; Chernevskaya, E. Blood Serum and Drainage Microbial and Mitochondrial Metabolites in Patients after Surgery for Pancreatic Cancer. Metabolites 2023, 13, 1198. [Google Scholar] [CrossRef]
- Chernevskaya, E.; Beloborodova, N.; Klimenko, N.; Pautova, A.; Shilkin, D.; Gusarov, V.; Tyakht, A. Serum and fecal profiles of aromatic microbial metabolites reflect gut microbiota disruption in critically ill patients: A prospective observational pilot study. Crit. Care 2020, 24, 312. [Google Scholar] [CrossRef] [PubMed]
- Grauslys, A.; Phelan, M.M.; Broughton, C.; Baines, P.B.; Jennings, R.; Siner, S.; Paulus, S.C.; Carrol, E.D. Title NMR-Based Metabolic Profiling Provides Diagnostic and Prognostic Information in Critically Ill Children with Suspected Infection. Sci. Rep. 2020, 10, 20198. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, A.M.; Edin, A.; Ziegler, I.; Mölling, P.; Sjöstedt, A.; Gylfe, Å.; Strålin, K.; Johansson, A. Metabolites in Blood for Prediction of Bacteremic Sepsis in the Emergency Room. PLoS ONE 2016, 11, e0147670. [Google Scholar] [CrossRef] [PubMed]
- Schmerler, D.; Neugebauer, S.; Ludewig, K.; Bremer-Streck, S.; Brunkhorst, F.M.; Kiehntopf, M. Targeted metabolomics for discrimination of systemic infl ammatory disorders in critically ill patients. J. Lipid Res. 2012, 53, 1369–1375. [Google Scholar] [CrossRef]
- Neugebauer, S.; Giamarellos-Bourboulis, E.J.; Pelekanou, A.; Marioli, A.; Baziaka, F.; Tsangaris, I.; Bauer, M.; Kiehntopf, M. Metabolite Profiles in Sepsis: Developing Prognostic Tools Based on the Type of Infection. Crit. Care Med. 2016, 44, 1649–1662. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic Changes in Amino Acid Concentration Profiles in Patients with Sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar] [CrossRef]
- Lin, S.-H.; Fan, J.; Zhu, J.; Zhao, Y.-S.; Wang, C.-J.; Zhang, M.; Xu, F. Exploring Plasma Metabolomic Changes in Sepsis: A Clinical Matching Study Based on Gas Chromatography–Mass Spectrometry. Ann. Transl. Med. 2020, 8, 1568. [Google Scholar] [CrossRef]
- Pautova, A.K.; Samokhin, A.S.; Beloborodova, N.V.; Revelsky, A.I. Multivariate Prognostic Model for Pre-dicting the Outcome of Critically Ill Patients Using the Aromatic Metabolites Detected by Gas Chromatog-raphy-Mass Spectrometry. Molecules 2022, 27, 4784. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Teng, S.; Li, K. Prediction of Sepsis Mortality Using Metabolite Biomarkers in the Blood: A Meta-Analysis of Death-Related Pathways and Prospective Validation. BMC Med. 2020, 18, 83. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Duggan, G.E.; Winston, B.W.; Doig, C.; Kubes, P.; Vogel, H.J. Metabolic Profiling of Serum Samples by 1H Nuclear Magnetic Resonance Spectroscopy as a Potential Diagnostic Approach for Septic Shock. Crit. Care Med. 2014, 42, 1140–1149. [Google Scholar] [CrossRef]
- Rubnitz, Z.; Sun, Y.; Agulnik, A.; Merritt, P.; Allison, K.; Ferrolino, J.; Dallas, R.; Tang, L.; Wolf, J. Prediction of attributable mortality in pediatric patients with cancer admitted to the intensive care unit for suspected infection: A comprehensive evaluation of risk scores. Cancer Med. 2023, 12, 21287–21292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Downes, K.J. Procalcitonin in Pediatric Sepsis: What Is It Good for? J. Pediatric Infect. Dis. Soc. 2021, 10, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, S.; Yuan, X.; Wang, H.; Wen, F. Evaluation of Inflammatory Biomarkers in Pediatric Hematology-Oncology Patients with Bloodstream Infection. J. Pediatr. Hematol. Oncol. 2021, 43, e596–e600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kukes, V.G.; Marinin, V.F.; Olefir, Y.V.; Shih, E.V.; Prokofiev, A.B.; Grapov, D.O.; Verdieva, D.A.; Rumyantsev, N.A. Presepsin—A New Biological Marker for Sepsis Diagnosing and Monitoring the Effect of Treatment. Med. News North Cauc. 2018, 13, 573–576. [Google Scholar] [CrossRef]
- Cerasi, S.; Leardini, D.; Lisanti, N.; Belotti, T.; Pierantoni, L.; Zama, D.; Lanari, M.; Prete, A.; Masetti, R. The Role of Presepsin in Pediatric Patients with Oncological and Hematological Diseases Experiencing Febrile Neutropenia. Sci. Rep. 2023, 13, 6464. [Google Scholar] [CrossRef] [PubMed]
- Guskova, N.K.; Morozova, A.A.; Rozenko, D.A.; Alyoshkina, A.V.; Skopintsev, A.M.; Selyutina, O.N.; Golomeeva, N.V.; Guskova, E.A.; Donskaya, A.K.; Tselishcheva, I.V.; et al. Presepsin as a Marker of Sepsis in Oncological Patients after Surgical Interventions. South. Russ. J. Cancer 2022, 3, 6–13. [Google Scholar] [CrossRef]
- Lee, M.J.; Han, W.H.; Chun, J.Y.; Kim, S.Y.; Kim, J.H. Presepsin in the Rapid Response System for Cancer Patients: A Retrospective Analysis. J. Clin. Med. 2021, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Y.; Li, C.; Liu, C.; Yao, Y.; Su, M.; Shou, S. The utility of presepsin in diagnosis and risk stratification for the emergency patients with sepsis. Am. J. Emerg. Med. 2018, 8, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.E.; Yoon, S.J.; Park, C.M.; Lee, N.Y.; Shin, T.G.; Kang, E.S. No Significant Differences in Presepsin Levels According to the Causative Microorganism of Bloodstream Infection. Infect. Chemother. 2024, 56, 47–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moustafa, R.; Albouni, T.; Aziz, G. The role of procalcitonin and presepsin in the septic febrile neutropenia in acute leukemia patients. PLoS ONE 2021, 16, e0253842. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Healthy Children (n = 18) | Patients without Complications (n = 40) | Patients with Complications (n = 31) |
|---|---|---|---|
| Gender, males, n (%) | 9 (50.0%) | 26 (65.0%) | 19 (61.3%) |
| Gender, females, n (%) | 9 (50.0%) | 14 (35.0%) | 12 (38.7%) |
| Age, years (Me (IQR)) | 13.5 (8.0; 16.0) | 9.0 (4.0; 12.0) | 9.0 (4.0; 15.0) |
| Cancer stage, n (%) | - | I-8 (20.0%) II-10 (25.0%) III-9 (22.5%) IV-13 (32.5%) | I-9 (29.0%) II-12 (38.7%) III-5 (16.1%) IV-5 (16.1%) |
| Bone marrow transplantation, n (%) | - | 1 (2.5%) | 2 (6.5%) |
| Leukemia, n (%) | - | 12 (30.0%) | 14 (45.0%) |
| Systemic pathology, n (%) | - | - | Aplasia—15 (48.4%), cardiovascular system—8 (25.8%), digestive—8 (25.8%), respiratory organs—7 (22.6%), kidneys—5 (16.1%), CNS—3 (9.7%), SPON—1 (3.2%), liver—1 (3.2%) |
| Complications after treatment, n (%) | - | - | Enterocolitis—8 (25.8%), coagulopathy—5 (16.1%), mucositis—4 (12.9%), stomatitis—4 (12.9%), pleurisy—2 (6.5%), bronchitis—2 (6.5%), hemorrhagic syndrome—1 (3.2%), shock—1 (3.2%), epilepsy—1 (3.2%), pyoderma—1 (3.2%) |
| Metabolites, μmol/L/Biomarkers | Healthy Children (n = 18) | Patients without Complications (n = 40) | Patients with Complications (n = 31) | p-Value |
|---|---|---|---|---|
| PhLA | <0.5 (<0.5; <0.5) | <0.5 (<0.5; <0.5) | <0.5 (<0.5; 0.9) | - |
| p-HPhAA | <0.5 (<0.5; <0.5) | <0.5 (<0.5; 0.6) | <0.5 (<0.5; 1.0) | - |
| p-HPhLA | 1.4 (1.1; 1.8) | 0.9 (0.7; 1.1) | 1.1 (0.8; 1.7) | <0.001 |
| Σ3AMM | 2.2 (1.5; 2.6) | 1.5 (1.1; 1.8) | 2.0 (1.6; 3.3) | 0.001 |
| Succinic acid | 3.4 (2.7; 4.8) | 2.8 (2.5; 3.6) | 1.9 (1.3; 3.1) | <0.001 |
| Fumaric acid | 1.1 (0.9; 1.2) | 0.7 (0.6; 0.9) | 0.8 (0.4; 1.2) | 0.002 |
| CRP (mg/L) | 0.3 (0.2; 3.8) | 1.35 (0.9; 2.4) | 128 (66; 201) | <0.001 |
| PCT (ng/mL) | 0.06 (0.03; 0.07) | 0.07 (0.05; 0.12) | 4.2 (0.6; 11.8) | <0.001 |
| PSP (pg/mL) | 93 (72; 111) | 108 (91; 122) | 526 (331; 917) | <0.001 |
| Metabolites, μmol/L/Biomarkers | Patient Point 1 (n = 31) | Patient Point 2 (n = 31) | Patient Point 3 (n = 31) | p-Value |
|---|---|---|---|---|
| PhLA | <0.5 (<0.5; 0.9) | <0.5 (<0.5; 0.7) | <0.5 (<0.5; <0.5) | - |
| p-HPhAA | <0.5 (<0.5; 1) | <0.5 (<0.5; 0.9) | <0.5 (<0.5; 0.5) | - |
| p-HPhLA | 1.1 (0.8; 1.7) | 1.0 (0.8; 1.5) | 1.1 (0.8; 1.4) | 0.411 |
| Succinic acid | 1.9 (1.3; 3.1) | 2.0 (1.2; 2.9) | 1.6 (1.3; 2.7) | 0.949 |
| Fumaric acid | 0.8 (<0.5; 1.2) | 0.7 (<0.5; 1.3) | 0.6 (0.5; 1.4) | 0.841 |
| CRP (mg/L) | 128 (66; 201) | 89 (40; 128) | 52 (13; 98) | 0.013 |
| PCT (ng/mL) | 5.24 (3; 16.2) | 4.2 (1; 13.1) | 2.6 (0.9; 6.8) | 0.003 |
| PSP (pg/mL) | 526 (331; 917) | 517 (213; 941) | 483 (278; 685) | 0.034 |
| Metabolites, μmol/L/Biomarkers | Patients with Sepsis/Septic Shock (n = 24) | Patients with Inflammation (n = 7) | p-Value |
|---|---|---|---|
| Point 1 | |||
| PhLA | <0.5 (<0.5; 0.9) | 0.5 (<0.5; 0.7) | 0.872 |
| p-HPhAA | <0.5 (<0.5; 0.9) | 0.6 (<0.5; 1.0) | 0.216 |
| p-HPhLA | 1.1 (0.9; 1.7) | 1.3 (0.8; 1.7) | 0.729 |
| Σ3AMM | 1.7 (1.4; 3.5) | 2.3 (2.1; 2.9) | 0.274 |
| Succinic acid | 2.0 (1.3; 2.8) | 1.8 (1.4; 3.1) | 0.908 |
| Fumaric acid | 0.7 (<0.5; 1.2) | 0.8 (0.5; 1.2) | 0.945 |
| CRP (mg/L) | 147 (67; 202) | 119 (64; 128) | 0.234 |
| PCT (ng/mL) | 5.3 (3.2; 17.3) | 0.45 (0.28; 0.88) | 0.003 |
| PSP (pg/mL) | 662 (416; 940) | 316 (179; 526) | 0.029 |
| Metabolites, μmol/L/Biomarkers | Patients Who Survived (n = 28) | Non-Survivor Patients (n = 3) | p-Value |
|---|---|---|---|
| PhLA | <0.5 (<0.5; 0.7) | 1.4 (0.6; 3.7) | - |
| p-HPhAA | <0.5 (<0.5; 0.9) | 1.02 (<0.5; 1.59) | - |
| p-HPhLA | 1.1 (0.8; 1.4) | 2.1 (1.9; 10.6) | 0.007 |
| Σ3AMM | 1.9 (1.5; 2.8) | 3.5 (3.5; 15.8) | 0.018 |
| Succinic acid | 1.8 (1.3; 2.4) | 3.1 (2.4; 3.6) | 0.065 |
| Fumaric acid | 0.7 (0.4; 1.2) | 1.1 (0.6; 1.7) | 0.385 |
| CRP (mg/L) | 120 (65; 185) | 239 (174; 317) | 0.030 |
| PCT (ng/mL) | 4.1 (0.5; 11.8) | 6.9 (0.7; 161) | 0.663 |
| PSP (pg/mL) | 474 (324; 740) | 942 (938; 2331) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getsina, M.; Chernevskaya, E.; Beloborodova, N.; Golovnya, E.; Polyakov, P.; Kushlinskii, N. Features of Metabolites and Biomarkers in Inflammatory and Infectious Complications of Childhood Cancers. Biomedicines 2024, 12, 2101. https://doi.org/10.3390/biomedicines12092101
Getsina M, Chernevskaya E, Beloborodova N, Golovnya E, Polyakov P, Kushlinskii N. Features of Metabolites and Biomarkers in Inflammatory and Infectious Complications of Childhood Cancers. Biomedicines. 2024; 12(9):2101. https://doi.org/10.3390/biomedicines12092101
Chicago/Turabian StyleGetsina, Maria, Ekaterina Chernevskaya, Natalia Beloborodova, Evgeniy Golovnya, Petr Polyakov, and Nicolai Kushlinskii. 2024. "Features of Metabolites and Biomarkers in Inflammatory and Infectious Complications of Childhood Cancers" Biomedicines 12, no. 9: 2101. https://doi.org/10.3390/biomedicines12092101







