Impact of Mild COVID-19 History on Oral-Gut Microbiota and Serum Metabolomics in Adult Patients with Crohn’s Disease: Potential Beneficial Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Outcomes
2.4. Sample Collection
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Metabolomic Data Generation and Analysis
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
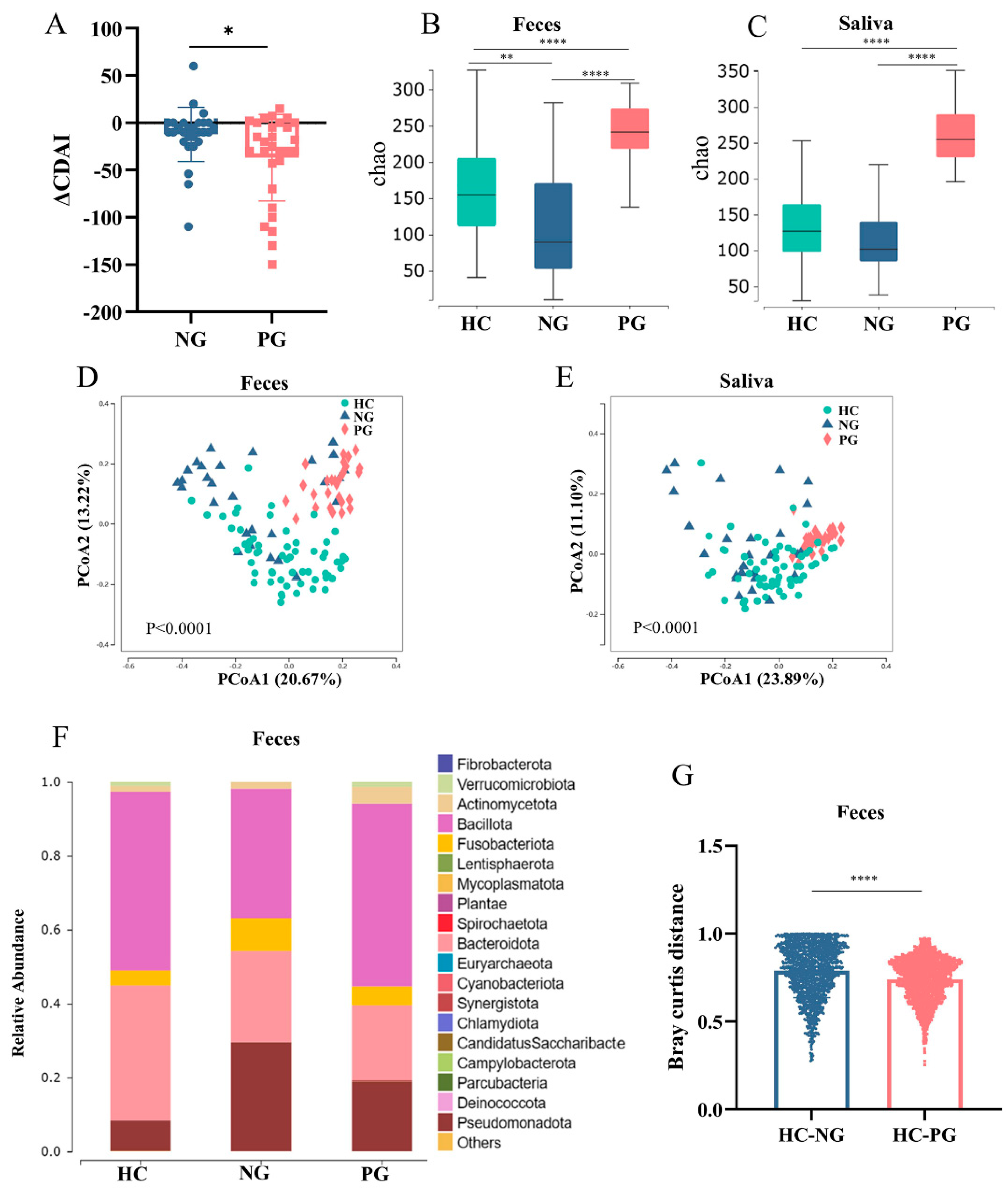
3.2. Altered Oral and Gut Microbiota Composition in COVID-19-Infected Patients with CD
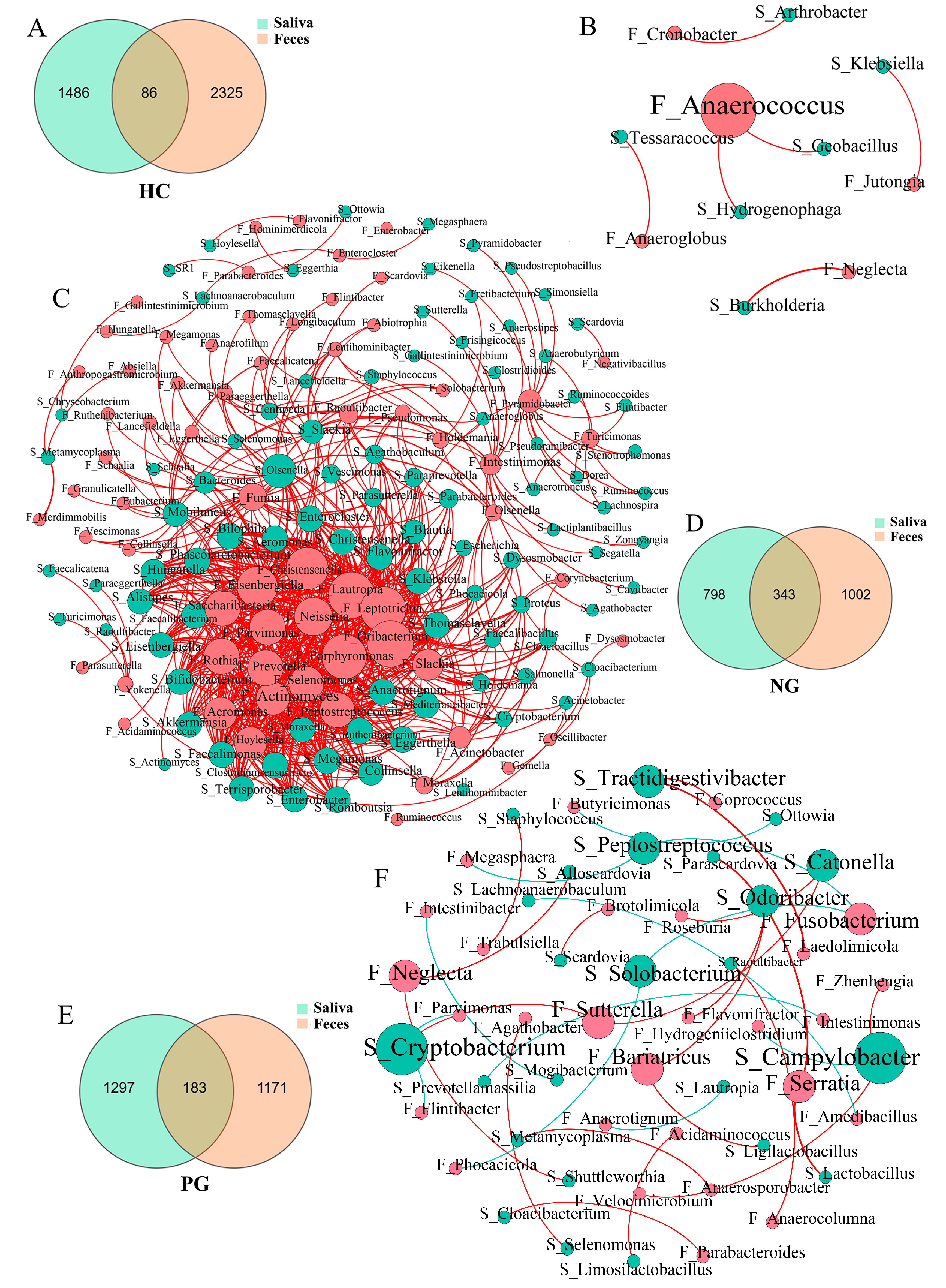
3.3. Interaction between the Oral and Gut Microbial Communities
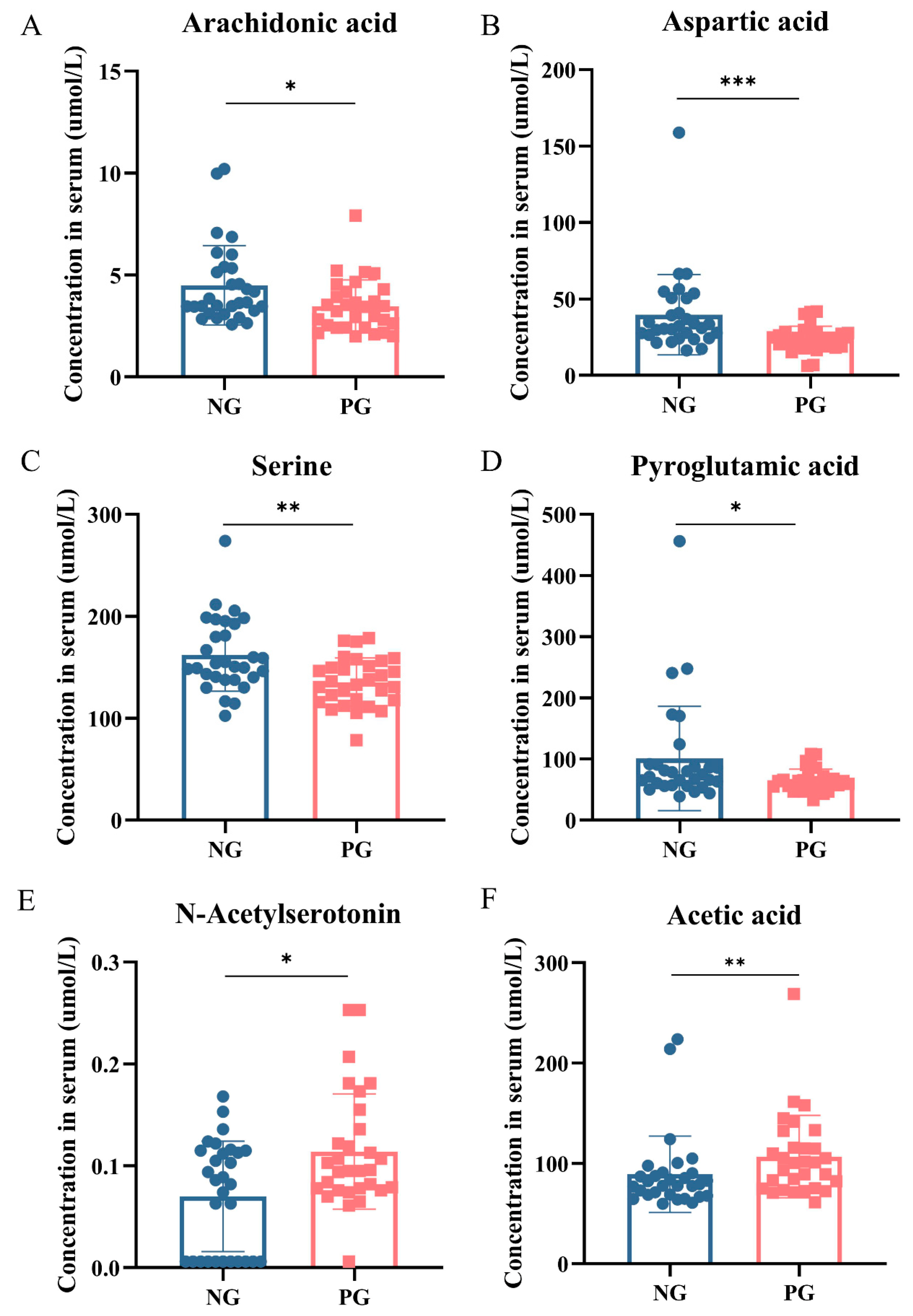
3.4. Metabolomic Changes in Participant Serum
3.5. Correlations between Microbiota and Serum Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Fiorino, G.; Zallot, C.; Furfaro, F.; Gilardi, D.; Radice, S.; Danese, S.; Peyrin-Biroulet, L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients From the Nancy and Milan Cohorts. Clin. Gastroenterol. Hepatol. 2020, 18, 2134–2135. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.S.; Wang, X.Y.; Yang, X.K.; Liu, Y.C.; Fu, Z.Y.; Zhang, L.Z.; Wang, Z.X.; Ni, J.; Shuai, Z.W.; Pan, H.F. COVID-19 and inflammatory bowel disease crosstalk: From emerging association to clinical proposal. J. Med. Virol. 2022, 94, 5640–5652. [Google Scholar] [CrossRef]
- King, D.S.; Trudgill, N.J.; Adderley, N.J. Editorial: Increasing IBD prevalence and its complications in the context of the COVID-19 pandemic. Authors’ reply. Aliment. Pharmacol. Ther. 2020, 51, 1442–1443. [Google Scholar] [CrossRef]
- Cui, G.Y.; Rao, B.C.; Zeng, Z.H.; Wang, X.M.; Ren, T.; Wang, H.Y.; Luo, H.; Ren, H.Y.; Liu, C.; Ding, S.Y.; et al. Characterization of oral and gut microbiome and plasma metabolomics in COVID-19 patients after 1-year follow-up. Mil. Med. Res. 2022, 9, 32. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, G.; Wang, X.; Guo, M.; Zeng, W.; Xu, Z.; Cao, D.; Pan, A.; Wang, Y.; Zhang, K.; et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med. Microecol. 2020, 5, 100023. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., 3rd; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e414. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F., Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Heinstein, C.; MacDonald, J.; Pettipas, J.; Hatchette, T.F.; Patriquin, G. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104442. [Google Scholar] [CrossRef]
- Kojima, N.; Turner, F.; Slepnev, V.; Bacelar, A.; Deming, L.; Kodeboyina, S.; Klausner, J.D. Self-Collected Oral Fluid and Nasal Swabs Demonstrate Comparable Sensitivity to Clinician Collected Nasopharyngeal Swabs for Coronavirus Disease 2019 Detection. Clin. Infect. Dis. 2021, 73, e3106–e3109. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Weisdorf, D.J.; Costalonga, M.; Staley, C. No evidence for colonization of oral bacteria in the distal gut in healthy adults. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Imai, J.; Ichikawa, H.; Kitamoto, S.; Golob, J.L.; Kaneko, M.; Nagata, J.; Takahashi, M.; Gillilland, M.G., 3rd; Tanaka, R.; Nagao-Kitamoto, H.; et al. A potential pathogenic association between periodontal disease and Crohn’s disease. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Somineni, H.K.; Weitzner, J.H.; Venkateswaran, S.; Dodd, A.; Prince, J.; Karikaran, A.; Sauer, C.G.; Abramowicz, S.; Zwick, M.E.; Cutler, D.J.; et al. Site- and Taxa-Specific Disease-Associated Oral Microbial Structures Distinguish Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 1889–1900. [Google Scholar] [CrossRef]
- Qian, J.; Lu, J.; Huang, Y.; Wang, M.; Chen, B.; Bao, J.; Wang, L.; Cui, D.; Luo, B.; Yan, F. Periodontitis Salivary Microbiota Worsens Colitis. J. Dent. Res. 2022, 101, 559–568. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Shkodra-Pula, B.; Kretzer, C.; Jordan, P.M.; Klemm, P.; Koeberle, A.; Pretzel, D.; Banoglu, E.; Lorkowski, S.; Wallert, M.; Höppener, S.; et al. Encapsulation of the dual FLAP/mPEGS-1 inhibitor BRP-187 into acetalated dextran and PLGA nanoparticles improves its cellular bioactivity. J. Nanobiotechnol. 2020, 18, 73. [Google Scholar] [CrossRef]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Ducker, G.S.; Billingham, L.K.; Martinez, C.A.; Mainolfi, N.; Suri, V.; Friedman, A.; Manfredi, M.G.; Weinberg, S.E.; Rabinowitz, J.D.; et al. Serine Metabolism Supports Macrophage IL-1β Production. Cell Metab. 2019, 29, 1003–1011.e1004. [Google Scholar] [CrossRef]
- He, L.; Ding, Y.; Zhou, X.; Li, T.; Yin, Y. Serine signaling governs metabolic homeostasis and health. Trends Endocrinol. Metab. TEM 2023, 34, 361–372. [Google Scholar] [CrossRef]
- Cioffi, I.; Di Vincenzo, O.; Imperatore, N.; Fisco, M.; Testa, A.; Scialò, F.; Castiglione, F.; Ruoppolo, M.; Pasanisi, F.; Santarpia, L. Amino acid profiles, disease activity, and protein intake in adult patients with Crohn’s disease. Front. Nutr. 2023, 10, 1245574. [Google Scholar] [CrossRef]
- Martin, F.P.; Su, M.M.; Xie, G.X.; Guiraud, S.P.; Kussmann, M.; Godin, J.P.; Jia, W.; Nydegger, A. Urinary metabolic insights into host-gut microbial interactions in healthy and IBD children. World J. Gastroenterol. 2017, 23, 3643–3654. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Ammar, N.M.; Abdelhameed, M.F.; Gendy, A.; Ragab, T.I.M.; Abd-ElGawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective Mechanism of Acacia saligna Butanol Extract and Its Nano-Formulations against Ulcerative Colitis in Rats as Revealed via Biochemical and Metabolomic Assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef]
- Li, M.; Zhang, R.; Xin, M.; Xu, Y.; Liu, S.; Yu, B.; Zhang, B.; Liu, J. Discovery and Validation of Potential Serum Biomarkers with Pro-Inflammatory and DNA Damage Activities in Ulcerative Colitis: A Comprehensive Untargeted Metabolomic Study. Metabolites 2022, 12, 997. [Google Scholar] [CrossRef]
- Kang, C.; Jeong, S.; Kim, J.; Ju, S.; Im, E.; Heo, G.; Park, S.; Yoo, J.W.; Lee, J.; Yoon, I.S.; et al. N-Acetylserotonin is an oxidation-responsive activator of Nrf2 ameliorating colitis in rats. J. Pineal Res. 2023, 74, e12835. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ariyannur, P.S.; Ribeiro, R.; Tanaka, M.; Moffett, J.R.; Kirmani, B.F.; Namboodiri, A.M.; Zhang, Y. Efficacy of N-Acetylserotonin and Melatonin in the EAE Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2016, 11, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Lopetuso, L.R.; Vetrone, L.M.; Gasbarrini, A.; Papa, A. SARS-CoV-2 infection as a potential trigger factor for de novo occurrence of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2022, 34, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Elbadry, M.; Medhat, M.A.; Zaky, S.; El Kassas, M. Ulcerative colitis as a possible sequela of COVID-19 Infection: The endless story. Arab J. Gastroenterol. 2022, 23, 134–137. [Google Scholar] [CrossRef]
- Hadi, Y.; Dulai, P.S.; Kupec, J.; Mohy-Ud-Din, N.; Jairath, V.; Farraye, F.A.; Kochhar, G.S. Incidence, outcomes, and impact of COVID-19 on inflammatory bowel disease: Propensity matched research network analysis. Aliment. Pharmacol. Ther. 2022, 55, 191–200. [Google Scholar] [CrossRef]
- Viganò, C.; Massironi, S.; Pirola, L.; Cristoferi, L.; Fichera, M.; Bravo, M.; Mauri, M.; Redaelli, A.E.; Dinelli, M.E.; Invernizzi, P. COVID-19 in Patients With Inflammatory Bowel Disease: A Single-center Observational Study in Northern Italy. Inflamm. Bowel Dis. 2020, 26, e138–e139. [Google Scholar] [CrossRef]
- Nobel, Y.R.; Phipps, M.; Zucker, J.; Lebwohl, B.; Wang, T.C.; Sobieszczyk, M.E.; Freedberg, D.E. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology 2020, 159, 373–375.e372. [Google Scholar] [CrossRef]
- Aghemo, A.; Piovani, D.; Parigi, T.L.; Brunetta, E.; Pugliese, N.; Vespa, E.; Omodei, P.D.; Preatoni, P.; Lleo, A.; Repici, A.; et al. COVID-19 Digestive System Involvement and Clinical Outcomes in a Large Academic Hospital in Milan, Italy. Clin. Gastroenterol. Hepatol. 2020, 18, 2366–2368.e2363. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, C.; Hegazy, A.N.; Glauben, R.; Siegmund, B. COVID-19-from mucosal immunology to IBD patients. Mucosal Immunol. 2021, 14, 566–573. [Google Scholar] [CrossRef]
- Livanos, A.E.; Jha, D.; Cossarini, F.; Gonzalez-Reiche, A.S.; Tokuyama, M.; Aydillo, T.; Parigi, T.L.; Ramos, I.; Dunleavy, K.; Lee, B.; et al. Gastrointestinal involvement attenuates COVID-19 severity and mortality. Medrxiv Prepr. Serv. Health Sci. 2020. [Google Scholar] [CrossRef]



| Parameter | HC | NG | PG | p |
|---|---|---|---|---|
| Sex (M/F) | 34/26 | 20/10 | 23/7 | a 0.567 b 0.069 c 0.494 |
| Age (years) | 21.8 (19.56–24.56) d | 30.37 ± 8.65 e | 33.13 ± 7.80 | a 0.199 b 0.543 c 0.114 |
| BMI (kg/m2) | 22.25 ± 3.51 | 18.77 ± 3.35 | 20.24 ± 2.82 | a 0.071 b 0.008 c 0.000 |
| Duration (years) | - | 3.32 ± 3.38 | 5.17 ± 4.65 | 0.08 |
| Smoking | - | 0 | 2 | 0.492 |
| Biologics | - - | 10 f 12.00 ± 9.15 g | 16 13.00 ± 9.28 | 0.192 0.79 |
| Immunosuppressants | - | 7 15.83 ± 8.23 | 3 18.00 ± 2.00 | 0.299 0.676 |
| Glucocorticoids | - | 3 6.00 ± 2.00 | 0 - | 0.237 - |
| Enteral nutritional agent | - | 3 5.83 ± 7.97 | 6 8.52 ± 15.26 | 0.472 0.79 |
| 5-ASA | - | 7 6.83 ± 5.42 | 3 2.00 ± 1.73 | 0.299 1.88 |
| Traditional Chinese medicine | - | 0 - | 2 10.00 ± 5.66 | 0.492 - |
| Non-use | - | 7 | 1 | 0.052 |
| CDAI < 150/CDAI ≥ 150 | - | 8/22 | 8/22 | 1.000 |
| Mild/Moderate/Severe | 14/8/0 | 15/7/0 | 1.000 | |
| Location (ileum/ileocolon/colon) | - | 9/20/1 | 13/17/0 | 0.422 |
| Stricturing | - | 2 | 6 | 0.254 |
| Penetrating | - | 6 | 5 | 1.000 |
| Stricturing and penetrating | - | 1 | 1 | 1.000 |
| Non-stricturing and non-penetrating | - | 21 | 18 | 0.589 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, B.; Zhang, Q.; Wu, H.; Lin, J.; Xu, Z.; Zhang, M.; Zhu, L.; Hu, J.; Zhi, M. Impact of Mild COVID-19 History on Oral-Gut Microbiota and Serum Metabolomics in Adult Patients with Crohn’s Disease: Potential Beneficial Effects. Biomedicines 2024, 12, 2103. https://doi.org/10.3390/biomedicines12092103
Xiang B, Zhang Q, Wu H, Lin J, Xu Z, Zhang M, Zhu L, Hu J, Zhi M. Impact of Mild COVID-19 History on Oral-Gut Microbiota and Serum Metabolomics in Adult Patients with Crohn’s Disease: Potential Beneficial Effects. Biomedicines. 2024; 12(9):2103. https://doi.org/10.3390/biomedicines12092103
Chicago/Turabian StyleXiang, Bingjie, Qi Zhang, Huibo Wu, Jue Lin, Zhaoyuan Xu, Min Zhang, Lixin Zhu, Jun Hu, and Min Zhi. 2024. "Impact of Mild COVID-19 History on Oral-Gut Microbiota and Serum Metabolomics in Adult Patients with Crohn’s Disease: Potential Beneficial Effects" Biomedicines 12, no. 9: 2103. https://doi.org/10.3390/biomedicines12092103







