Evaluating the Clinical Utility of Left Ventricular Strains in Severe AS: A Pilot Study with Feature-Tracking Cardiac Magnetic Resonance
Abstract
:1. Introduction
2. Materials and Methods
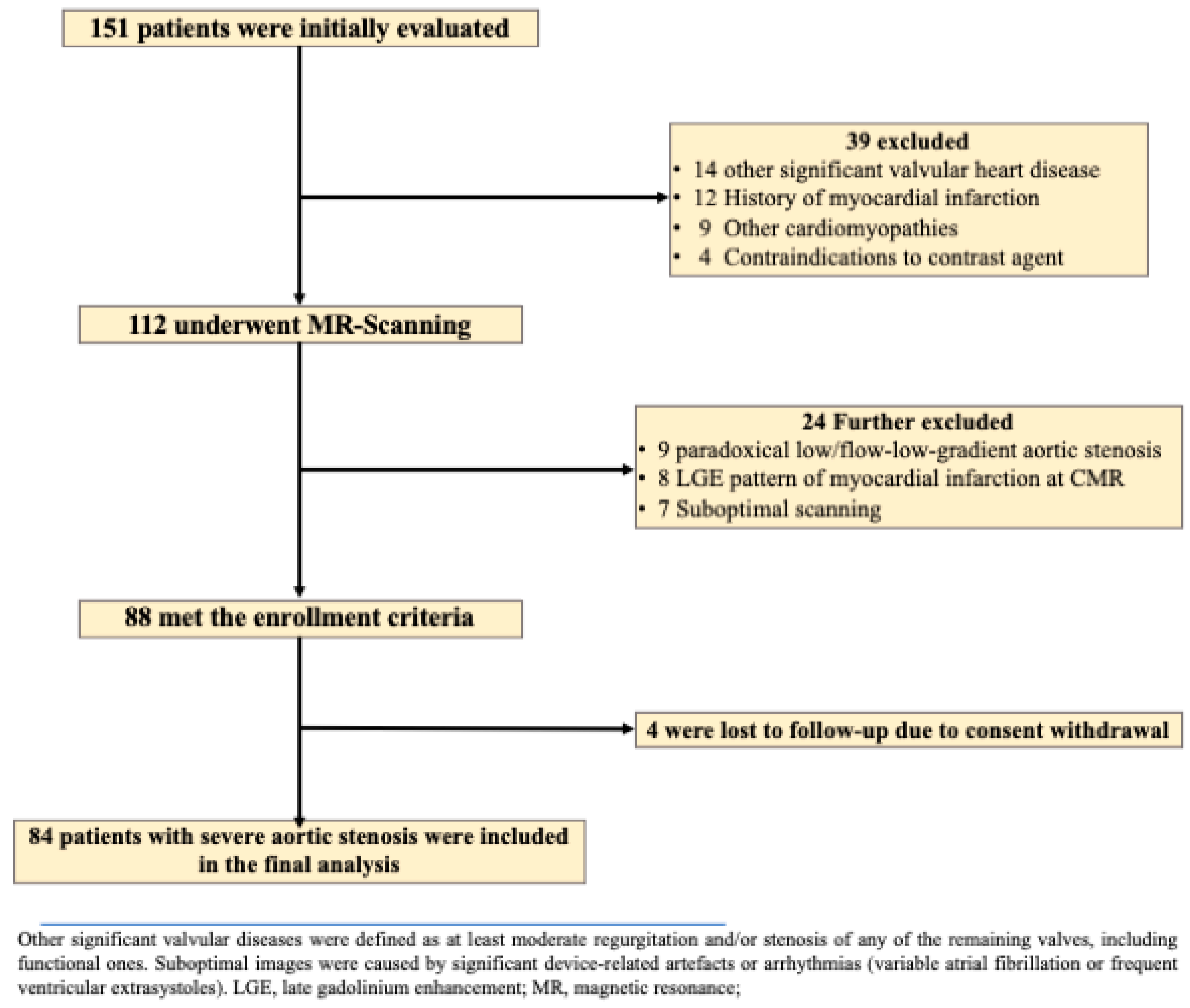
2.1. Study Population
2.2. Image Acquisition and Analysis of Standard CMR Metrics
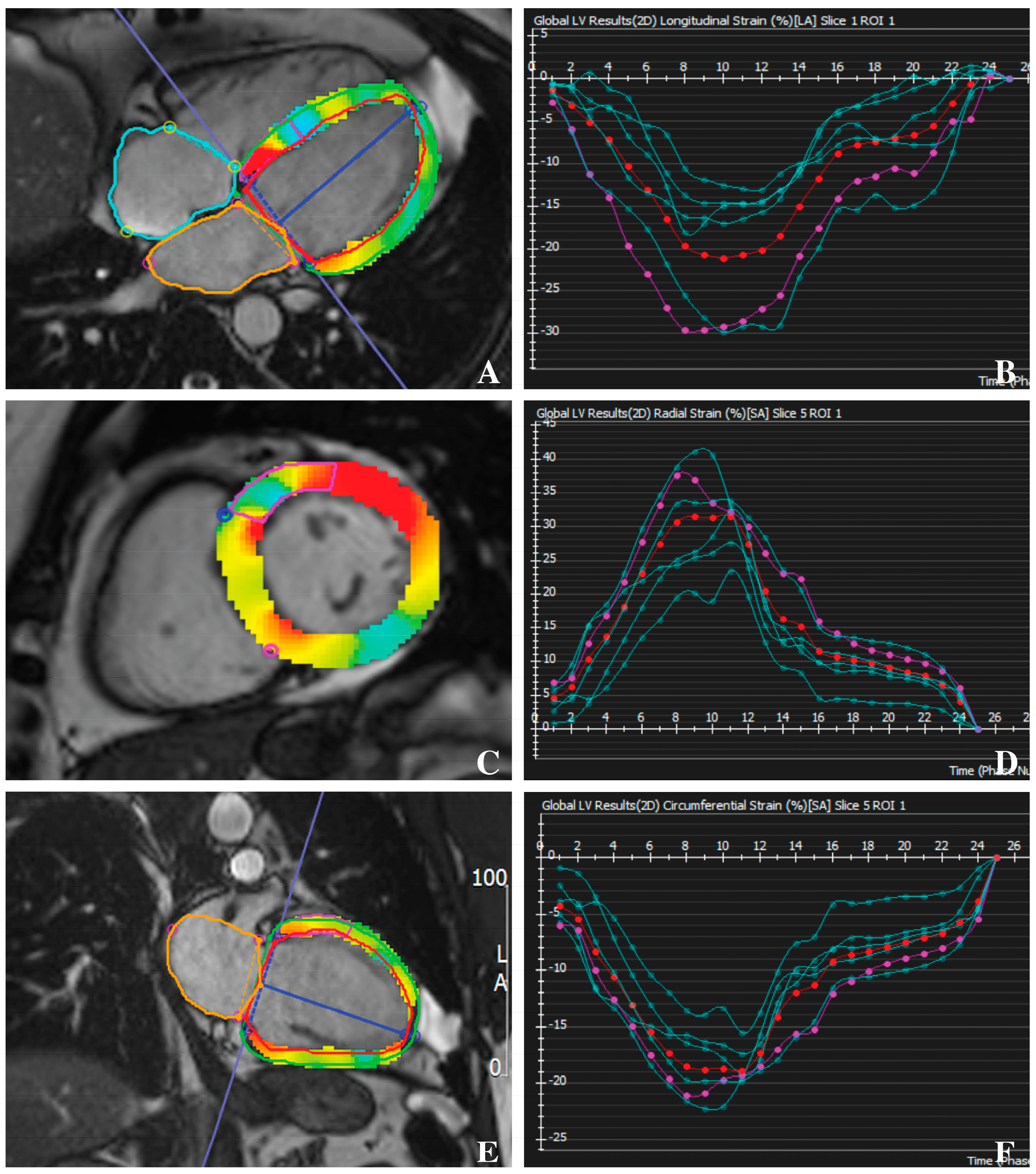
2.3. Feature-Tracking CMR
2.4. Clinical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. CMR Characteristics
3.3. Correlation between LV Strain and LV Functional Parameters
3.4. ROC Analysis of GLS and CMR Parameters for Predicting MACEs
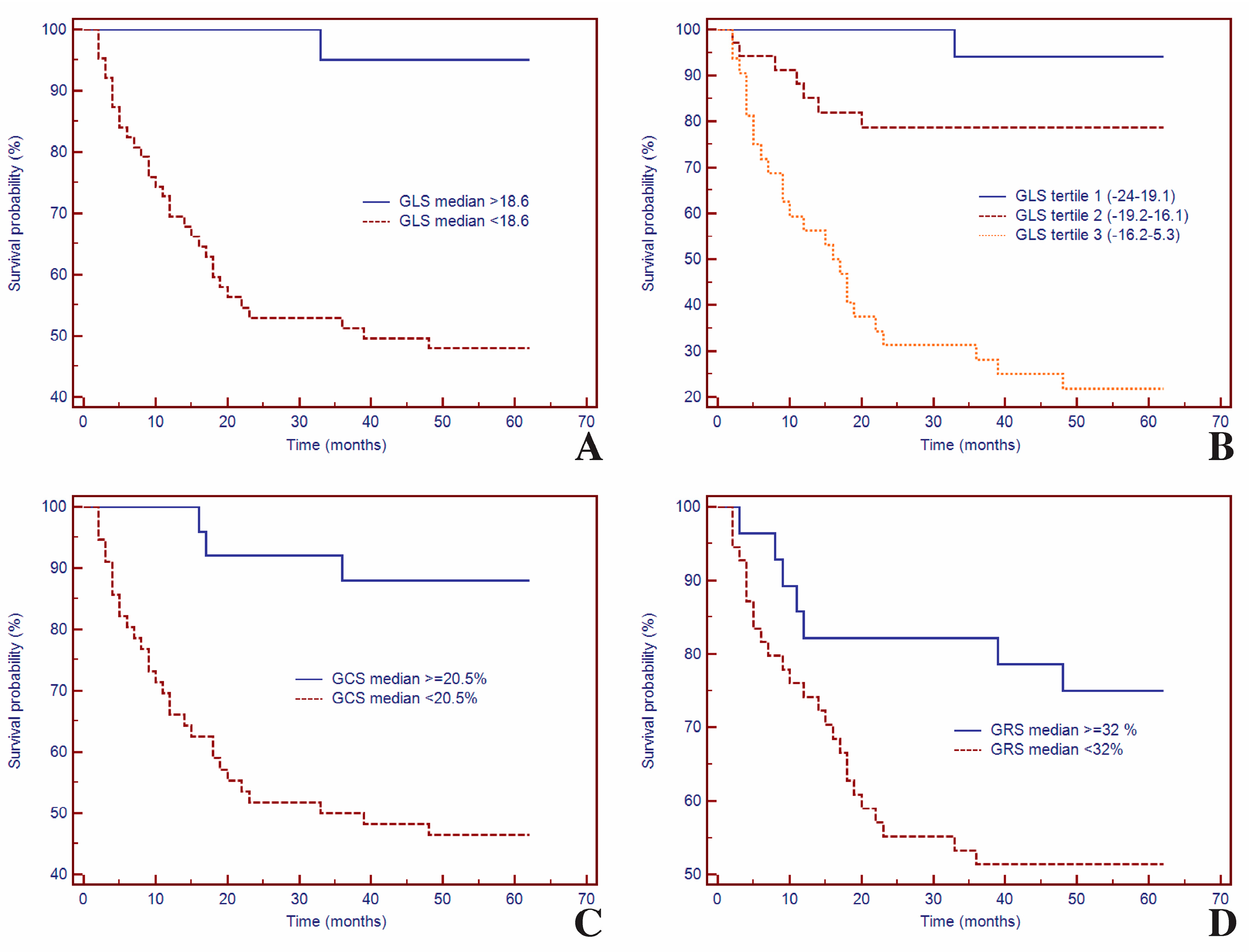
3.5. Survival Analysis of GLS and CMR Parameters for Predicting MACEs
3.6. Univariate and Multivariate Cox Analysis of LV Strain in Predicting MACEs
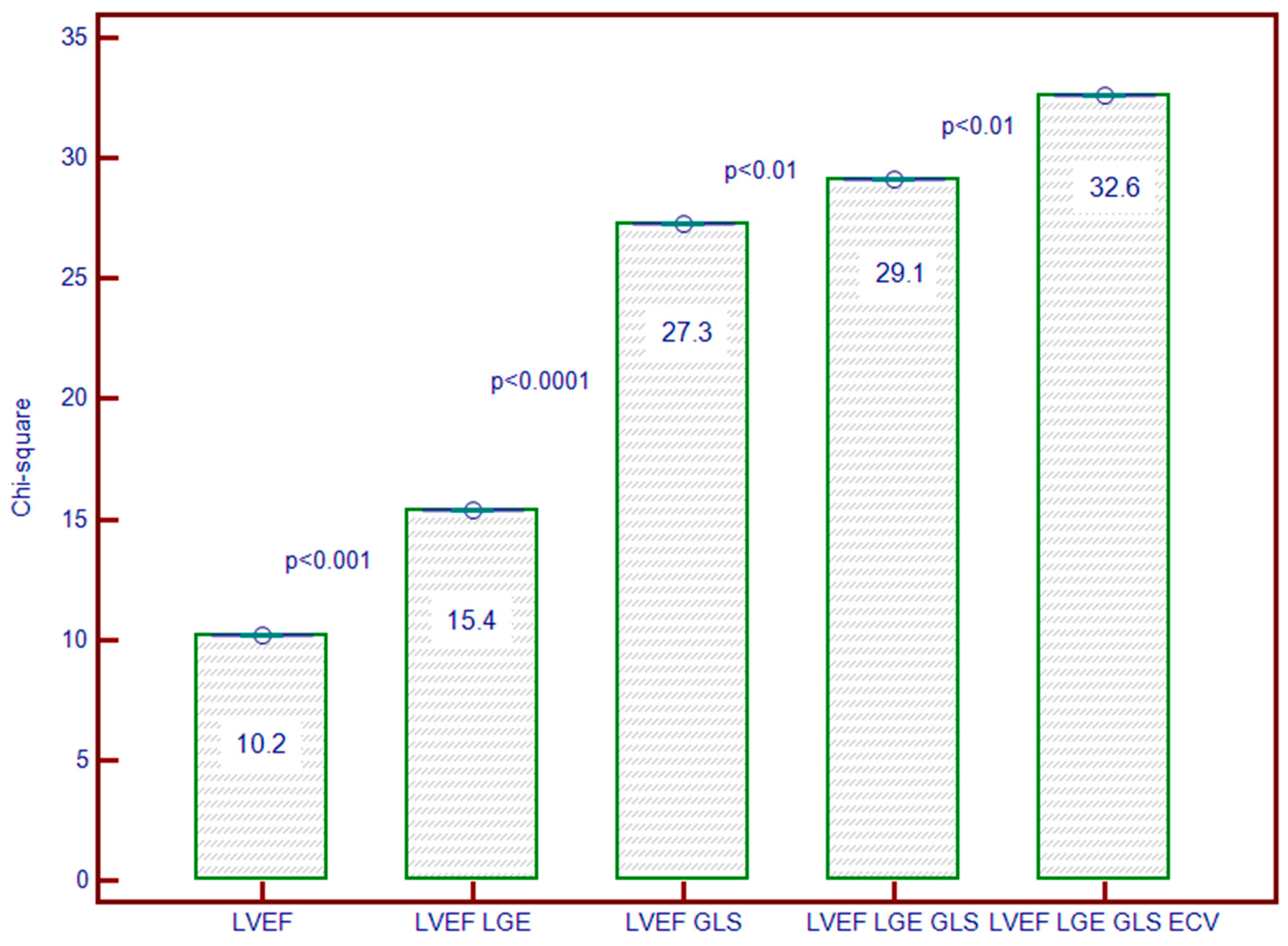
3.7. Incremental Predictive Ability of LV Strain for Predicting MACEs
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; McCann, G.P. Cardiac Magnetic Resonance Imaging for the Assessment of Aortic Stenosis. Heart 2019, 105, 489–497. [Google Scholar] [CrossRef]
- Treibel, T.A.; Kozor, R.; Schofield, R.; Benedetti, G.; Fontana, M.; Bhuva, A.N.; Sheikh, A.; López, B.; González, A.; Manisty, C.; et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 860–871. [Google Scholar] [CrossRef]
- Inui, K.; Tachi, M.; Saito, T.; Kubota, Y.; Murai, K.; Kato, K.; Takano, H.; Amano, Y.; Asai, K.; Shimizu, W. Superiority of the Extracellular Volume Fraction over the Myocardial T1 Value for the Assessment of Myocardial Fibrosis in Patients with Non-Ischemic Cardiomyopathy. Magn. Reson. Imaging 2016, 34, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.T.; Phan, H.T.; Pham, K.N.P.; Duong, H.N.H.; Ngo, T.N.M.; Palmer, C.; Nguyen, T.T.H.; Truong, B.H.; Vo, M.A.; Tretter, J.T.; et al. Normal Ranges of Left Ventricular Strain by Three-Dimensional Speckle-Tracking Echocardiography in Adults: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2019, 32, 1586–1597.e5. [Google Scholar] [CrossRef]
- Ouyang, D.; Carter, R.E.; Pellikka, P.A. Machine Learning in Imaging: What Is JASE Looking For? J. Am. Soc. Echocardiogr. 2024, 37, 273–275. [Google Scholar] [CrossRef]
- Stens, N.A.; van Iersel, O.; Rooijakkers, M.J.P.; van Wely, M.H.; Nijveldt, R.; Bakker, E.A.; Rodwell, L.; Pedersen, A.L.D.; Poulsen, S.H.; Kjønås, D.; et al. Prognostic Value of Preprocedural LV Global Longitudinal Strain for Post-TAVR-Related Morbidity and Mortality. JACC Cardiovasc. Imaging 2023, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial Strain of the Left Ventricle by Speckle Tracking Echocardiography: From Physics to Clinical Practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef]
- Zlibut, A.; Cojocaru, C.; Onciul, S.; Agoston-Coldea, L. Cardiac Magnetic Resonance Imaging in Appraising Myocardial Strain and Biomechanics: A Current Overview. Diagnostics 2023, 13, 553. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of Cardiovascular Magnetic Resonance Feature Tracking and Echocardiographic Speckle Tracking for Informed Clinical Use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef]
- Narang, A.; Addetia, K. An Introduction to Left Ventricular Strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.C.W.; Lee, N.H.C.; Poon, J.W.L.; Chin, C.W.L.; He, B.; Luo, L.; Chen, C.; Wan, E.Y.F.; Pennell, D.J.; Mohiaddin, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Derived Global Longitudinal Strain Analysis in Patients with Ischaemic and Non-Ischaemic Dilated Cardiomyopathy: A Systematic Review and Meta-Analysis. Int. J. Cardiovasc. Imaging 2022, 38, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of Myocardial Deformation with Cardiac Magnetic Resonance Strain Imaging Improves Risk Stratification in Patients with Dilated Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef]
- Agoston-Coldea, L.; Bheecarry, K.; Cionca, C.; Petra, C.; Strimbu, L.; Ober, C.; Lupu, S.; Fodor, D.; Mocan, T. Incremental Predictive Value of Longitudinal Axis Strain and Late Gadolinium Enhancement Using Standard CMR Imaging in Patients with Aortic Stenosis. J. Clin. Med. 2019, 8, 165. [Google Scholar] [CrossRef]
- Cionca, C.; Zlibut, A.; Orzan, R.-I.; Cojan-Minzat, B.O.; Horvat, D.; Muresan, I.D.; Kiss, E.; Gonciar, D.; Dirzu, D.; Seicean, S.; et al. Left Atrial Geometric and Functional Remodeling Parameters Measured by Cardiac Magnetic Resonance Imaging and Outcome Prediction in Patients with Severe Aortic Stenosis. Kardiol. Pol. 2023, 81, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Kim, N.Y.; Im, D.J.; Youn, J.-C.; Hong, Y.J.; Choi, B.W.; Kang, S.-M.; Lee, H.-J. Synthetic Extracellular Volume Fraction Derived Using Virtual Unenhanced Attenuation of Blood on Contrast-Enhanced Cardiac Dual-Energy CT in Nonischemic Cardiomyopathy. Am. J. Roentgenol. 2022, 218, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Cojan-Minzat, B.O.; Zlibut, A.; Muresan, I.D.; Cionca, C.; Horvat, D.; Kiss, E.; Revnic, R.; Florea, M.; Ciortea, R.; Agoston-Coldea, L. Left Ventricular Geometry and Replacement Fibrosis Detected by CMRI Are Associated with Major Adverse Cardiovascular Events in Nonischemic Dilated Cardiomyopathy. J. Clin. Med. 2020, 9, 1997. [Google Scholar] [CrossRef]
- Lim, C.; Blaszczyk, E.; Riazy, L.; Wiesemann, S.; Schüler, J.; von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Quantification of Myocardial Strain Assessed by Cardiovascular Magnetic Resonance Feature Tracking in Healthy Subjects—Influence of Segmentation and Analysis Software. Eur. Radiol. 2021, 31, 3962–3972. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain Imaging Using Cardiac Magnetic Resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Weise Valdés, E.; Barth, P.; Piran, M.; Laser, K.T.; Burchert, W.; Körperich, H. Left-Ventricular Reference Myocardial Strain Assessed by Cardiovascular Magnetic Resonance Feature Tracking and FSENC—Impact of Temporal Resolution and Cardiac Muscle Mass. Front. Cardiovasc. Med. 2021, 8, 764496. [Google Scholar] [CrossRef] [PubMed]
- Ochs, A.; Riffel, J.; Ochs, M.M.; Arenja, N.; Fritz, T.; Galuschky, C.; Schuster, A.; Bruder, O.; Mahrholdt, H.; Giannitsis, E.; et al. Myocardial Mechanics in Dilated Cardiomyopathy: Prognostic Value of Left Ventricular Torsion and Strain. J. Cardiovasc. Magn. Reson. 2021, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Janwetchasil, P.; Yindeengam, A.; Krittayaphong, R. Prognostic Value of Global Longitudinal Strain in Patients with Preserved Left Ventricular Systolic Function: A Cardiac Magnetic Resonance Real-World Stud. J. Cardiovasc. Magn. Reson. 2024, 26, 101057. [Google Scholar] [CrossRef]
- Porcari, A.; Merlo, M.; Baggio, C.; Gagno, G.; Cittar, M.; Barbati, G.; Paldino, A.; Castrichini, M.; Vitrella, G.; Pagnan, L.; et al. Global Longitudinal Strain by CMR Improves Prognostic Stratification in Acute Myocarditis Presenting with Normal LVEF. Eur. J. Clin. Investig. 2022, 52, e13815. [Google Scholar] [CrossRef]
- Reis Santos, R.; Abecasis, J.; Maltês, S.; Lopes, P.; Oliveira, L.; Freitas, P.; Ferreira, A.; Ribeiras, R.; Andrade, M.J.; Sousa Uva, M.; et al. Cardiac Magnetic Resonance Patterns of Left Ventricular Remodeling in Patients with Severe Aortic Stenosis Referred to Surgical Aortic Valve Replacement. Sci. Rep. 2024, 14, 7085. [Google Scholar] [CrossRef]

| Variables | AS All Patients n = 84 | Controls n = 84 | p-Value |
|---|---|---|---|
| Clinical characteristics | |||
| - Age, mean (SD), years | 66 (8.9) | 65 (8.5) | NS |
| - Male gender, n (%) | 46 (54.7) | 48 (57.1) | NS |
| - Body mass index, kg/m2, mean (SD) | 30.1 (4.9) | 28.5 (4.2) | 0.05 |
| - 6MWD, m, mean (SD) | 415 (138) | 597 (102) | <0.001 |
| - Smokers, n (%) | 33 (39.3) | 21 (25.0) | <0.01 |
| - Hypertension, n (%) | 58 (69.0) | 41 (51.2) | 0.05 |
| - Diabetes mellitus, n (%) | 35 (41.7) | 22 (26.2) | 0.05 |
| Medication | |||
| - Beta-blockers, n (%) | 58 (69.0) | 8 (9.5) | <0.001 |
| - ACEIs or ARBs, n (%) | 56 (66.6) | 11 (13.1) | <0.001 |
| - Calcium channel blockers, n (%) | 24 (28.5) | 6 (7.1) | <0.001 |
| - Diuretics, n (%) | 48 (57.1) | 6 (7.1) | <0.001 |
| - Anticoagulant, n (%) | 17 (20.2) | - | NA |
| - Antiarrhythmic, n (%) | 16 (19.0) | - | NA |
| Electrocardiogram | |||
| - Atrial fibrillation, n (%) | 8 (9.5) | - | NA |
| - Left bundle branch block, n (%) | 14 (16.6) | 3 (3.5) | <0.001 |
| - Right bundle branch block, n (%) | 13 (15.5) | 2 (2.8) | <0.001 |
| - Significant Q waves, n (%) | 4 (4.7) | - | NA |
| Echocardiography | |||
| - Peak aortic velocity, m/s, mean (SD) | 4.43 (0.44) | 1.34 (0.29) | <0.001 |
| - Peak transaortic gradient, mmHg, mean (SD) | 81.2 (17.1) | 7.6 (2.23) | <0.001 |
| - Mean transaortic gradient, mmHg, mean (SD) | 52.4 (13.9) | 3.7 (0.71) | <0.001 |
| - AVA index, cm2/m2, mean (SD) | 0.52 (0.08) | 3.2 (0.06) | <0.001 |
| Biomarkers | |||
| - NT-proBNP, pg/mL, median (IQR) | 673 (170–1960) | 210 (60–330) | <0.001 |
| CMR parameters | |||
| - LVEDV index, mL/m2, mean (SD) | 80.6 (20.2) | 62.2 (14.8) | <0.001 |
| - LVESV index, mL/m2, mean (SD) | 34.3 (15.2) | 21.2 (5.9) | <0.001 |
| - LVM index, g/m2, mean (SD) | 95.5 (23.5) | 62.1 (14.7) | <0.001 |
| - LVEF, %, mean (SD) | 59.0 (9.3) | 65.9 (4.7) | <0.001 |
| - GLS, %, mean (SD) | −15.8 (3.9) | −19.7 (1.2) | <0.001 |
| - GCS, %, mean (SD) | −17.9 (3.6) | −21.3 (1.4) | <0.001 |
| - GRS, %, mean (SD) | 28.9 (4.3) | 38.8 (7.1) | <0.001 |
| - T1 native, ms, mean (SD) | 1034 (80.2) | 971 (17.2) | <0.001 |
| - ECV, % mean (SD) | 27.3 (3.7) | 25.2 (2.5) | <0.001 |
| - LV-LGE, n (%) | 40 (47.6) | - | NA |
| - LV-LGE mass, g/m2, mean (SD) | 15.6 (6.2) | - | NA |
| Parameters | Coefficient Kappa 95% | Confidence Interval | Standard Error |
|---|---|---|---|
| Inter-reader | |||
| - LVEF | 0.94 | 0.908 to 0.970 | 0.028 |
| - GLS | 0.96 | 0.917 to 0.981 | 0.021 |
| - GCS | 0.92 | 0.897 to 0.956 | 0.036 |
| - GRS | 0.90 | 0.873 to 0.952 | 0.049 |
| - LGE | 0.89 | 0.801 to 0.934 | 0.074 |
| Intra-reader | |||
| - LVEF | 0.99 | 0.982 to 0.991 | 0.003 |
| - GLS | 0.96 | 0.958 to 0.984 | 0.017 |
| - GCS | 0.93 | 0.913 to 0.967 | 0.029 |
| - GRS | 0.92 | 0.911 to 0.944 | 0.038 |
| - LGE | 0.91 | 0.908 to 9.943 | 0.035 |
|
No Events n = 51 |
Events n = 33 | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
|
Unadjusted HR (95% CI) | p Value |
Adjusted HR (95% CI) | p Value | |||
| Age, years | 66 (10.2) | 67 (6.5) | 1.00 (0.96–1.04) | NS | ||
| Male gender, n, % | 24 (47.0) | 22 (66.7) | 0.51 (0.25–1.06) | NS | ||
| Body-mass index, kg/m2 | 31.0 (4.8) | 28.7 (4.9) | 0.89 (0.82–0.97) | 0.01 | ||
| Systolic blood pressure, mmHg | 130 (10.6) | 130 (15.0) | 1.00 (0.98–1.02) | NS | ||
| Smokers, n % | 19 (37.2) | 14 (42.4) | 0.98 (0.96–1.01) | NS | ||
| Hypertension, n % | 33 (64.7) | 25 (75.7) | 0.97 (0.68–1.03) | NS | ||
| Diabetes mellitus, n % | 20 (39.2) | 15 (45.4) | 1.02 (1.01–1.05) | 0.002 | 0.98 (0.87–1.02) | NS |
| NT-proBNP, pg/mL | 706 (170–935) | 1034 (234–1960) | 1.01 (1.00–1.02) | NS | ||
| 6MWD, m | 464 (134) | 338 (106) | 0.99 (0.99–1.00) | 0.001 | 0.97 (0.95–0.99) | NS |
| LVEDV index, mL/m2 | 75.2 (20.2) | 90.1 (16.3) | 1.02 (1.01–1.04) | 0.003 | ||
| LVESV index, mL/m2 | 29.6 (13.1) | 41.5 (15.4) | 1.04 (1.02–1.06) | 0.002 | ||
| LVM index, g/m2 | 93.3 (16.9) | 101.9 (18.3) | 1.01 (0.99–1.03) | NS | ||
| LVEF, % | 61.5 (8.1) | 55.1 (9.6) | 0.94 (0.90–0.98) | 0.001 | 0.99 (0.94–1.02) | NS |
| GLS, % | −17.6 (3.0) | −13.8 (3.1) | 1.21 (1.12–1.13) | <0.0001 | 1.19 (1.07–1.53) | 0.003 |
| GCS, % | −19.5 (3.2) | −15.6 (2.9) | 1.20 (1.11–1.30 | <0.0001 | ||
| GRS, % | 30.2 (3.1) | 27.6 (5.2) | 0.89 (0.82–0.96) | 0.005 | ||
| T1 native, ms | 1022 (87) | 1103 (70) | 1.03 (0.99–1.08) | <0.001 | 0.97 (0.96–1.01) | NS |
| ECV, % | 27.2 (3.9) | 27.4 (3.4) | 1.02 (0.98–1.11) | <0.01 | ||
| LGE mass index, g/m2 | 13.8 (2.3) | 17.3 (5.6) | 1.13 (1.01–1.42) | <0.001 | 0.97 (0.88–1.55) | 0.049 |
| Peak aortic velocity, m/s | 4.34 (0.34) | 4.55 (0.54) | 2.10 (1.12–3.9) | 0.033 | ||
| Peak aortic gradient, mmHg | 78.8 (13.5) | 84.8 (21.1) | 1.04 (0.98–1.11) | NS | ||
| Mean aortic gradient, mmHg | 51.9 (12.9) | 53.2 (15.6) | 1.00 (0.98–1.02) | NS | ||
| AVA index, cm2/m2 | 0.51 (0.08) | 0.51 (0.08) | 1.48 (0.29–7.37) | NS | ||
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| HR 95% | HR 95% | HR 95% | HR 95% | HR 95% | HR 95% | |
| Age | 1.01 (0.96–1.39) | 0.95 (0.91–0.99) | 1.01 (0.97–1.05) | 1.00 (0.96–1.04) | 0.99 (0.95–1.03) | 1.01 (0.97–1.05) |
| 6MWD | 0.99 (0.98–0.99) * | |||||
| LVEF | 0.94 (0.90–0.96) | |||||
| ECV | 1.02 (0.93–1.12) | |||||
| LGE | 1.02 (1.01–1.36) ** | |||||
| GLS | 1.22 (1.12–1.32) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cionca, C.; Zlibut, A.; Agoston, R.; Agoston-Coldea, L.; Orzan, R.I.; Mocan, T. Evaluating the Clinical Utility of Left Ventricular Strains in Severe AS: A Pilot Study with Feature-Tracking Cardiac Magnetic Resonance. Biomedicines 2024, 12, 2104. https://doi.org/10.3390/biomedicines12092104
Cionca C, Zlibut A, Agoston R, Agoston-Coldea L, Orzan RI, Mocan T. Evaluating the Clinical Utility of Left Ventricular Strains in Severe AS: A Pilot Study with Feature-Tracking Cardiac Magnetic Resonance. Biomedicines. 2024; 12(9):2104. https://doi.org/10.3390/biomedicines12092104
Chicago/Turabian StyleCionca, Carmen, Alexandru Zlibut, Renata Agoston, Lucia Agoston-Coldea, Rares Ilie Orzan, and Teodora Mocan. 2024. "Evaluating the Clinical Utility of Left Ventricular Strains in Severe AS: A Pilot Study with Feature-Tracking Cardiac Magnetic Resonance" Biomedicines 12, no. 9: 2104. https://doi.org/10.3390/biomedicines12092104
APA StyleCionca, C., Zlibut, A., Agoston, R., Agoston-Coldea, L., Orzan, R. I., & Mocan, T. (2024). Evaluating the Clinical Utility of Left Ventricular Strains in Severe AS: A Pilot Study with Feature-Tracking Cardiac Magnetic Resonance. Biomedicines, 12(9), 2104. https://doi.org/10.3390/biomedicines12092104






