Abstract
Background/Objectives: Anaplastic oligodendrogliomas (AOs) are central nervous system (CNS) World Health Organization (WHO) grade 3 gliomas characterized by isocitrate dehydrogenase (IDH) mutation (m)IDH and 1p/19q codeletion. AOs are typically treated with surgery and chemoradiation. However, chemoradiation can cause detrimental late neurocognitive morbidities and an accelerated disease course. The recently regulatory-approved vorasidenib, a brain-penetrating oral inhibitor of IDH1/2, has altered the treatment paradigm for recurrent/residual non-enhancing surgically resected CNS WHO grade 2 mIDH gliomas. Though vorasidenib can delay the time to chemoradiation for grade 2 gliomas, the implications for vorasidenib in non-grade 2 mIDH gliomas are not well understood. Results: We present a case of a 71-year-old male with a grade 3 non-enhancing oligodendroglioma successfully treated with vorasidenib with an 11% reduction in residual tumor volume. Vorasidenib was well tolerated in our patient with a mild elevation in his liver transaminases that resolved following a brief interruption in treatment. Conclusions: Our case suggests that vorasidenib may impart therapeutic benefits in this setting. This case illustrates the need for further investigation into these less commonly addressed scenarios and treatment strategies that extend beyond current guidelines.
1. Introduction
Anaplastic oligodendrogliomas (AOs) are central nervous system (CNS) World Health Organization (WHO) grade 3 gliomas characterized by isocitrate dehydrogenase mutations (mIDHs) and 1p/19q codeletion [1,2]. The diagnostic criteria for oligodendrogliomas have undergone multiple revisions with the integration of molecular criteria to improve upon diagnostic accuracy. However, the grading criteria in the current classification schema for oligodendrogliomas continues to predominantly rely upon histopathologic examination; the differentiating criterion between grade 2 and grade 3 oligodendrogliomas are the presence of conspicuous microvascular proliferation and/or 6 mitoses per 10 high-power field [3,4,5,6,7,8,9,10,11]. These criteria are subject to inter-rater variability, and accordingly, the reliability of these benchmarks as a predictor of patient outcome have been called into question [7,12,13,14]. To ameliorate this variability, molecular alterations, including homozygous loss of cyclin-dependent kinase inhibitor (CDKN)2A and mutations in phosphatase and tensin homolog (PTEN) and NOTCH1 have been investigated as adjunctive markers for risk stratification. Nevertheless, their prognostic role and clinical relevance are yet to be determined [15,16,17].
The distinction between grade 2 and grade 3 oligodendrogliomas carries therapeutic relevance. While maximal safe resection remains the initial treatment for oligodendrogliomas, the implications for adjuvant chemotherapy and radiation are reliant upon risk stratification [18,19,20,21,22]. To date, consensus has not been reached on the risk stratification criteria for oligodendrogliomas (Table 1). Various risk factors have been identified across multiple trials, including the European Organization for Research and Treatment of Cancer (EORTC) 228445, 228446, and Radiation Therapy Oncology Group (RTOG) 0424 trials. RTOG 9802 and other studies further emphasize the impact of tumor extent of resection (EOR) as a prognostic factor [23,24,25,26,27,28]. Consequently, there is a growing focus on residual tumor volume (RTV) as a principal prognostic factor [10,27,29,30].

Table 1.
Variable criteria for risk stratification for oligodendrogliomas.
2. Detailed Case Description
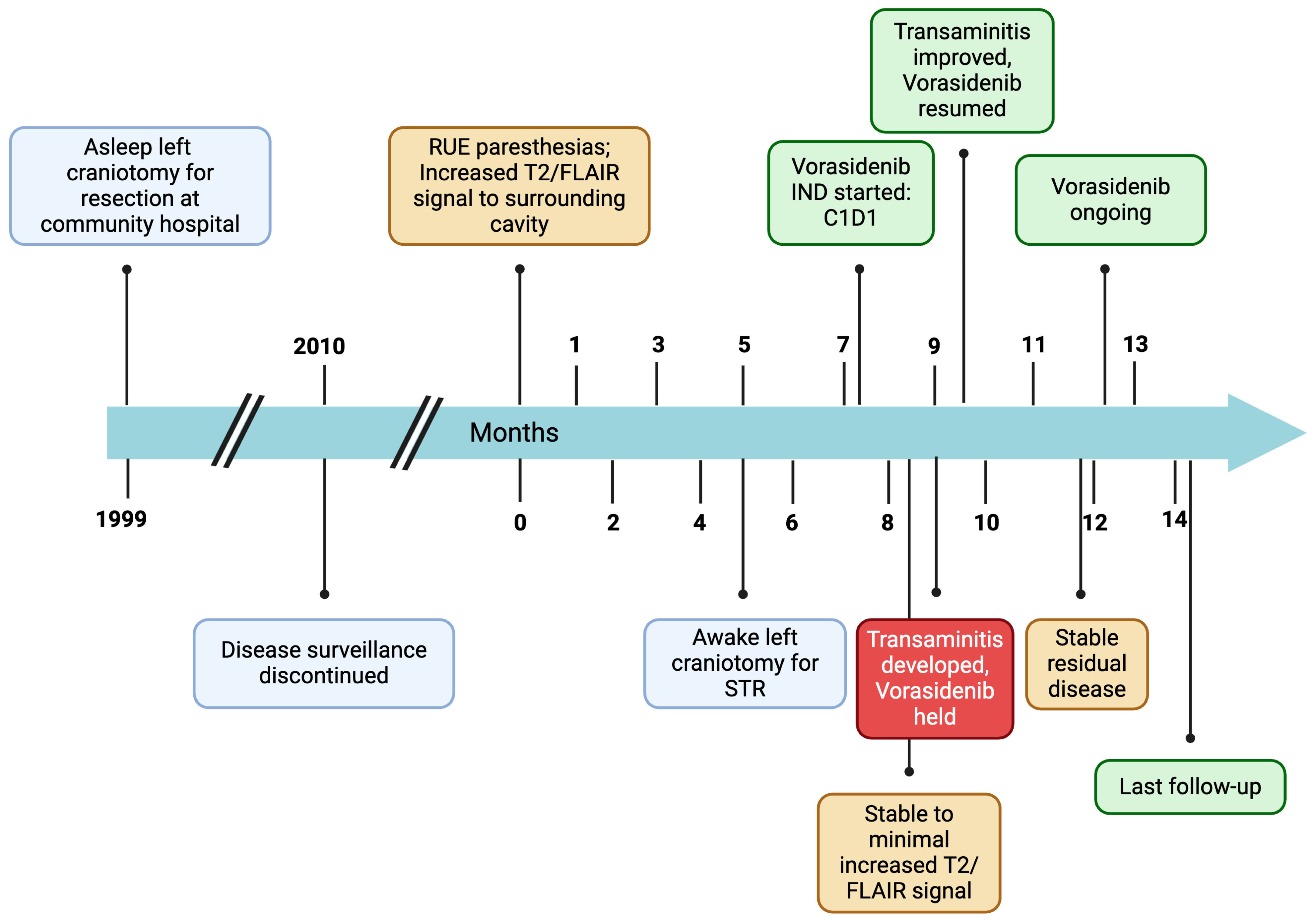
Twenty-five years prior to presentation, a 71-year-old ambidextrous male presented to an outside hospital with syncope (Figure 1). He was incidentally found to have a left parietal non-enhancing mass. He underwent resection at a community hospital with a diagnosis of grade 2 oligodendroglioma. The EOR is unknown. He was monitored for 11 years with no evidence of recurrence (Figure 2A,B), and surveillance was discontinued.
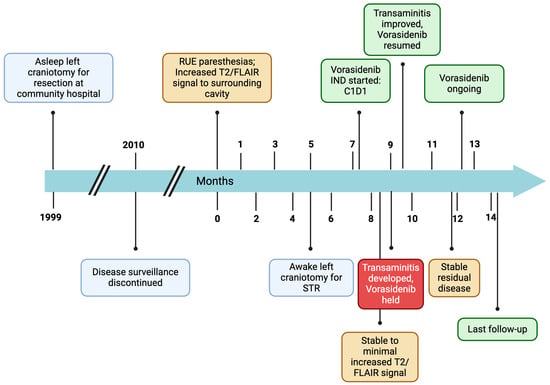
Figure 1.
Patient disease course timeline. RUE = right upper extremity; STR = subtotal resection; IND = investigational new drug; FLAIR = fluid attenuated inversion recovery.
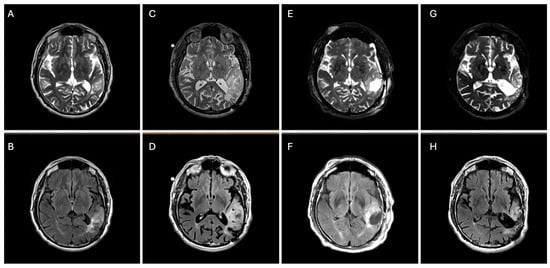
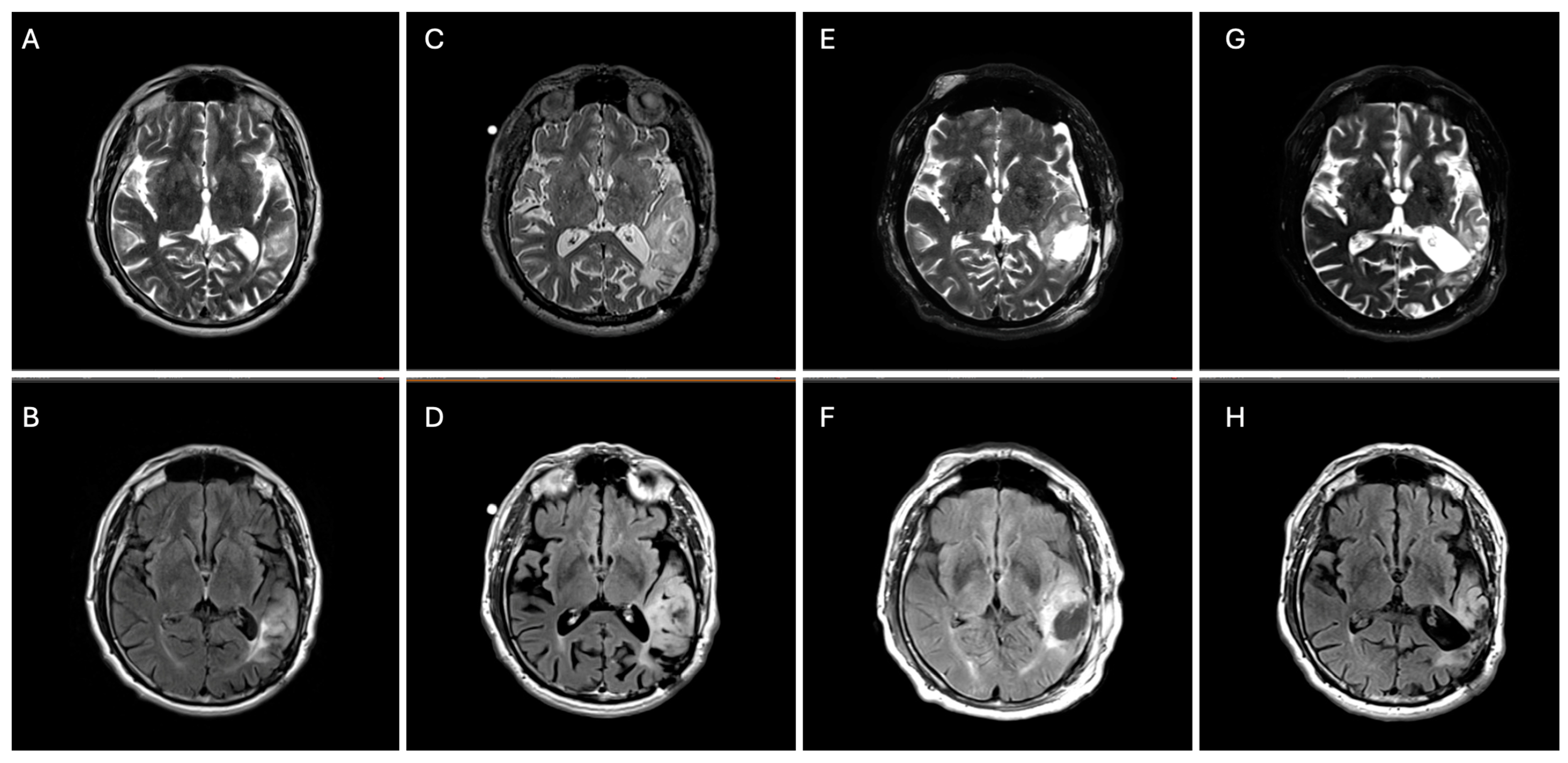
Figure 2.
Axial T2 (top panels) and FLAIR (bottom panels) brain MRIs demonstrating an interval increase in left parietal–temporal T2/FLAIR-hyperintensity from 2017 (A,B) to 2024 (C,D) with subsequent surgical debulking (residual tumor volume 45 cm3) (E,F) followed by 8 months of vorasidenib (residual tumor volume 40 cm3) (G,H).
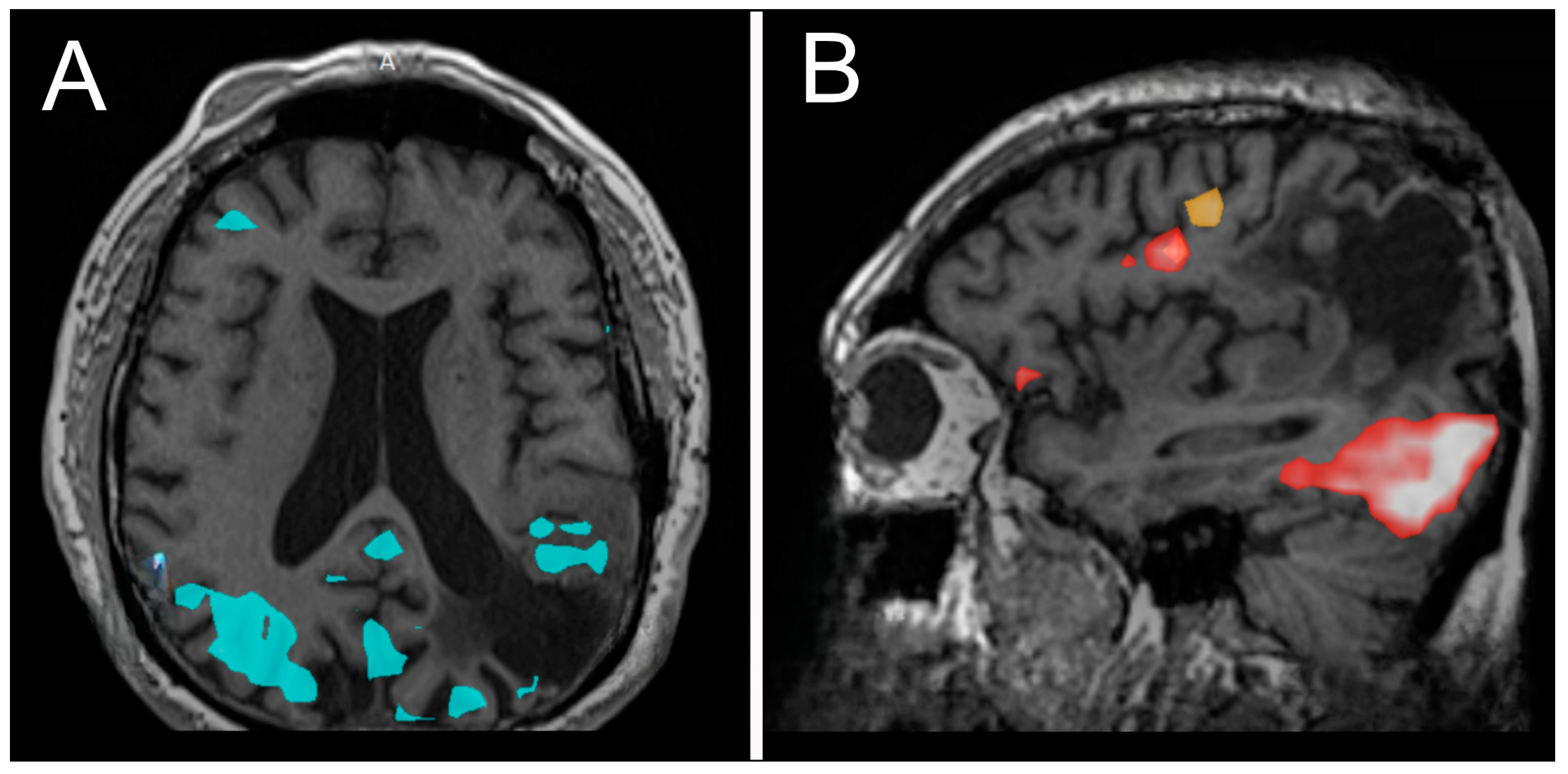
At year 24, he presented to our institution for evaluation of right arm numbness. His neurological exam showed decreased upper-right extremity sensation to light touch. Magnetic resonance imaging (MRI) of the brain showed an interval increase in T2/FLAIR hyperintensity surrounding the resection cavity with no correlative enhancement (Figure 2C–F). A functional MRI showed left-sided language lateralization at Broca’s area (Figure 3). During lip movement, there was overlap in activation of the left primary motor cortex with T2/FLAIR signal hyperintensity, but foot and hand movements were distant from the lesion. His clinical and radiographic progression warranted re-resection and tissue diagnosis to guide adjuvant therapy. A subtotal resection was achieved with approximately 2 cm of residual disease and an RTV of 45 cm3 remaining in eloquent structures (arcuate fasciculus, superior longitudinal fasciculus).
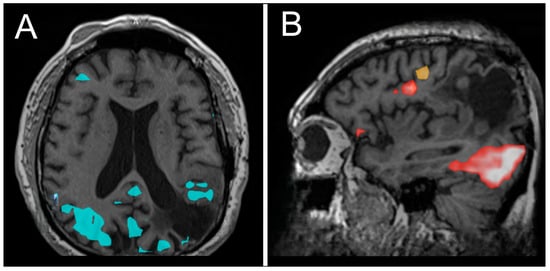
Figure 3.
Functional MRI (fMRI) demonstrating areas of activation during lip movement (A), word production, and verb comprehension (B).
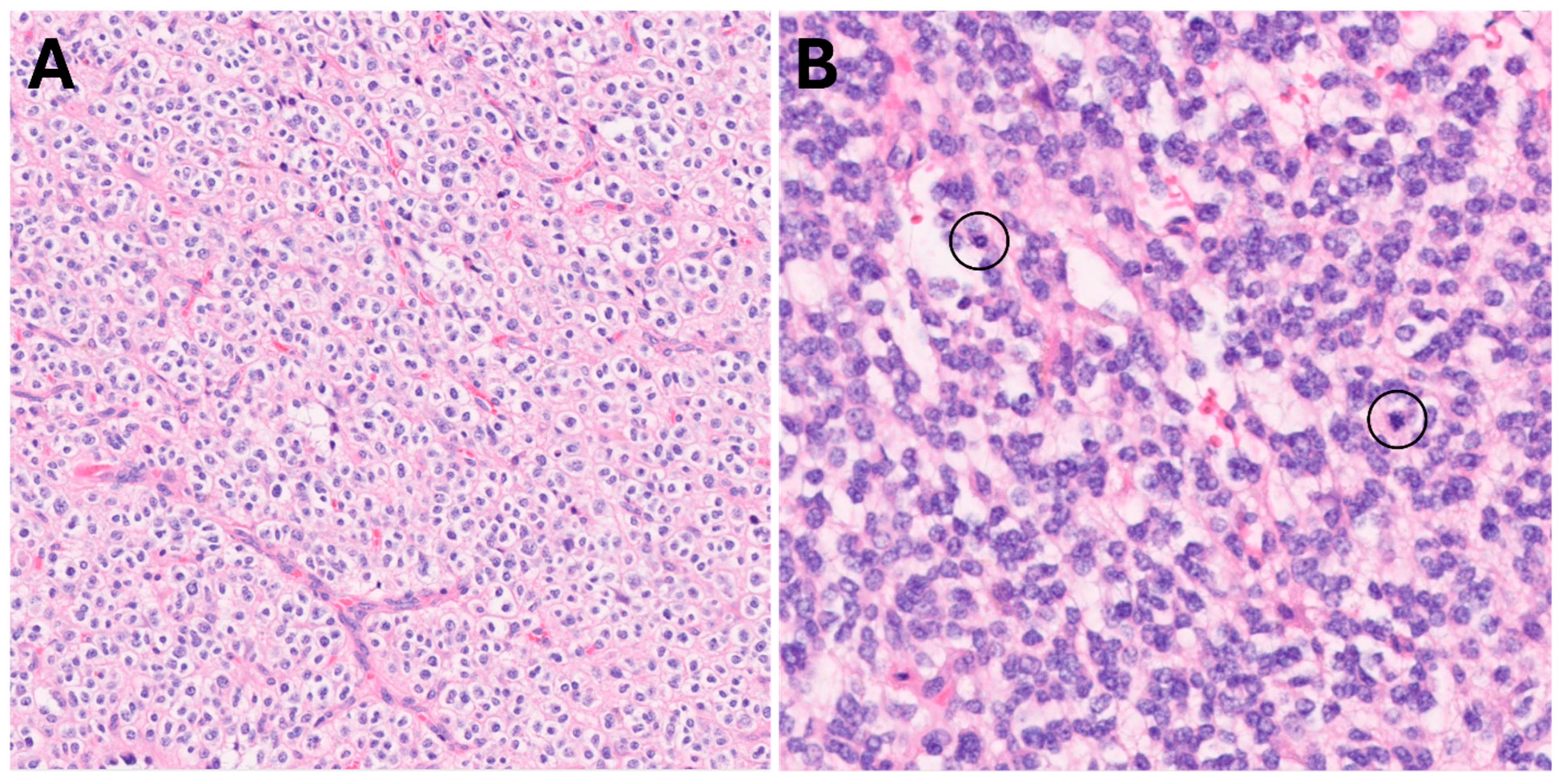
Pathology (reviewed independently by two board-certified, fellowship-trained neuropathologists) showed a widely infiltrative glioma with classic oligodendroglial morphology, including round to oval nuclei, perinuclear halos, and thin intervening capillaries (Figure 4A). High-grade features were also present (Figure 4B), characterized by markedly cellular areas with brisk mitotic activity (up to 10 mitoses in 10 high power fields). No necrosis or microvascular proliferation was seen. Immunohistochemistry demonstrated positivity for IDH1 R132H mutant protein and ATRX expression was retained in tumor nuclei. 1p/19q co-deletion was detected by fluorescence in situ hybridization (FISH) and Tempus xT 648 gene panel reported genomic variants in IDH1, TERT, CIC, and FUBP1, as expected for an oligodendroglioma. These findings yielded a final diagnosis of oligodendroglioma, IDH-mutant and 1p/19q-codeleted, CNS WHO grade 3.
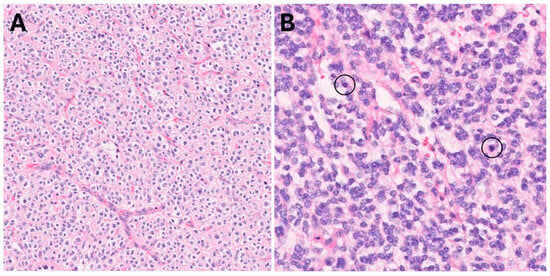
Figure 4.
Classic oligodendroglial morphology, 200× (A). High-grade areas (B) showed brisk mitotic activity (circles) and hypercellularity, 400×.
The patient’s postoperative course was uncomplicated. His mild right upper extremity weakness, paresthesia, and incoordination improved. Given his grade 3 tumor designation, chemoradiation versus vorasidenib was discussed. A consensus was reached at our multidisciplinary Neuro-Oncology Tumor Board to recommend vorasidenib. The patient decided to proceed with 40 mg of vorasidenib. Vorasidenib was well-tolerated, with the exception of asymptomatic transaminitis, which subsequently resolved spontaneously after a two-week treatment interruption. At 8-month follow-up, he remains clinically stable with radiographic findings of an 11% reduction in RTV to 40 cm3 (Figure 2G,H).
3. Discussion
The management of oligodendrogliomas following surgery is influenced by several key factors, including age, tumor grade, and EOR. Together, these components stratify the risk of recurrence (Table 1). The median age of diagnosis for oligodendrogliomas is 49.5 years of age [17], which corroborates with our patient’s age of initial diagnosis at 47 years. The role of age as a prognostic indicator for oligodendrogliomas remains a matter of debate with a historical threshold of ≥ 40 years as the benchmark for high-risk classification in grade 2 gliomas [10]. Recent studies suggest that an older age cutoff (45, >50, or ≥60) is a more reliable indicator for identifying high-risk LGG patients [8,16,31]. Based on RTOG 9802 and EORTC 22033–26033 criteria, our patient is categorized as high-risk on the basis of his age at diagnosis. On the contrary, he is stratified to a low-risk classification under the EORTC 22844/5 and RTOG 0424 criteria (oligodendroglioma lineage, and absence of pre-operative neurological deficit, unknown preoperative tumor diameter) [10,24,28,32,33,34,35].
Our patient defied survival expectations with a disease trajectory that continues over 24 years from diagnosis, and he remains alive at time of publication. Under the “wait-and-see” strategy, the anticipated median OS is 13.2–14.2 years with a median PFS of 3.4 years for grade 2 LGGs [10,33]. His unexpected longevity may stem from the persistent lack of enhancement of his tumor at recurrence, which may indicate a less aggressive glioma compared to an enhancing glioma [36]. Despite historical controversy regarding surgical management of LGGs, early maximal safe resection is now favored over the watchful waiting approach [27,37,38,39,40]. In our patient, there was minimal residual tumor observed on his 10-year MRI, suggesting an initial near-total or gross-total resection. Early and maximal surgery offers three related benefits of reducing malignant transformation rate, reducing recurrence rate, and improving OS [23,29,30,39,40,41]. Results from a 20-year investigation conducted by Hervey-Jumper et al. suggest that ≥75% EOR positively influences survival in these tumors [42]. Our patient’s tumor also exhibited an insidious growth pattern. Based on his available imaging, his estimated growth rate was 0.27 cm3 per month, or a volume of diametric expansion (VDE) of 0.87 mm per year. VDE, the rate of change in tumor diameter over time, is an independent predictor of outcome in LGGs [43,44,45]. This insidious growth rate may have contributed to the protracted quiescent period prior to his tumor’s malignant transformation. Gozé et al. showed that LGGs with a VDE of < 8 mm/year are less likely to undergo malignant transformation compared to LGGs with accelerated growth rates (36.1% vs. 73.9%, respectively) and exhibit prolonged time to malignant transformation (71.2 vs. 47.1 months, p < 0.001) [43]. Lastly, our patient had a low RTV following his initial surgery. The benefit of a smaller RTV is substantiated in several studies and associated with a decreased risk of recurrence and improved OS [29,30]. RTV > 10–15 cm3 is associated with earlier progression and decreased survival [29,30]. Following his second resection, our patient’s RTV was 45 cm3 with a 2 cm diameter owing to tumor in eloquent language cortex.
At the time of initial diagnosis, our patient’s oligodendroglioma diagnosis was established based on the WHO 1993 grading criteria, which were exclusively based on histomorphology. Though molecular analyses were not obtained for our patient’s initial tumor, molecular analyses obtained from his re-resection confirmed that his tumor harbors the canonical IDH1-R132H and codeletion of 1p/19q. Further, our patient’s tumor underwent malignant degeneration to grade 3 with findings of brisk mitotic activity, though microvascular proliferation and necrosis were absent and thus fulfilling only one criterion for a grade 3 designation [14]. Our patient’s tumor harbored mutations in TERT, FUBP1, and CIC, which are common alterations in oligodendroglioma, but not clinically relevant [46,47]. A rare finding in oligodendrogliomas, CDKN2A/B is the sole molecular marker to distinguish grade 2 from grade 3 oligodendrogliomas [17] but was not present in our patient’s tumor. Accordingly, our patient’s tumor met the criteria for a grade 3 designation based on a solitary histologic feature alone.
Our patient would have been ineligible for the INDIGO trial and is ineligible for the regulatory-approved drug based on his tumor’s grade 3 designation. At the time of recurrence, our patient was elderly, his tumor malignantly transformed, and he had residual tumor. Taken together, adjuvant therapy was warranted. In view of the well-documented grade 3 and 4 hematologic toxicities associated with chemoradiation, we reasoned that vorasidenib was the optimal therapy for our patient due to its low toxicity profile, his non-enhancing residual disease, and a grade 3 determination based on brisk mitotic activity alone in the absence of microvascular proliferation, necrosis, and intact CDKN2A/B. His tumor is conceivably an intermediate-risk oligodendroglioma, a proposed new category of grade 3 oligodendrogliomas [48]. On these bases, vorasidenib was favored over sequential chemoradiation with the goal of halting disease progression and prolonging the time to his next intervention. Arguably, the majority of cases in the INDIGO trial were high-risk gliomas warranting chemoradiation [48]. However, despite this high-risk assignment, PFS was improved in the vorasidenib group [49].
At last follow-up, our patient was clinically stable with radiographic improvement of an 11% reduction in RTV (40 cm3). Although a defined RTV cutoff for benefit with maintenance vorasidenib has yet to be determined, our case suggests that vorasidenib has a beneficial effect despite a substantial RTV. He continues to tolerate treatment well. His transient grade 1 liver transaminase elevation resolved with a brief interruption in treatment, which can be observed in 9.6% of patients who develop ≥ grade 3 elevated alanine aminotransferase [49]. Limited evidence suggests that IDHi responders lack NOTCH1 mutations [50]. Though our patient’s tumor lacks NOTCH1 mutations, it is unclear if this is linked to his response to vorasidenib. Vorasidenib is FDA indicated for use in grade 2 oligodendroglioma but may have greater potential, including in non-enhancing grade 3 oligodendrogliomas as substantiated by our case.
We acknowledge that the limitations of this study are the nature of a case report as a single case is not generalizable to all grade 3 oligodendrogliomas. Though a 11% reduction in RTV was demonstrated in our case, we could not evaluate for long-term response given the short follow-up. Subsequent analyses from the INDIGO trial demonstrated decreased tumor growth rate as measured by percent change in volume in 6-month intervals [49]. Given our patient’s prolonged disease course, and initial diagnosis during the pre-molecular era, molecular analysis was not performed for the patient’s initial tumor tissue. Moreover, DNA methylation profiling was not performed on his tumor tissue, which may have been informative to identify the favorable glioma-CpG island (G-CIMP)-high methylated phenotype from a less favorable G-CIMP-low phenotype [51,52,53]. Lastly, we could not confirm the initial RTV nor determine his initial preoperative VDE given that he was under active surveillance at an outside institution with irregular monitoring.
Future investigations should be directed at recognition of additional cohorts of LGG patients who may benefit from vorasidenib and identifying the utility of IDHis in non-grade 2 and enhancing gliomas. Accordingly, investigations with safusidenib (DS-1001) and olutasidenib, both of which are oral brain-penetrant IDH inhibitors with activity in enhancing gliomas, are underway. Safusidenib is a selective IDH R132 inhibitor that has explicitly demonstrated benefit in an enhancing grade 3 oligodendroglioma [54]. This is a notable finding, given that there are differences in outcome with vorasidenib in canonical mIDH1 R132H gliomas compared to non-canonical mIDH gliomas [55]. In patients treated with vorasidenib, it is unknown if IDH inhibition and alteration of this pathway will modify the tumor’s biology and if this inhibition will confer resistance to future chemoradiation [48]. Conversely, another open area of investigation is the role of maintenance vorasidenib in patients previously treated with chemoradiation. Forthcoming studies should also be directed at identifying epigenetic changes within additional affected signaling pathways, such as RB and PI3K [56]. These studies may detect actionable targets for use in combinatorial therapy with vorasidenib [56,57]. As our understanding of IDH-mutant gliomas tumorigenesis evolve, therapies will also continue to advance.
4. Conclusions
The grading criteria for oligodendrogliomas is predominantly based on subjective histopathologic features. Oligodendrogliomas may be classified as grade 3 based on brisk mitotic activity alone. We present the successful use of vorasidenib in a novel clinical context of an individual with a recurrent malignantly transformed chemoradiation-naïve oligodendroglioma with an acceptable toxicity profile. Vorasidenib may have indications that extend beyond current guidelines.
Author Contributions
C.A.Y.—study concept, data collection, analysis, interpretation, manuscript drafting, revision, and final approval. A.S.H.—data collection, analysis, interpretation, manuscript drafting, revision, and final approval. J.W.C.—data analysis, interpretation, and manuscript final approval. E.C.—data collection, analysis, interpretation, and manuscript final approval. M.A.P.-R.—data collection, analysis, interpretation, and manuscript final approval. M.Z.—data collection and final approval. S.M.—data collection and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the University of California, Irvine, Institutional Review Board.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.
Acknowledgments
We would like to thank Servier for their support in production of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 2-HG | 2-hydroxyglutarate |
| AO | anaplastic oligodendroglioma |
| CDKN | cyclin-dependent kinase inhibitor |
| CNS | central nervous system |
| EOR | extent of resection |
| EORTC | European Organization for Research and Treatment of Cancer |
| FLAIR | fluid attenuated inversion recovery |
| fMRI | functional MRI |
| G-CIMP | glioma-CpG island |
| IDH | isocitrate dehydrogenase |
| IDHis | isocitrate dehydrogenase inhibitors |
| IND | investigational new drug |
| LGG | low-grade glioma |
| mIDH | isocitrate dehydrogenase mutations |
| MRI | magnetic resonance imaging |
| OS | overall survival |
| PCV | procarbazine, CCNU, and vincristine |
| PFS | progression-free survival |
| PTEN | phosphatase and tensin homolog |
| RTOG | Radiation Therapy Oncology Group |
| RTV | residual tumor volume |
| RUE | right upper extremity |
| STR | subtotal resection |
| TMZ | temozolomide |
| VDE | volume of diametric expansion |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; A Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef]
- Jaeckle, K.A.; Ballman, K.V.; Van Den Bent, M.; Giannini, C.; Galanis, E.; Brown, P.D.; Jenkins, R.B.; Cairncross, J.G.; Wick, W.; Weller, M.; et al. CODEL: Phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro-Oncol. 2021, 23, 457–467. [Google Scholar] [CrossRef]
- Aboud, O.; Shah, R.; Vera, E.; Burton, E.; Theeler, B.; Wu, J.; Boris, L.; Quezado, M.; Reyes, J.; Wall, K.; et al. Challenges of imaging interpretation to predict oligodendroglioma grade: A report from the Neuro-Oncology Branch. CNS Oncol. 2022, 11, CNS83. [Google Scholar] [CrossRef]
- Malta, T.M.; Sabedot, T.S.; Morosini, N.S.; Datta, I.; Garofano, L.; Vallentgoed, W.; Varn, F.S.; Aldape, K.; D’Angelo, F.; Bakas, S.; et al. The Epigenetic Evolution of Glioma Is Determined by the IDH1 Mutation Status and Treatment Regimen. Cancer Res. 2024, 84, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Cimino, P.J.; Zager, M.; McFerrin, L.; Wirsching, H.G.; Bolouri, H.; Hentschel, B.; von Deimling, A.; Jones, D.; Reifenberger, G.; Holland, E.C.; et al. Multidimensional scaling of diffuse gliomas: Application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol. Commun. 2017, 5, 39. [Google Scholar] [CrossRef]
- Dubbink, H.J.; Atmodimedjo, P.N.; Kros, J.M.; French, P.J.; Sanson, M.; Idbaih, A.; Wesseling, P.; Enting, R.; Spliet, W.; Tijssen, C.; et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: A report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro-Oncol. 2016, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Alfaro-Munoz, K.D.; Heathcock, L.E.; van Thuijl, H.F.; Gilbert, M.R.; Armstrong, T.S.; Sulman, E.P.; Cahill, D.P.; Vera-Bolanos, E.; et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015, 129, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Carstam, L.; Latini, F.; Solheim, O.; Bartek, J.; Pedersen, L.K.; Zetterling, M.; Beniaminov, S.; Sjåvik, K.; Ryttlefors, M.; Jensdottir, M.; et al. Long-term follow up of patients with WHO grade 2 oligodendroglioma. J. Neuro-Oncol. 2023, 164, 65–74. [Google Scholar] [CrossRef]
- van den Bent, M.J. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: A clinician’s perspective. Acta Neuropathol. 2010, 120, 297–304. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; French, P.J.; Brat, D.; Tonn, J.C.; Touat, M.; Ellingson, B.M.; Young, R.J.; Pallud, J.; von Deimling, A.; Sahm, F.; et al. The biological significance of tumor grade, age, enhancement and extent of resection in IDH mutant gliomas: How should they inform treatment decision in the era of IDH inhibitors? Neuro-Oncol. 2024, 26, 1805–1822. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Lassman, A.B. Indications for Treatment: Is Observation or Chemotherapy Alone a Reasonable Approach in the Management of Low-Grade Gliomas? Semin. Radiat. Oncol. 2015, 25, 203–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figarella-Branger, D.; Mokhtari, K.; Dehais, C.; Jouvet, A.; Uro-Coste, E.; Colin, C.; Carpentier, C.; Forest, F.; Maurage, C.-A.; Vignaud, J.-M.; et al. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro-Oncol. 2014, 16, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. WHO Classification of Tumours of the Central Nervous System, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Giannini, C.; Scheithauer, B.W.; Weaver, A.L.; Burger, P.C.; Kros, J.M.; Mork, S.; Graeber, M.B.; Bauserman, S.; Buckner, J.C.; Altermatt, H.; et al. Oligodendrogliomas: Reproducibility and prognostic value of histologic diagnosis and grading. J. Neuropathol. Exp. Neurol. 2001, 60, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Fleischeuer, R.; Dirven, C.M.F.; Vincent, A.J.P.E.; Van Den Bent, M.J. Prognostic relevance of mutations and copy number alterations assessed with targeted next generation sequencing in IDH mutant grade II glioma. J. Neuro-Oncol. 2018, 139, 349–357. [Google Scholar] [CrossRef]
- Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F.; Shiina, S.; Yamamoto, T.; et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro-Oncol. 2018, 20, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Maurage, C.A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Ducray, F.; Idbaih, A.; Figarella-Branger, D.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Lassman, A.B.; Hoang-Xuan, K.; Polley, M.-Y.C.; Brandes, A.A.; Cairncross, J.G.; Kros, J.M.; Ashby, L.S.; Taphoorn, M.J.; Souhami, L.; Dinjens, W.N.; et al. Joint Final Report of EORTC 26951 and RTOG 9402: Phase III Trials With Procarbazine, Lomustine, and Vincristine Chemotherapy for Anaplastic Oligodendroglial Tumors. J. Clin. Oncol. 2022, 40, 2539–2545. [Google Scholar] [CrossRef]
- Van Den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.J.A.; Frenay, M.; Tijssen, C.C.; Hoang-Xuan, K.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef]
- Cairncross, G.; Wang, M.; Shaw, E.; Jenkins, R.; Brachman, D.; Buckner, J.; Fink, K.; Souhami, L.; Laperriere, N.; Mehta, M.; et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J. Clin. Oncol. 2013, 31, 337–343. [Google Scholar] [CrossRef]
- Weller, M.; Van Den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Wick, W.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Schiff, D.; Van den Bent, M.; Vogelbaum, M.A.; Wick, W.; Miller, C.R.; Taphoorn, M.; Pope, W.; Brown, P.D.; Platten, M.; Jalali, R.; et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro-Oncol. 2019, 21, 837–853. [Google Scholar] [CrossRef]
- Shaw, E.G.; Berkey, B.; Coons, S.W.; Bullard, D.; Brachman, D.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: Results of a prospective clinical trial. J. Neurosurg. 2008, 109, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Hegi, M.E.; van den Bent, M.J.; von Deimling, A.; Gorlia, T.; Hoang-Xuan, K.; Brandes, A.A.; Kantor, G.; Taphoorn, M.J.B.; Ben Hassel, M.; et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016, 17, 1521–1532. [Google Scholar] [CrossRef]
- Pignatti, F.; Van Den Bent, M.; Curran, D.; Debruyne, C.; Sylvester, R.; Therasse, P.; Afra, D.; Cornu, P.; Bolla, M.; Vecht, C.; et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 2002, 20, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.B.; Brown, P.D.; Felten, S.J.; Wu, W.; Buckner, J.C.; Arusell, R.M.; Curran, W.J.; Abrams, R.A.; Schiff, D.; Shaw, E.G. Validation of EORTC prognostic factors for adults with low-grade glioma: A report using intergroup 86-72-51. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.W.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef]
- Roelz, R.; Strohmaier, D.; Jabbarli, R.; Kraeutle, R.; Egger, K.; Coenen, V.A.; Weyerbrock, A.; Reinacher, P.C. Residual Tumor Volume as Best Outcome Predictor in Low Grade Glioma - A Nine-Years Near-Randomized Survey of Surgery vs. Biopsy. Sci. Rep. 2016, 6, 32286. [Google Scholar] [CrossRef]
- Berger, M.S.; Deliganis, A.V.; Dobbins, J.; Keles, G.E. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 1994, 74, 1784–1791. [Google Scholar] [CrossRef]
- Reuss, D.E.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C.; Korshunov, A.; von Deimling, A.; et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation little difference and in survival: A grading problem for, W.H.O. Acta Neuropathol. 2015, 129, 867–873. [Google Scholar] [CrossRef]
- Fisher, B.J.; Hu, C.; Macdonald, D.R.; Lesser, G.J.; Coons, S.W.; Brachman, D.G.; Ryu, S.; Bahary, J.-P.; Chakravarti, A.; Mehta, M.; et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: Preliminary results of Radiation Therapy Oncology Group 0424. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 497–504. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Afra, D.; De Witte, O.; Hassel, M.B.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.-O.; Collette, L.; Piérart, M.; Karim, A.B.M.F.; et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Karim, A.B.; Maat, B.; Hatlevoll, R.; Menten, J.; Rutten, E.H.; Thomas, D.G.; Mascarenhas, F.; Horiot, J.C.; Parvinen, L.M.; van Reijn, M.; et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.H.; Zhang, P.; Shaw, E.G.; Buckner, J.C.; Barger, G.R.; Bullard, D.E.; Mehta, M.P.; Gilbert, M.R.; Brown, P.D.; Stelzer, K.J.; et al. Comprehensive Genomic Analysis in NRG Oncology/RTOG 9802: A Phase III Trial of Radiation Versus Radiation Plus Procarbazine, Lomustine (CCNU), and Vincristine in High-Risk Low-Grade Glioma. J. Clin. Oncol. 2020, 38, 3407–3417. [Google Scholar] [CrossRef]
- Suchorska, B.; Schüller, U.; Biczok, A.; Lenski, M.; Albert, N.L.; Giese, A.; Kreth, F.-W.; Ertl-Wagner, B.; Tonn, J.-C.; Ingrisch, M. Contrast enhancement is a prognostic factor in IDH1/2 mutant, but not in wild-type WHO grade II/III glioma as confirmed by machine learning. Eur. J. Cancer 2019, 107, 15–27. [Google Scholar] [CrossRef]
- Chaichana, K.L.; McGirt, M.J.; Laterra, J.; Olivi, A.; Quiñones-Hinojosa, A. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J. Neurosurg. 2010, 112, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Wick, W.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgňrd, G.; Solheim, O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinoñes-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–707, Author reply 7–8. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Guyotat, J.; Duffau, H.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Zhang, Y.; Phillips, J.J.; Morshed, R.A.; Young, J.S.; McCoy, L.; Lafontaine, M.; Luks, T.; Ammanuel, S.; Kakaizada, S.; et al. Interactive Effects of Molecular, Therapeutic, and Patient Factors on Outcome of Diffuse Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 2029–2042. [Google Scholar] [CrossRef]
- Gozé, C.; Blonski, M.; Le Maistre, G.; Bauchet, L.; Dezamis, E.; Page, P.; Varlet, P.; Capelle, L.; Devaux, B.; Pallud, J.; et al. Imaging growth and isocitrate dehydrogenase 1 mutation are independent predictors for diffuse low-grade gliomas. Neuro-Oncol. 2014, 16, 1100–1109. [Google Scholar] [CrossRef]
- Pallud, J.; Blonski, M.; Mandonnet, E.; Audureau, E.; Fontaine, D.; Sanai, N.; Bauchet, L.; Peruzzi, P.; Frénay, M.; Colin, P.; et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro-Oncol. 2013, 15, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Pallud, J.; Taillandier, L.; Capelle, L.; Fontaine, D.; Peyre, M.; Ducray, F.; Matthieu, P.; François, D.; Hugues, D.; Mandonnet, E.; et al. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: A plea for systematic measurement of growth rates. Neurosurgery 2012, 71, 729–739; Discussion 39–40. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, P.; Harter, P.N.; Tönjes, M.; Capper, D.; Blank, A.E.; Sahm, F.; von Deimling, A.; Kolluru, V.; Schwamb, B.; Mittelbronn, M.; et al. Loss of FUBP1 expression in gliomas predicts FUBP1 mutation and is associated with oligodendroglial differentiation, IDH1 mutation and 1p/19q loss of heterozygosity. Neuropathol. Appl. Neurobiol. 2014, 40, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.D.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [PubMed]
- Dipasquale, A.; Franceschi, E.; Lombardi, G.; Simonelli, M. Vorasidenib in IDH mutant WHO grade 2 gliomas: Time to stop sitting on the fence? Neuro-Oncol. Adv. 2024, 6, vdae003. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, I.K.; van den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef]
- Spitzer, A.; Gritsch, S.; Nomura, M.; Jucht, A.; Fortin, J.; Raviram, R.; Weisman, H.R.; Castro, L.N.G.; Druck, N.; Chanoch-Myers, R.; et al. Mutant IDH inhibitors induce lineage differentiation in IDH-mutant oligodendroglioma. Cancer Cell 2024, 42, 904–914.e9. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sahm, F.; Koelsche, C.; Wefers, A.; Reinhardt, A.; Huang, K.; et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018, 136, 153–166. [Google Scholar] [CrossRef]
- de Souza, C.F.; Sabedot, T.S.; Malta, T.M.; Stetson, L.; Morozova, O.; Sokolov, A.; Laird, P.W.; Wiznerowicz, M.; Iavarone, A.; Snyder, J.; et al. A Distinct DNA Methylation Shift in a Subset of Glioma CpG Island Methylator Phenotypes during Tumor Recurrence. Cell Rep. 2018, 23, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; de Souza, C.F.; Sabedot, T.S.; Silva, T.C.; Mosella, M.S.; Kalkanis, S.N.; Snyder, J.; Castro, A.V.B.; Noushmehr, H. Glioma CpG island methylator phenotype (G-CIMP): Biological and clinical implications. Neuro-Oncol. 2018, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Arakawa, Y.; Narita, Y.; Sugiyama, K.; Hata, N.; Muragaki, Y.; Shinojima, N.; Kumabe, T.; Saito, R.; Motomura, K.; et al. The first-in-human phase I study of a brain-penetrant mutant IDH1 inhibitor DS-1001 in patients with recurrent or progressive IDH1-mutant gliomas. Neuro-Oncol. 2023, 25, 326–336. [Google Scholar] [CrossRef]
- Tesileanu, C.M.S.; Vallentgoed, W.R.; Sanson, M.; Taal, W.; Clement, P.M.; Wick, W.; Brandes, A.A.; Baurain, J.F.; Chinot, O.L.; French, P.J.; et al. Non-IDH1-R132H IDH1/2 mutations are associated with increased DNA methylation and improved survival in astrocytomas, compared to IDH1-R132H mutations. Acta Neuropathol. 2021, 141, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Tesileanu, C.M.S.; Vallentgoed, W.R.; French, P.J.; van den Bent, M.J. Molecular markers related to patient outcome in patients with IDH-mutant astrocytomas grade 2 to 4: A systematic review. Eur. J. Cancer 2022, 175, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Rongen, M.; Schmitz, R.; Brunn, A.; Gesk, S.; Richter, J.; Hong, K.; Wiestler, O.D.; Siebert, R.; Küppers, R.; Deckert, M. Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol. 2010, 120, 529–535. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).