Risk Factors for the Development of Malignancies Post-Transplantation in Kidney Transplant Recipients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Clinical Characteristics and Immunosuppressive Treatment of Kindey Transplant Recipients with and Without Malignancy
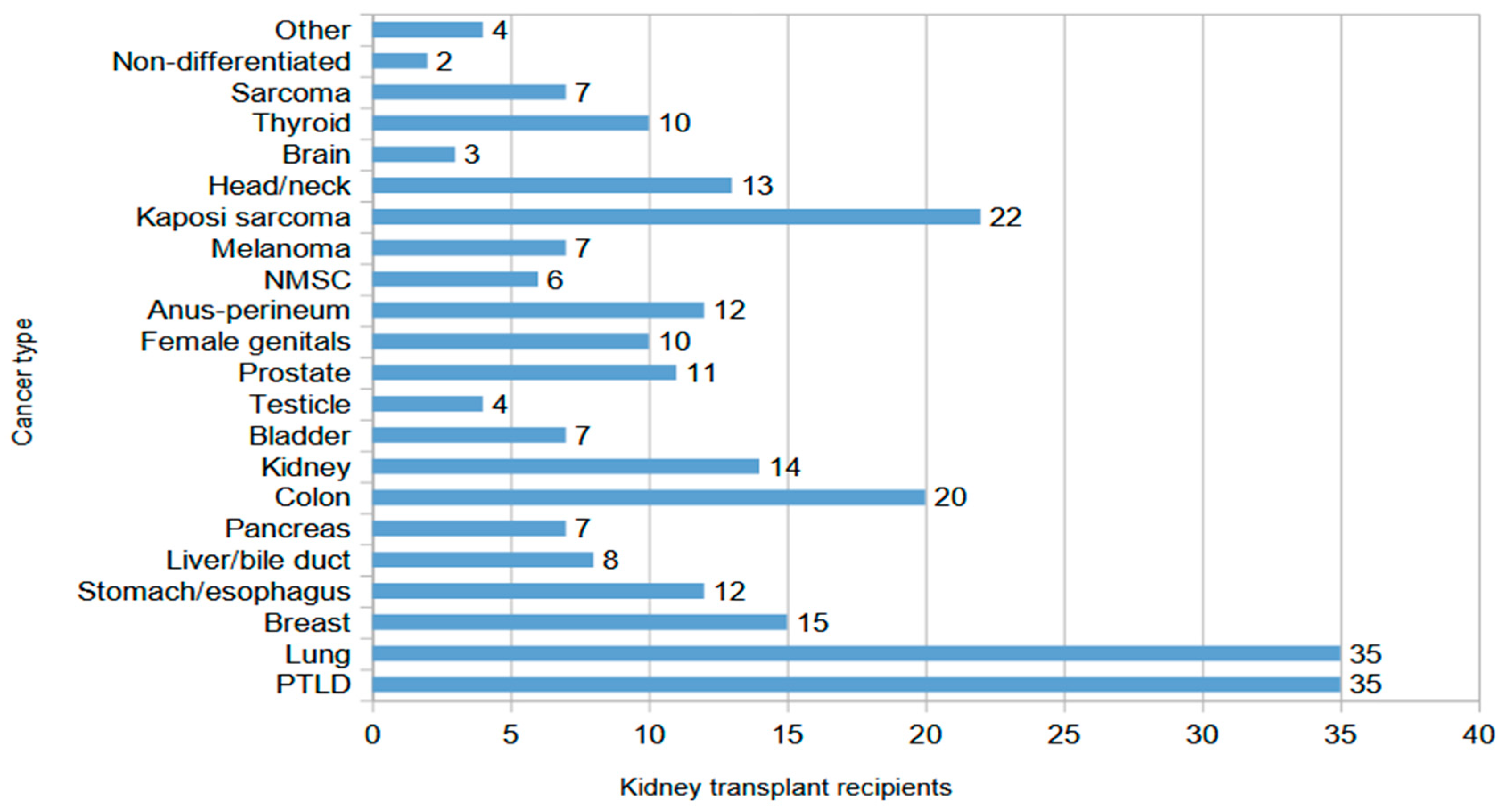
3.2. Characteristics of Kidney Transplant Recipients with Malignancies
3.3. Risk Factors for Cancer After Kidney Transplantation
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Merion, R.M.; Goodrich, N.P.; Johnson, R.J.; McDonald, S.P.; Russ, G.R.; Gillespie, B.W.; Collett, D. Kidney Transplant Graft Outcomes in 379257 Recipients on 3 Continents. Am. J. Transplant. 2018, 18, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.A.; Niu, J.; Pan, J.S.; Erickson, K.F.; Mandayam, S.; Winkelmayer, W.C.; Navaneethan, S.D.; Ramanathan, V. Trends in the Causes of Death among Kidney Transplant Recipients in the United States (1996–2014). Am. J. Nephrol. 2018, 48, 472–481. [Google Scholar] [CrossRef]
- Villanego, F.; Vigara, L.A.; López, V.; Gracia, M.d.C.d.; Rodríguez-Benot, A.; Bernal, G.; Castro, P.; Mazuecos, A. Changes over Time in the Causes of Death with a Functioning Graft in Kidney Transplantation Recipients. Nefrologia 2023, 43, 91–101. [Google Scholar] [CrossRef]
- Ying, T.; Shi, B.; Kelly, P.J.; Pilmore, H.; Clayton, P.A.; Chadban, S.J. Death after Kidney Transplantation: An Analysis by Era and Time Post-Transplant. J. Am. Soc. Nephrol. 2020, 31, 2887–2899. [Google Scholar] [CrossRef]
- UK Renal Registry. UK Renal Registry 25th Annual Report–Data to 31/12/2021; UK Renal Registry: Bristol, UK, 2023. [Google Scholar]
- Australia and New Zealand Dialysis and Transplant Registry. Australia and New Zealand Organ Donation Registry: Section 3: Mortality in Kidney Failure with Replacement Therapy. In ANZDATA Registry 44th Annual Report; Australia and New Zealand Dialysis and Transplant Registry: Adelaide, Australia, 2021. [Google Scholar]
- Au, E.; Wong, G.; Chapman, J.R. Cancer in Kidney Transplant Recipients. Nat. Rev. Nephrol. 2018, 14, 508–520. [Google Scholar] [CrossRef]
- Buell, J.F.; Gross, T.G.; Woodle, E.S. Malignancy after Transplantation. Transplantation 2005, 80, S254–S264. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Tang, S.C.W. An Update on Cancer after Kidney Transplantation. Nephrol. Dial. Transplant. 2019, 34, 914–920. [Google Scholar] [CrossRef]
- Wong, G.; Chapman, J.R. Cancers after Renal Transplantation. Transplant. Rev. 2008, 22, 141–149. [Google Scholar] [CrossRef]
- Collett, D.; Mumford, L.; Banner, N.R.; Neuberger, J.; Watson, C. Comparison of the Incidence of Malignancy in Recipients of Different Types of Organ: A UK Registry Audit. Am. J. Transplant. 2010, 10, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Hortlund, M.; Arroyo Mühr, L.S.; Storm, H.; Engholm, G.; Dillner, J.; Bzhalava, D. Cancer Risks after Solid Organ Transplantation and after Long-Term Dialysis. Int. J. Cancer 2017, 140, 1091–1101. [Google Scholar] [CrossRef]
- Grulich, A.E.; van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of Cancers in People with HIV/AIDS Compared with Immunosuppressed Transplant Recipients: A Meta-Analysis. Lancet 2007, 370, 59–67. [Google Scholar] [CrossRef]
- Sprangers, B.; Nair, V.; Launay-Vacher, V.; Riella, L.V.; Jhaveri, K.D. Risk Factors Associated with Post–Kidney Transplant Malignancies: An Article from the Cancer-Kidney International Network. Clin. Kidney J. 2018, 11, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Turner, R.M.; Chapman, J.R.; Howell, M.; Lim, W.H.; Webster, A.C.; Craig, J.C. Time on Dialysis and Cancer Risk After Kidney Transplantation. Transplantation 2013, 95, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H.; Vajdic, C.M.; van Leeuwen, M.T.; Amin, J.; Webster, A.C.; Chapman, J.R.; McDonald, S.P.; Grulich, A.E.; McCredie, M.R.E. The Pattern of Excess Cancer in Dialysis and Transplantation. Nephrol. Dial. Transplant. 2009, 24, 3225–3231. [Google Scholar] [CrossRef]
- Rosales, B.M.; De La Mata, N.; Vajdic, C.M.; Kelly, P.J.; Wyburn, K.; Webster, A.C. Cancer Mortality in Kidney Transplant Recipients: An Australian and New Zealand Population-Based Cohort Study, 1980–2013. Int. J. Cancer 2020, 146, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.T.; Wang, C. Cancer after Kidney Transplantation in the United States. Am. J. Transplant. 2004, 4, 905–913. [Google Scholar] [CrossRef]
- Benoni, H.; Eloranta, S.; Dahle, D.O.; Svensson, M.H.S.; Nordin, A.; Carstens, J.; Mjøen, G.; Helanterä, I.; Hellström, V.; Enblad, G.; et al. Relative and Absolute Cancer Risks among Nordic Kidney Transplant Recipients-a Population-Based Study. Transpl. Int. 2020, 33, 1700–1710. [Google Scholar] [CrossRef]
- Villeneuve, P.; Schaubel, D.; Fenton, S.; Shepherd, F.; Jiang, Y.; Mao, Y. Cancer Incidence Among Canadian Kidney Transplant Recipients. Am. J. Transplant. 2007, 7, 941–948. [Google Scholar] [CrossRef]
- Piselli, P.; Serraino, D.; Cimaglia, C.; Furian, L.; Biancone, L.; Busnach, G.; Bossini, N.; Todeschini, P.; Iaria, M.; Citterio, F.; et al. Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period. Cancers 2023, 15, 1347. [Google Scholar] [CrossRef]
- Oliveras, L.; Pareja, L.; Ribes, J.; Comas, J.; Couceiro, C.; Favà, À.; Codina, S.; Coloma, A.; Manonelles, A.; Lloberas, N.; et al. Cancer Risks in People on Dialysis and Kidney Transplant Recipients: A Catalan Cohort Study, 2003–2021. Clin. Kidney J. 2025, 18, sfaf077. [Google Scholar] [CrossRef] [PubMed]
- OECD. EU Country Cancer Profiles, EU Country Cancer Profile: Greece 2023; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Global Observatory on Donation and Transplantation International Figures on Donation and Transplantation 2023–2024. Available online: https://www.transplant-observatory.org/ (accessed on 15 April 2025).
- Webster, A.C.; Craig, J.C.; Simpson, J.M.; Jones, M.P.; Chapman, J.R. Identifying High Risk Groups and Quantifying Absolute Risk of Cancer After Kidney Transplantation: A Cohort Study of 15 183 Recipients. Am. J. Transplant. 2007, 7, 2140–2151. [Google Scholar] [CrossRef]
- Vajdic, C.M.; McDonald, S.P.; McCredie, M.R.E.; van Leeuwen, M.T.; Stewart, J.H.; Law, M.; Chapman, J.R.; Webster, A.C.; Kaldor, J.M.; Grulich, A.E. Cancer Incidence Before and After Kidney Transplantation. JAMA 2006, 296, 2823–2831. [Google Scholar] [CrossRef]
- van de Wetering, J.; Roodnat, J.I.; Hemke, A.C.; Hoitsma, A.J.; Weimar, W. Patient Survival after the Diagnosis of Cancer in Renal Transplant Recipients: A Nested Case-Control Study. Transplantation 2010, 90, 1542–1546. [Google Scholar] [CrossRef]
- Hall, E.C.; Pfeiffer, R.M.; Segev, D.L.; Engels, E.A. Cumulative Incidence of Cancer after Solid Organ Transplantation. Cancer 2013, 119, 2300–2308. [Google Scholar] [CrossRef]
- Allen, U.D.; Preiksaitis, J.K. The AST Infectious Diseases Community of Practice Post-Transplant Lymphoproliferative Disorders, Epstein-Barr Virus Infection, and Disease in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13652. [Google Scholar] [CrossRef]
- Guba, M.; Graeb, C.; Jauch, K.-W.; Geissler, E.K. Pro and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004, 77, 1777–1782. [Google Scholar] [CrossRef]
- Ume, A.C.; Pugh, J.M.; Kemp, M.G.; Williams, C.R. Calcineurin Inhibitor (CNI)-Associated Skin Cancers: New Insights on Exploring Mechanisms by Which CNIs Downregulate DNA Repair Machinery. Photodermatol. Photoimmunol. Photomed. 2020, 36, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Fernandes, M.; Habbab, F.; de Edwardes, M.D.B.; Loertscher, R.; Meterissian, S. Malignancy after Renal Transplantation: Incidence and Role of Type of Immunosuppression. Ann. Surg. Oncol. 2002, 9, 785–788. [Google Scholar] [CrossRef]
- Herman, M.; Weinstein, T.; Korzets, A.; Chagnac, A.; Ori, Y.; Zevin, D.; Malachi, T.; Gafter, U. Effect of Cyclosporin A on DNA Repair and Cancer Incidence in Kidney Transplant Recipients. J. Lab. Clin. Med. 2001, 137, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dantal, J.; Hourmant, M.; Cantarovich, D.; Giral, M.; Blancho, G.; Dreno, B.; Soulillou, J.-P. Effect of Long-Term Immunosuppression in Kidney-Graft Recipients on Cancer Incidence: Randomised Comparison of Two Cyclosporin Regimens. Lancet 1998, 351, 623–628. [Google Scholar] [CrossRef]
- Offman, J.; Opelz, G.; Doehler, B.; Cummins, D.; Halil, O.; Banner, N.R.; Burke, M.M.; Sullivan, D.; Macpherson, P.; Karran, P. Defective DNA Mismatch Repair in Acute Myeloid Leukemia/Myelodysplastic Syndrome after Organ Transplantation. Blood 2004, 104, 822–828. [Google Scholar] [CrossRef]
- Swann, P.F.; Waters, T.R.; Moulton, D.C.; Xu, Y.-Z.; Zheng, Q.; Edwards, M.; Mace, R. Role of Postreplicative DNA Mismatch Repair in the Cytotoxic Action of Thioguanine. Science 1996, 273, 1109–1111. [Google Scholar] [CrossRef]
- Bunea, M.-C.; Diculescu, V.-C.; Enculescu, M.; Oprea, D.; Enache, T.A. Influence of the Photodegradation of Azathioprine on DNA and Cells. Int. J. Mol. Sci. 2022, 23, 14438. [Google Scholar] [CrossRef]
- Ingvar, Å.; Smedby, K.E.; Lindelöf, B.; Fernberg, P.; Bellocco, R.; Tufveson, G.; Höglund, P.; Adami, J. Immunosuppressive Treatment after Solid Organ Transplantation and Risk of Post-Transplant Cutaneous Squamous Cell Carcinoma. Nephrol. Dial. Transplant. 2010, 25, 2764–2771. [Google Scholar] [CrossRef]
- Zwart, E.S.; Yüksel, E.; Pannekoek, A.; de Vries, R.; Mebius, R.E.; Kazemier, G. De Novo Carcinoma after Solid Organ Transplantation to Give Insight into Carcinogenesis in General—A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Döhler, B.; Ruhenstroth, A.; Cinca, S.; Unterrainer, C.; Stricker, L.; Scherer, S.; Gombos, P.; Süsal, C.; Daniel, V.; et al. The Collaborative Transplant Study Registry. Transplant. Rev. 2013, 27, 43–45. [Google Scholar] [CrossRef]
- Robson, R.; Cecka, J.M.; Opelz, G.; Budde, M.; Sacks, S. Prospective Registry-Based Observational Cohort Study of the Long-Term Risk of Malignancies in Renal Transplant Patients Treated with Mycophenolate Mofetil. Am. J. Transplant. 2005, 5, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Lamy, F.X.; Quelen, C.; Dantal, J.; Lebranchu, Y.; Lang, P.; Velten, M.; Moulin, B. Epidemiology of Posttransplant Lymphoproliferative Disorders in Adult Kidney and Kidney Pancreas Recipients: Report of the French Registry and Analysis of Subgroups of Lymphomas. Am. J. Transplant. 2012, 12, 682–693. [Google Scholar] [CrossRef]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.S.; Zhao, P.; Yan, P. Overview of Research into mTOR Inhibitors. Μοlecules 2022, 27, 5295. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Wei, L.; Huang, J. mTOR Signaling, Function, Novel Inhibitors, and Therapeutic Targets. J. Nucl. Med. 2011, 52, 497. [Google Scholar] [CrossRef]
- Budde, K.; Lehner, F.; Sommerer, C.; Reinke, P.; Arns, W.; Eisenberger, U.; Wüthrich, R.P.; Mühlfeld, A.; Heller, K.; Porstner, M.; et al. Five-Year Outcomes in Kidney Transplant Patients Converted from Cyclosporine to Everolimus: The Randomized ZEUS Study. Am. J. Transplant. 2015, 15, 119–128. [Google Scholar] [CrossRef]
- Opelz, G.; Unterrainer, C.; Süsal, C.; Döhler, B. Immunosuppression with Mammalian Target of Rapamycin Inhibitor and Incidence of Post-Transplant Cancer in Kidney Transplant Recipients. Nephrol. Dial. Transplant. 2016, 31, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.-U.; Kasiske, B.L.; Zeier, M.G. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [Google Scholar] [CrossRef]
- Francis, A.; Johnson, D.W.; Craig, J.C.; Wong, G. Incidence and Predictors of Cancer Following Kidney Transplantation in Childhood. Am. J. Transplant. 2017, 17, 2650–2658. [Google Scholar] [CrossRef]
- Flechner, S.M.; Glyda, M.; Cockfield, S.; Grinyó, J.; Legendre, C.; Russ, G.; Steinberg, S.; Wissing, K.M.; Tai, S.S. The ORION Study: Comparison of Two Sirolimus-Based Regimens versus Tacrolimus and Mycophenolate Mofetil in Renal Allograft Recipients. Am. J. Transplant. 2011, 11, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.P.; Kelly, P.J.; Jardine, M.; Perkovic, V.; Cass, A.; Craig, J.C.; Eris, J.; Webster, A.C. Long-Term Cancer Risk of Immunosuppressive Regimens after Kidney Transplantation. J. Am. Soc. Nephrol. 2010, 21, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Vock, D.M.; Matas, A.J.; Robiner, W.N.; Nevins, T.E. Medication Adherence Is Associated with an Increased Risk of Cancer in Kidney Transplant Recipients: A Cohort Study. Nephrol. Dial. Transplant. 2019, 34, 364–370. [Google Scholar] [CrossRef]
- Saowapa, S.; Polpichai, N.; Siladech, P.; Wannaphut, C.; Tanariyakul, M.; Wattanachayakul, P.; Lalitnithi, P. Evaluating Kaposi Sarcoma in Kidney Transplant Patients: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e52527. [Google Scholar] [CrossRef]
- Chung, E.Y.M.; Palmer, S.C.; Strippoli, G.F.M. Interventions to Prevent Nonmelanoma Skin Cancers in Recipients of a Solid Organ Transplant: Systematic Review of Randomized Controlled Trials. Transplantation 2019, 103, 1206–1215. [Google Scholar] [CrossRef]
- Stallone, G.; Schena, A.; Infante, B.; Di Paolo, S.; Loverre, A.; Maggio, G.; Ranieri, E.; Gesualdo, L.; Schena, F.P.; Grandaliano, G. Sirolimus for Kaposi’s Sarcoma in Renal-Transplant Recipients. N. Engl. J. Med. 2005, 352, 1317–1323. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Sant, A.J.; McMichael, A. Revealing the Role of CD4+ T Cells in Viral Immunity. J. Exp. Med. 2012, 209, 1391–1395. [Google Scholar] [CrossRef]
- Wang, L.; Motter, J.; Bae, S.; Ahn, J.B.; Kanakry, J.A.; Jackson, J.; Schnitzler, M.A.; Hess, G.; Lentine, K.L.; Stuart, E.A.; et al. Induction Immunosuppression and the Risk of Incident Malignancies among Older and Younger Kidney Transplant Recipients: A Prospective Cohort Study. Clin. Transplant. 2020, 34, e14121. [Google Scholar] [CrossRef]
- Acharya, S.; Lama, S.; Kanigicherla, D.A. Anti-Thymocyte Globulin for Treatment of T-Cell-Mediated Allograft Rejection. World J. Transpl. 2023, 13, 299–308. [Google Scholar] [CrossRef]
- Massicotte-Azarniouch, D.; Detwiler, R.K.; Hu, Y.; Falk, R.J.; Saha, M.K.; Hogan, S.L.; Derebail, V.K. Malignancy Risk in Kidney Transplant Recipients Exposed to Immunosuppression Pre-Transplant for the Treatment of Glomerulonephritis. Nephrol. Dial. Transplant. 2023, 38, 2009–2018. [Google Scholar] [CrossRef]
- Chukwu, C.A.; Wu, H.H.L.; Pullerits, K.; Garland, S.; Middleton, R.; Chinnadurai, R.; Kalra, P.A. Incidence, Risk Factors, and Outcomes of De Novo Malignancy Following Kidney Transplantation. J. Clin. Med. 2024, 13, 1872. [Google Scholar] [CrossRef]
- Papadimitriou, J.C.; Randhawa, P.; Rinaldo, C.H.; Drachenberg, C.B.; Alexiev, B.; Hirsch, H.H. BK Polyomavirus Infection and Renourinary Tumorigenesis. Am. J. Transplant. 2016, 16, 398–406. [Google Scholar] [CrossRef]
- British Transplantation Society. UK Guideline on Prevention and Management of Cytomegalovirus (CMV) Infection and Disease Following Solid Organ Transplantation; British Transplantation Society: Sheffield, UK, 2022. [Google Scholar]
- Caillard, S.; Dharnidharka, V.; Agodoa, L.; Bohen, E.; Abbott, K. Posttransplant Lymphoproliferative Disorders after Renal Transplantation in the United States in Era of Modern Immunosuppression. Transplantation 2005, 80, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Bigotte Vieira, M.; Arai, H.; Nicolau, C.; Murakami, N. Cancer Screening and Cancer Treatment in Kidney Transplant Recipients. Kidney360 2024, 5, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
| Kidney Transplant Recipients with Malignancy (Ν = 268) | Kidney Transplant Recipients Without Malignancy (Ν = 268) | p-Value | |
|---|---|---|---|
| Sex (male) | 159 (59.3) | 159 (59.3) | 1.000 |
| Age at transplantation (years) | 48 (39–57) | 48 (39–57) | 0.804 |
| Dialysis vintage (months) | 31.5 (9.5–70) | 31 (15–72) | 0.722 |
| Primary kidney disease | 0.392 | ||
| ● glomerulonephritis | 72 (26.8) | 61 (22.8) | |
| ● polycystic kidney disease | 43 (16.1) | 50 (18.7) | |
| ● hypoplastic kidneys/congenital nephropathy | 19 (7.1) | 26 (9.7) | |
| ● obstructive nephropathy/lithiasis | 20 (7.4) | 13 (4.8) | |
| ● diabetic nephropathy | 7 (2.6) | 11 (4.1) | |
| ● hypertensive nephropathy | 5 (1.9) | 7 (2.6) | |
| ● other | 5 (1.9) | 5 (1.9) | |
| ● unknown | 97 (36.2) | 95 (35.4) | |
| Smoking (yes) | 99 (36.9) | 85 (31.7) | 0.210 |
| BMI (kg/m2) | 23.6 (22–25.6) | 23.9 (22–26) | 0.210 |
| HBV infection | 18 (6.7) | 18 (6.7) | 0.441 |
| HCV infection | 23 (8.6) | 29 (10.8) | 0.720 |
| Donor (living/deceased) | 119/149 (44.4/55.6) | 112/156 (41.8/58.2) | 0.562 |
| Transplant number | 0.960 | ||
| ● 1st | 259 (96.6) | 251 (93.6) | |
| ● 2nd | 8 (3) | 16 (6) | |
| ● 3rd | 1 (0.4) | 1 (0.4) | |
| Transplant year | 0.317 | ||
| ● 1979–1989 | 40 (14.9) | 40 (14.9) | |
| ● 1990–1999 | 91 (33.9) | 92 (34.3) | |
| ● 2000–2009 | 85 (31.7) | 86 (32.1) | |
| ● 2010–2019 | 50 (18.7) | 48 (17.9) | |
| ● 2020–2023 | 2 (0.8) | 2 (0.8) | |
| PRAs (%) | 0.548 | ||
| ● <5% | 208 (77.6) | 212 (79.1) | |
| ● 5–70% | 46 (17.2) | 39 (14.5) | |
| ● >70% | 14 (5.2) | 17 (6.4) | |
| DSA (yes) | 28 (10.4) | 31 (11.6) | 0.766 |
| ABO incompatibility | 6 (2.2) | 6 (2.2) | 1.000 |
| eGFR 1 year post Τx (mL/min/1.73 m2) | 53 (43–66) | 56 (46–69) | 0.103 |
| Years of follow-up | 8 (4–16) | 13 (8–17) | <0.001 |
| eGFR at follow-up (mL/min/1.73 m2) | 50 (35–66) | 39 (25–57) | <0.001 |
| Rejection (yes) | 46 (17.2) | 32 (11.9) | 0.103 |
| Rejection type | 0.256 | ||
| ● T-cell-mediated | 38 (14.2) | 26 (9.7) | |
| ● antibody-mediated | 6 (2.2) | 4 (1.5) | |
| ● mixed | 2 (0.8) | 2 (0.8) | |
| CMV infection | 25 (9.3) | 49 (18.3) | 0.003 |
| BKV infection | 7 (2.6) | 9 (3.4) | 0.593 |
| Kidney Transplant Recipients with Malignancy (Ν = 268) | Kidney Transplant Recipients Without Malignancy (Ν = 268) | p-Value | |
|---|---|---|---|
| Induction treatment | |||
| Anti-IL2Ra antibodies | 39 (14.5) | 35 (13) | 0.618 |
| ATG/OKT3 | 139 (51.9) | 145 (54.1) | 0.505 |
| No induction | 90 (33.6) | 88 (32.8) | 0.880 |
| Maintenance treatment | |||
| Steroids | 231 (86.2) | 221 (82) | 0.282 |
| Calcineurin inhibitor (CNI) | 252 (94) | 247 (92.3) | 0.848 |
| ● cyclosporin A | 141 (52.6) | 130 (48.5) | 0.764 |
| ● tacrolimus | 111 (41.2) | 114 (42.5) | 0.910 |
| Azathioprine (Aza) | 51 (19) | 49 (18.3) | 0.874 |
| Μycophenolate (MMF/MPA) | 195 (72.8) | 198 (73.8) | 0.703 |
| mTOR inhibitors | 21 (7.8) | 24 (9) | 0.296 |
| Antirejection treatment | |||
| Steroid pulses | 39 (14.5) | 28 (10.4) | 0.101 |
| ATG/OKT3 | 10 (3.7) | 8 (3) | 0.617 |
| Rituximab±plasma exchange | 3 (6.7) | 4 (7.5) | 0.887 |
| Immunosuppressive regimens | |||
| ΜΜF/MPA+CNI±MP | 184 (66.7) | 184 (66.7) | 0.897 |
| Aza+CNI±MP | 46 (17.1) | 42 (15.7) | |
| MMF/MPA+mTORi±MP | 8 (3) | 13 (4.8) | |
| CNI+mTORi±MP | 13 (4.8) | 11 (4.1) | |
| CNI+MP | 9 (3.4) | 10 (3.7) | |
| Aza+MP | 5 (1.9) | 7 (2.6) | |
| Other | 3 (1.1) | 1 (0.4) |
| Odds Ratio (OR) | 95% CI | p-Value | |
|---|---|---|---|
| Immunosuppressive Regimen | |||
| ΜΜF/MPA+CNI±MP (reference) | |||
| Aza+CNI±MP | 1.16 | (0.66, 2.04) | 0.593 |
| MMF/MPA+mTORi±MP | 0.62 | (0.24, 1.60) | 0.330 |
| CNI+mTORi±MP | 1.14 | (0.52, 2.53) | 0.623 |
| CNI+MP | 0.97 | (0.36, 2.60) | 0.958 |
| Aza+MP | 0.80 | (0.23, 2.74) | 0.724 |
| Induction treatment | |||
| No induction (reference) | |||
| ATG/OKT3 | 1.01 | (0.54, 1.81) | 0.990 |
| Anti-IL2Ra Abs | 0.82 | (0.48, 1.34) | 0.405 |
| Rejection | 1.75 | (1.04, 2.94) | 0.034 |
| eGFR 1 year post Τx (mL/min/1.73 m2) | 0.99 | (0.98, 1.01) | 0.085 |
| Dialysis vintage (months) | 1.00 | (0.99, 1.01) | 0.890 |
| Primary kidney disease | |||
| unknown (reference) | |||
| glomerulonephritis | 2.23 | (1.06, 4.67) | 0.034 |
| polycystic kidney disease | 0.83 | (0.49, 1.39) | 0.478 |
| hypoplastic kidneys/congenital nehropathy | 0.78 | (0.39, 1.55) | 0.493 |
| obstructive nephropathy/lithiasis | 1.23 | (0.78, 1.96) | 0.358 |
| diabetic nephropathy | 0.69 | (0.24, 1.91) | 0.480 |
| hypertensive nephropathy | 0.78 | (0.23, 2.67) | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallianou, K.; Bellos, I.; Filiopoulos, V.; Skalioti, C.; Lagiou, P.; Benetou, V.; Marinaki, S. Risk Factors for the Development of Malignancies Post-Transplantation in Kidney Transplant Recipients. Biomedicines 2025, 13, 2346. https://doi.org/10.3390/biomedicines13102346
Vallianou K, Bellos I, Filiopoulos V, Skalioti C, Lagiou P, Benetou V, Marinaki S. Risk Factors for the Development of Malignancies Post-Transplantation in Kidney Transplant Recipients. Biomedicines. 2025; 13(10):2346. https://doi.org/10.3390/biomedicines13102346
Chicago/Turabian StyleVallianou, Kalliopi, Ioannis Bellos, Vassilis Filiopoulos, Chrysanthi Skalioti, Pagona Lagiou, Vassiliki Benetou, and Smaragdi Marinaki. 2025. "Risk Factors for the Development of Malignancies Post-Transplantation in Kidney Transplant Recipients" Biomedicines 13, no. 10: 2346. https://doi.org/10.3390/biomedicines13102346
APA StyleVallianou, K., Bellos, I., Filiopoulos, V., Skalioti, C., Lagiou, P., Benetou, V., & Marinaki, S. (2025). Risk Factors for the Development of Malignancies Post-Transplantation in Kidney Transplant Recipients. Biomedicines, 13(10), 2346. https://doi.org/10.3390/biomedicines13102346









