Differential Pattern of Circulating MicroRNA Expression in Patients with Intracranial Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Statistical Analysis
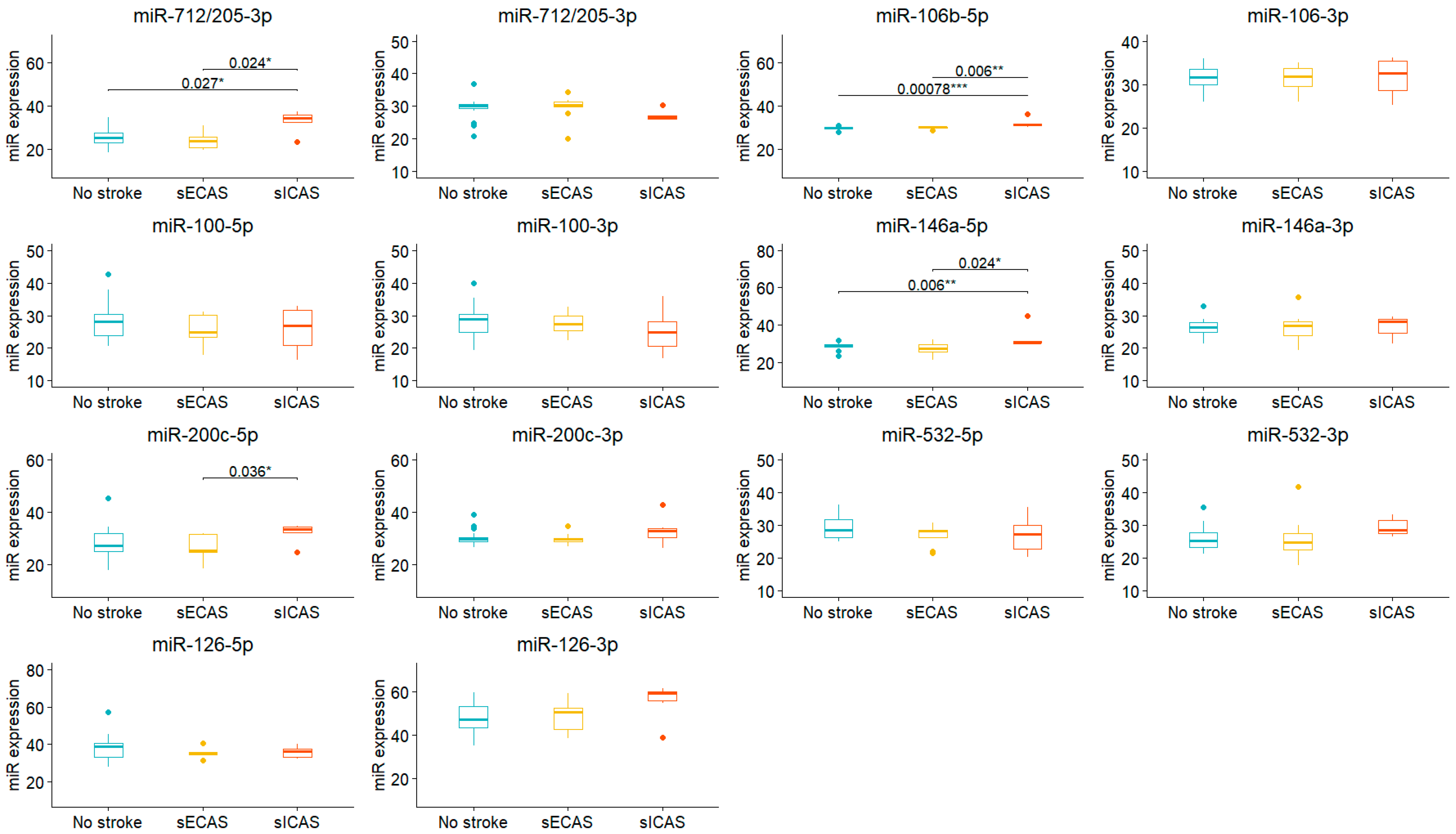
3. Results
4. Discussion
| Study | Patient Population | Data on ICAS | Examined miRs | Relevant Conclusion | Commentary |
|---|---|---|---|---|---|
| Long G. et al. (2013) [46] | Stroke patients (n = 197), incl. large-vessel atherosclerosis (n = 51); control (n = 50) | None | Circulating levels of miR-30a, miR-126, and let-7b | Circulating let-7b was lower in patients with large-vessel atherosclerosis than healthy volunteers | Possibly in some patients with LAA stroke could be attributed to ICAS |
| Dong H. et al. (2015) [25] | Alzheimer’s disease (n = 127), mild cognitive impairment (n = 30), and vascular dementia (VD, n = 30) | None | miR-31, miR-93a, and miR-146a | miR-31, miR-93, and miR-146a levels were significantly higher in the VD patients than in the controls | Some cases of VD could be due to ICAS |
| Volný O. et al. (2015) [31] | NA (review) | NA | miR-17-92 cluster, miR-126, miR-143/145 cluster, miR-155, miR-21, and miR-221 | miR-155 knockout or inhibition in experimental models of atherosclerosis led to reduction of atherosclerotic plaque size and decreased macrophage accumulation; levels of miR-21 and miR-221 were shown to be higher in patients with carotid atherosclerosis; urinary levels of miR-29b were shown to correlate with cIMT in patients with type 2 diabetes mellitus | No division into extra- and intracranial atherosclerosis |
| Sima X. et al. (2015) [47] | 305 patients with intracranial aneurysms and 401 healthy controls | None | rs10877887 and rs13293512 polymorphisms in the promoters of let-7 family | Association of the rs13293512 polymorphism in the promoter region of let-7 with intracranial aneurysms | Data on intracranial vessel wall miRs may be relevant to ICAS study |
| Zhong H. et al. (2016) [27] | 297 patients with atherosclerotic cerebral infarction (ACI) and 300 matched healthy individuals | None | miR-146a expression | The C allele of rs2910164 of the APOE4 gene may reduce miR-146a expression and subsequently weaken anti-inflammatory action in the pathogenesis of ACI; ACI patients with the ApoEε4 allele exhibited reduced miR-146a expression compared with controls | Some patients could have ICAS; possible molecular interplay between Alzheimer’s disease and atherosclerosis |
| Jeong H. et al. (2017) [20] | 36 atherosclerotic patients, who had severe stenosis in the intracranial and extracranial vessels, and 37 non-atherosclerotic patients, who had no stenosis in the evaluated vessels | ICAS was defined as >3 vessels having >50% of stenosis on 11 intracranial vessels (middle cerebral arteries, anterior cerebral arteries, posterior cerebral arteries, intracranial internal carotid arteries, vertebral arteries of both sides, and basilar artery) on time of flight magnetic resonance angiography; extracranial atherosclerosis: >2 vessels having >50% stenotic area on carotid duplex sonography | 14 miRNAs (miR-30c, -99b, -152, -181, -212, -222, -301b, -372, -454, -502, -576, -27a, -744, and -888) showed differential expression between non-atherosclerotic and atherosclerotic patients | The study identified miR-212 as a novel marker that enhanced the estimation power of three established cardiovascular risk markers (HbA1c, HDL-C, and lipoprotein(a)) for atherosclerosis presence in ischemic stroke patients. | Here, patients simultaneously had extra- and intracranial atherosclerosis, with very strict inclusion criteria. One of the few studies with ICAS as the main focus |
| Li Z. et al. (2017) [48] | 23 patients with atherosclerotic cerebral infarction and 32 healthy individuals | None | miR-223 | Mean methylation levels of a total of nine CpGs of miR-223 promoter were significantly lower in ACI patients than in healthy individuals, and were also significantly lower in individuals with carotid atherosclerosis than those without carotid atherosclerosis | A relevant proportion of patients could have ICAS |
| Prabhakar P. et al. (2017) [49] | 204 patients with VD, 200 control patients | None | plasma miRNA profiling | Plasma miR-409-3p, miR-502-3p, miR-486-5p, and miR-451a could be used to differentiate small vessel VaD patients from healthy controls | Some cases of VD may attributed to ICAS |
| Gao J. et al. (2019) [24] | LAA stroke patients (n = 57), Atherosclerosis group (n = 41), control group (n = 50) | None | Plasma miR-126 and miR-143 | miR-143 and miR-126 might participate in the atherosclerosis process and are minimally affected by cerebral infarction | Possibly some cases with ICAS (not explicitly) |
| Li J. et al. (2019) [50] | NA (review) | NA | miR-155, miR-27a/b, miR-342-5p, miR-21, miR-124, miR-223, miR-146a, miR-181b, miR-126, miR-143, and let-7b | Described are multiple roles of the mentioned miRs in regulating the progression of atherosclerosis involved with systemic and local inflammatory activities in cerebral arteries | Great review, but most findings are from general atherosclerosis or carotid atherosclerosis studies |
| Jiang H. et al. (2019) [21] | Patients with severe ICAS (≥70% stenosis) of any intracranial large artery (n = 74): 29 patients had recurrent ischemic events despite intensive medical management during 6-month follow-up (non-responders) and 45 patients had no ischemic events (responders) | All ICAS patients | Exosomal expression of miR-27b-3p, miR-122-5p, miR-16-5p, miR-30c-5p, miR-486-5p, miR-10a-5p, miR-10b-5p, miR-101-3p, miR-24-3p, miR-192-5p, miR-30c-5p, miR-425-5p, and miR-191-5p | This study suggests a specific circulating e-miRNA expression profile that is associated with antiangiogenesis and is a novel biomarker for recurrent ischemic events despite IMM in severe ICAD | Very informative study with a special focus on treatment; a large number of miRs have shown differential expression, possibly a novel target biomarker for therapy modification in resistant ICAS |
| Xuan J. et al. (2021) [51] | Cerebral atherosclerosis group (n = 52), control group (n = 46) | Possibly, all ICAS patients (due to enrollment criteria) | miR-137 | miR-137 expression was decreased in ICAS; miR-137 was independently correlated with the occurrence of cerebrovascular adverse events | Possibly, antiatherogenic factor |
| Zhang H. et al. (2020) [30] | 97 ischemic stroke patients (LAA, n = 35); 76 healthy controls | None | EMVs-miR-155 | EMVs and EMVs-miR-155 levels were higher in LAA and cardioembolic stroke subtypes | Possibly, some patients with LAA had ICAS |
| Yang S. et al. (2022) [52] | Cerebral atherosclerosis patients (stenosis≥ 50%) (n = 46); patients with no AS or vascular stenosis < 50% were included in the control group (n = 46) | ICAS (due to enrollment criteria) | LncRNA SNHG16, miR-30c-5p and SDC2 expression | miR-30c-5p was downregulated in ICAS; downregulating lncRNA SNHG16 inhibits VSMC proliferation and migration in AS by targeting the miR-30c-5p/SDC2 axis | This study examines a novel non-coding RNAs target interplay involved in the crucial aspect of atherosclerosis development—VSMC migration |
| Jiang H. et al. (2022) [53] | Patients with intracranial aneurysms (n = 58), control group (n = 44) | None | Serum levels of miR-1246 | Serum levels of miR-1246 were elevated in intracranial aneurysm patients | Again, data on miR-1246 may be relevant to ICAS, as it is involved in inflammatory response, lipid, and atherosclerotic signaling pathways |
| Xu J. et al. (2022) [54] | Cerebral atherosclerosis (n = 74), control group (n = 62) | Probably, most ICAS | Serum miR-130a-3p | Cerebral atherosclerosis patients had elevated serum miR-130a-3p compared with controls; high serum miR-130a-3p was independently related with high probability of cerebrovascular events | A prospective study utilizing a novel significant prognostic marker for stroke in ICAS patients |
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, K.S.; Caplan, L.R.; Kim, J.S. Stroke Mechanisms. Front. Neurol. Neurosci. 2016, 40, 58–71. [Google Scholar] [CrossRef]
- de Havenon, A.; Zaidat, O.O.; Amin-Hanjani, S.; Nguyen, T.N.; Bangad, A.; Abbasi, M.; Anadani, M.; Almallouhi, E.; Chatterjee, R.; Mazighi, M.; et al. Large Vessel Occlusion Stroke due to Intracranial Atherosclerotic Disease: Identification, Medical and Interventional Treatment, and Outcomes. Stroke 2023, 54, 1695–1705. [Google Scholar] [CrossRef]
- Kim, J.S.; Caplan, L.R.; Wong, K.S. Intracranial Atherosclerosis: Pathophysiology, Diagnosis, and Treatment; Frontiers of Neurology and Neuroscience; Karger: Basel, Switzerland, 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, R.; Liu, G.; Cao, C.; Chen, F.; Jin, K.; Ji, X.; Cao, G. Intracranial atherosclerotic disease. Neurobiol. Dis. 2019, 124, 118–132. [Google Scholar] [CrossRef]
- Resch, J.A.; Baker, A.B. Etiologic mechanisms in cerebral atherosclerosis. Preliminary study of 3,839 cases. JAMA Neurol. 1964, 10, 617–628. [Google Scholar] [CrossRef]
- Tanashyan, M.M.; Lagoda, O.V.; Raskurazhev, A.A.; Annushkin, V.A.; Mazur, A.S.; Sinitsyn, I.A. Extra- versus intracranial atherosclerosis: Two facets of the same problem. Russ. Neurol. J. 2022, 27, 11–19. [Google Scholar] [CrossRef]
- Tariq, M.B.; Kaneko, N.; Prochilo, G.; Hinman, J.D.; Liebeskind, D.S. Arterial Lesion Location and Outcomes of Intracranial Atherosclerotic Disease. Stroke Vasc. Interv. Neurol. 2024, 4, e001344. [Google Scholar] [CrossRef]
- Kasner, S.E.; Chimowitz, M.I.; Lynn, M.J.; Howlett-Smith, H.; Stern, B.J.; Hertzberg, V.S.; Frankel, M.R.; Levine, S.R.; Chaturvedi, S.; Benesch, C.G.; et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006, 113, 555–563. [Google Scholar] [CrossRef]
- Qin, G.; Wang, L.; Hua, Y.; Hou, H.; Zou, Q.; Wang, D.; Hu, Z.; Lu, D. Comparative morphology of the internal elastic lamina of cerebral and peripheral arteries. Int. J. Clin. Exp. Pathol. 2020, 13, 764–770. [Google Scholar]
- Ritz, K.; Denswil, N.P.; Stam, O.C.; van Lieshout, J.J.; Daemen, M.J. Cause and mechanisms of intracranial atherosclerosis. Circulation 2014, 130, 1407–1414. [Google Scholar] [CrossRef]
- Kang, D.W.; Kim, D.Y.; Kim, J.; Baik, S.H.; Jung, C.; Singh, N.; Song, J.W.; Bae, H.-J.; Kim, B.J. Emerging Concept of Intracranial Arterial Diseases: The Role of High Resolution Vessel Wall MRI. J. Stroke 2024, 26, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Witztum, J.L.; de Nigris, F.; Palumbo, G.; D’armiento, F.P.; Palinski, W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation 1999, 99, 2003–2010. [Google Scholar] [CrossRef]
- D’Armiento, F.P.; Bianchi, A.; de Nigris, F.; Capuzzi, D.M.; D’Armiento, M.R.; Crimi, G.; Abete, P.; Palinski, W.; Condorelli, M.; Napoli, C.; et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 2001, 32, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Bargieł, W.; Cierpiszewska, K.; Maruszczak, K.; Pakuła, A.; Szwankowska, D.; Wrzesińska, A.; Gutowski, Ł.; Formanowicz, D. Recognized and Potentially New Biomarkers—Their Role in Diagnosis and Prognosis of Cardiovascular Disease. Medicina 2021, 57, 701. [Google Scholar] [CrossRef] [PubMed]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef] [PubMed]
- Tanashyan, M.M.; Shabalina, A.A.; Annushkin, V.A.; Mazur, A.S.; Kuznetsova, P.I.; Raskurazhev, A.A. Circulating microRNAs in Carotid Atherosclerosis: Complex Interplay and Possible Associations with Atherothrombotic Stroke. Int. J. Mol. Sci. 2024, 25, 10026. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Xiao, J.; Cen, S.Y.; Liu, X.; Wu, F.; Schlick, K.; Li, D.; Yang, Q.; Song, S.S.; Fan, Z. Sex Differences in Intracranial Atherosclerosis in Patients with Hypertension With Acute Ischemic Stroke. J. Am. Heart Assoc. 2022, 11, e025579. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, J.Y.; Lee, S.H.; Hwang, J.; Shin, J.W.; Song, K.S.; Lee, S.; Kim, J. Synergy of circulating miR-212 with markers for cardiovascular risks to enhance estimation of atherosclerosis presence. PLoS ONE 2017, 12, e0177809, Erratum in PLoS ONE 2017, 12, e0184600. [Google Scholar] [CrossRef]
- Jiang, H.; Toscano, J.F.; Song, S.S.; Schlick, K.H.; Dumitrascu, O.M.; Pan, J.; Lyden, P.D.; Saver, J.L.; Gonzalez, N.R. Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antiangiogenesis. Sci. Rep. 2019, 9, 19429. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, H.; Gong, H. microRNA-212 promotes lipid accumulation and attenuates cholesterol efflux in THP-1 human macrophages by targeting SIRT1. Gene 2018, 643, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Gao, J.; Yang, S.; Wang, K.; Zhong, Q.; Ma, A.; Pan, X. Plasma miR-126 and miR-143 as Potential Novel Biomarkers for Cerebral Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2019, 28, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K.; et al. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers 2015, 2015, 625659. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Zhong, H.; Cai, Y.; Cheng, J.; Cai, D.; Chen, L.; Su, C.; Li, K.; Chen, P.; Xu, J.; Cui, L. Apolipoprotein E Epsilon 4 Enhances the Association between the rs2910164 Polymorphism of miR-146a and Risk of Atherosclerotic Cerebral Infarction. J. Atheroscler. Thromb. 2016, 23, 819–829. [Google Scholar] [CrossRef]
- Chu, T.; Xu, X.; Ruan, Z.; Wu, L.; Zhou, M.; Zhu, G. miR-146a contributes to atherosclerotic plaque stability by regulating the expression of TRAF6 and IRAK-1. Mol. Biol. Rep. 2022, 49, 4205–4216. [Google Scholar] [CrossRef] [PubMed]
- Raitoharju, E.; Lyytikäinen, L.P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Qiu, W.; Pan, Q.; Chen, Y.; Chen, Y.; Ma, X. Plasma endothelial microvesicles and their carrying miRNA-155 serve as biomarkers for ischemic stroke. J. Neurosci. Res. 2020, 98, 2290–2301. [Google Scholar] [CrossRef]
- Volný, O.; Kašičková, L.; Coufalová, D.; Cimflová, P.; Novák, J. MicroRNAs in Cerebrovascular Disease. In microRNA: Medical Evidence; Advances in Experimental Medicine and Biology (AEMB); Springer: Cham, Switzerland, 2015; Volume 888. [Google Scholar] [CrossRef]
- Son, D.J.; Kumar, S.; Takabe, W.; Kim, C.W.; Ni, C.-W.; Alberts-Grill, N.; Jang, I.-H.; Kim, S.; Kim, W.; Kang, S.W.; et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat. Commun. 2013, 4, 3000. [Google Scholar] [CrossRef]
- Dosta, P.; Tamargo, I.; Ramos, V.; Kumar, S.; Kang, D.W.; Borrós, S.; Jo, H. Delivery of Anti-microRNA-712 to Inflamed Endothelial Cells Using Poly(β-amino ester) Nanoparticles Conjugated with VCAM-1 Targeting Peptide. Adv. Healthc. Mater. 2021, 10, e2001894. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE−/− Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Li, R.; Shen, Y.Q.; Wang, X.-C.; Liu, Q.-J.; Wang, H.-Y.; Li, Q.; Yao, G.-E.; Xie, P. Importance of lipid ratios for predicting intracranial atherosclerotic stenosis. Lipids Health Dis. 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Song, X.; Lv, Z. Knockdown of circ_0004104 Alleviates Oxidized Low-Density Lipoprotein-Induced Vascular Endothelial Cell Injury by Regulating miR-100/TNFAIP8 Axis. J. Cardiovasc. Pharmacol. 2021, 78, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Sun, B. MicroRNA-532-5p protects against atherosclerosis through inhibiting vascular smooth muscle cell proliferation and migration. Cardiovasc. Diagn. Ther. 2020, 10, 481–489. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, B. Effects of miR-532-5p on human brain microvascular endothelial cells damage induced by ox-LDL via down-regulating CLIC4 expression. Pak. J. Pharm. Sci. 2020, 33, 1435–1441. [Google Scholar] [PubMed]
- Gao, Y.; Li, H.; Zhou, Y.; Lv, H.; Chen, Y. PDCD4 expression in coronary atherosclerosis rat models and its mechanism. Exp. Ther. Med. 2019, 17, 3150–3154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, H.; Ramírez, C.M.; Lee, S.-M.; Hoe, H.-S.; Fernández-Hernando, C.; Kim, J. miR-106b impairs cholesterol efflux and increases Aβ levels by repressing ABCA1 expression. Exp. Neurol. 2012, 235, 476–483. [Google Scholar] [CrossRef]
- Giannella, A.; Castelblanco, E.; Zambon, C.F.; Basso, D.; Hernandez, M.; Ortega, E.; Alonso, N.; Mauricio, D.; Avogaro, A.; Ceolotto, G.; et al. Circulating Small Noncoding RNA Profiling as a Potential Biomarker of Atherosclerotic Plaque Composition in Type 1 Diabetes. Diabetes Care 2023, 46, 551–560. [Google Scholar] [CrossRef]
- Telkoparan-Akillilar, P.; Cevik, D. Identification of miR-17, miR-21, miR-27a, miR-106b and miR-222 as endoplasmic reticulum stress-related potential biomarkers in circulation of patients with atherosclerosis. Mol. Biol. Rep. 2021, 48, 3503–3513. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.D.; Xu, X.; Cai, Y.-B.; Zhu, Z.-X.; Zhu, L.; Ren, K. LINC00657 promoted pyroptosis in THP-1-derived macrophages and exacerbated atherosclerosis via the miR-106b-5p/TXNIP/NLRP3 axis. Int. J. Biol. Macromol. 2023, 253 Pt 4, 126953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Xu, J.; Kong, X.; Zou, L. Up-regulated miR-106b inhibits ox-LDL-induced endothelial cell apoptosis in atherosclerosis. Braz. J. Med. Biol. Res. 2020, 53, e8960. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Wang, F.; Li, H.; Yin, Z.; Sandip, C.; Lou, Y.; Wang, Y.; Chen, C.; Wang, D.W. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 2013, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Sima, X.; Sun, H.; Zhou, P.; You, C. A Potential Polymorphism in the Promoter of Let-7 is Associated With an Increased Risk of Intracranial Aneurysm: A Case-Control Study. Medicine 2015, 94, e2267. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, F.; Zhou, X.; Zeng, S.; Zhan, Q.; Yuan, M.; Yang, Q.; Liu, Y.; Xia, J. Promoter hypomethylation of microRNA223 gene is associated with atherosclerotic cerebral infarction. Atherosclerosis 2017, 263, 237–243. [Google Scholar] [CrossRef]
- Prabhakar, P.; Chandra, S.R.; Christopher, R. Circulating microRNAs as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Ageing 2017, 46, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, K.; Chen, X. Inflammation-regulatory microRNAs: Valuable targets for intracranial atherosclerosis. J. Neurosci. Res. 2019, 97, 1242–1252. [Google Scholar] [CrossRef]
- Xuan, J.; Shang, M.; Li, X. Serum MicroRNA-137 Serves as a Novel Biomarker for Cerebral Atherosclerosis Diagnosis and Cerebrovascular Event Prediction. J. Cardiovasc. Pharmacol. 2021, 78, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-N.; Zhong, L.-Y.; Sun, Y.-H.; Wang, C.; Ru, W.-J.; Liu, R.-Z.; Dai, W.; Xie, X.-M.; Li, S.-D. Downregulation of lncRNA SNHG16 inhibits vascular smooth muscle cell proliferation and migration in cerebral atherosclerosis by targeting the miR-30c-5p/SDC2 axis. Heart Vessels 2022, 37, 1085–1096. [Google Scholar] [CrossRef]
- Jiang, H.; Ding, Y.; Wu, L.; Jiang, C.; Wang, C. The roles and diagnostic value of miRNA-1246 in the serum of patients with intracranial aneurysms. Transl. Neurosci. 2022, 13, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, F. Circulating miR-130a-3p is elevated in patients with cerebral atherosclerosis and predicts 2-year risk of cerebrovascular events. BMC Neurol. 2022, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Hurford, R.; Feng, X.; Chan, K.L.; Wolters, F.J.; Li, L.; Soo, Y.O.; Wong, K.S.L.; Mok, V.C.; Leung, T.W.; et al. Intracranial arterial stenosis in Caucasian versus Chinese patients with TIA and minor stroke: Two contemporaneous cohorts and a systematic review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 590–597. [Google Scholar] [CrossRef] [PubMed]
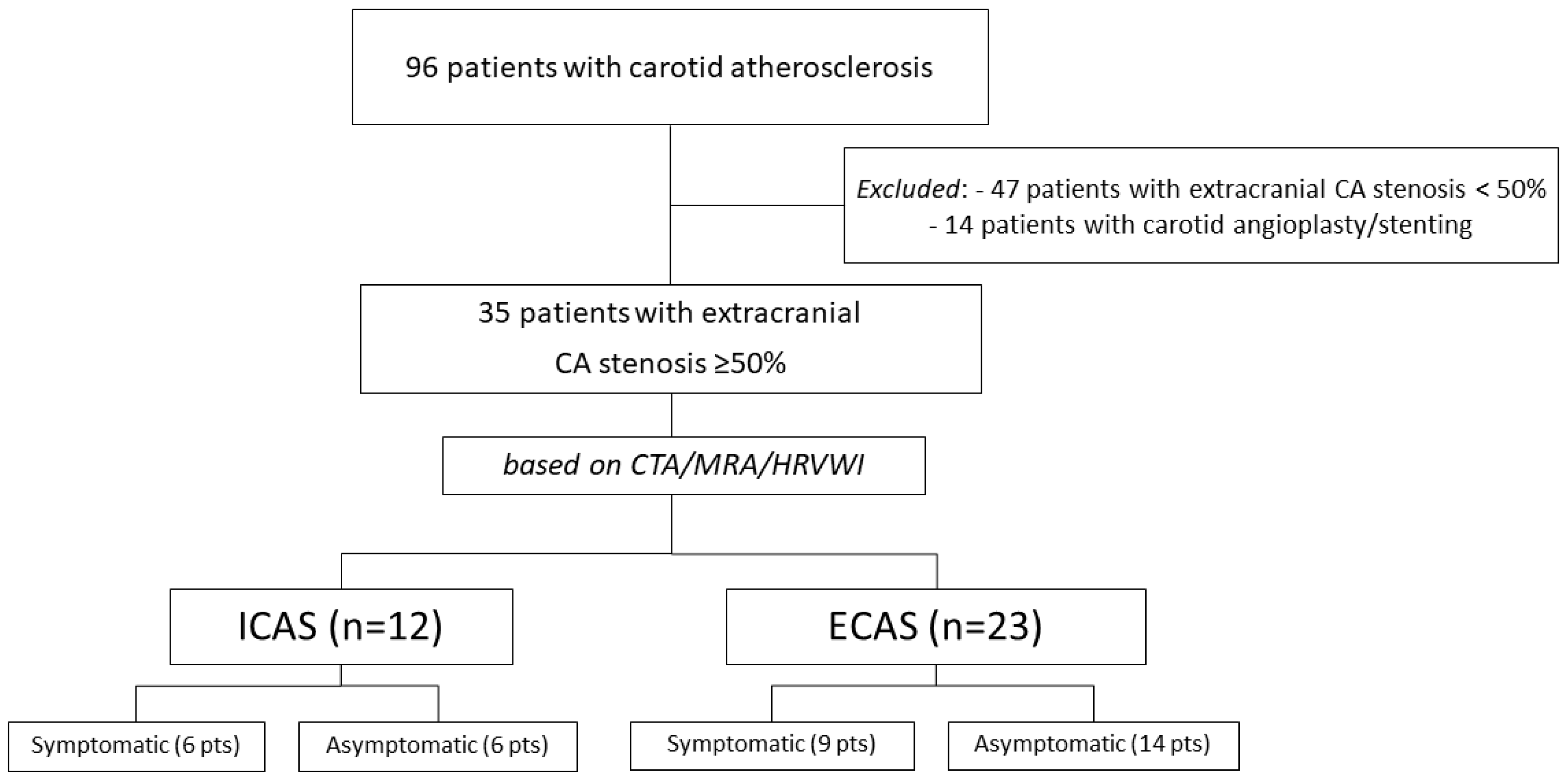

| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Clinical and Laboratory Findings | ICAS | p-Value 2 | |
|---|---|---|---|
| No (n = 23) 1 | Yes (n = 12) 1 | ||
| Age, years | 67 (62, 72) | 65 (59.5, 66.5) | 0.16 |
| M | 9 (39%) | 10 (83%) | 0.033 |
| Smoking | 10 (43%) | 5 (42%) | >0.9 |
| DM | 8 (35%) | 3 (25%) | 0.8 |
| Stroke | 9 (39%) | 6 (50%) | 0.5 |
| Stenosis 3,% | 75 (70, 80) | 70 (70, 90) | >0.9 |
| BMI, kg/m2 | 27.0 (26.0, 34.0) | 27.0 (25.7, 28.4) | 0.2 |
| RBC, ×1012/L | 4.60 (4.34, 4.90) | 4.76 (4.51, 5.26) | 0.12 |
| WBC, ×109/L | 7.90 (6.30, 9.40) | 8.20 (6.95, 8.55) | >0.9 |
| Platelets, ×109/L | 216 (188, 281) | 228 (200, 263) | 0.7 |
| LDL-C, mmol/L | 1.83 (1.34, 2.72) | 1.78 (1.20, 2.23) | 0.23 |
| miR-712/205-5p | 23.9 (21.0, 27.9) | 30.0 (24.2, 34.8) | 0.034 |
| miR-712/205-3p | 30.20 (29.47, 30.49) | 27.87 (26.20, 29.81) | 0.037 |
| miR-106b-3p | 31.48 (28.82, 33.38) | 32.26 (27.41, 34.34) | 0.5 |
| miR-106b-5p | 30.03 (29.68, 30.26) | 30.74 (30.24, 31.33) | 0.006 |
| miR-146a-3p | 26.11 (24.19, 28.25) | 27.57 (23.27, 28.68) | 0.8 |
| miR-146a-5p | 28.58 (26.19, 29.47) | 30.06 (29.66, 30.99) | <0.001 |
| miR-100-3p | 28.8 (25.4, 31.0) | 24.7 (22.2, 29.0) | 0.073 |
| miR-100-5p | 26.7 (23.3, 29.9) | 26.7 (23.1, 32.3) | 0.7 |
| miR-200c-3p | 29.64 (28.36, 30.01) | 30.21 (29.25, 33.33) | 0.2 |
| miR-200c-5p | 25.3 (24.6, 31.6) | 32.5 (25.1, 34.4) | 0.019 |
| miR-532-3p | 24.9 (22.8, 27.6) | 28.4 (24.8, 31.8) | 0.071 |
| miR-532-5p | 28.4 (26.2, 30.7) | 27.7 (24.8, 30.2) | 0.5 |
| miR-126-3p | 50 (39, 53) | 55 (45, 59) | 0.073 |
| miR-126-5p | 36.0 (34.6, 40.1) | 36.2 (32.6, 39.7) | 0.7 |
| Non-Smokers (n = 13) 1 | Smokers (n = 10) 1 | p-Value 2 | |
|---|---|---|---|
| Stroke | 3 (23%) | 6 (60%) | 0.10 |
| M | 2 (15%) | 7 (70%) | 0.013 |
| Age, years | 69 (64, 72) | 62 (49, 69) | 0.067 |
| LDL-C, mmol/L | 2.34 (1.44, 3.49) | 1.53 (1.37, 2.34) | 0.2 |
| BMI, kg/m2 | 26.0 (24.4, 27.0) | 26.5 (25.9, 34.0) | 0.5 |
| DM | 5 (38%) | 3 (30%) | >0.9 |
| miR-712-5p | 23.8 (22.4, 27.6) | 26.4 (20.5, 29.6) | 0.8 |
| miR-712-3p | 30.2 (29.9, 30.5) | 30.2 (27.7, 30.6) | >0.9 |
| miR-106b-3p | 31.71 (30.38, 33.74) | 31.13 (26.76, 31.78) | 0.3 |
| miR-106b-5p | 29.98 (29.64, 30.23) | 30.09 (29.91, 30.26) | 0.4 |
| miR-146a-3p | 26.0 (25.0, 28.1) | 27.2 (23.6, 28.7) | 0.8 |
| miR-146a-5p | 28.13 (26.19, 28.63) | 29.18 (28.58, 29.68) | 0.15 |
| miR-100-3p | 28.8 (23.7, 31.3) | 28.9 (27.4, 29.8) | 0.8 |
| miR-100-5p | 26.3 (23.3, 28.5) | 30.3 (23.3, 31.3) | 0.3 |
| miR-200c-3p | 29.32 (28.56, 30.51) | 29.54 (27.68, 29.72) | 0.6 |
| miR-200c-5p | 25.3 (24.6, 28.2) | 27.5 (24.6, 31.7) | 0.7 |
| miR-494-3p | 32.7 (26.8, 38.5) | 32.0 (29.6, 33.9) | >0.9 |
| miR-494-5p | 35 (31, 41) | 36 (32, 42) | >0.9 |
| miR-532-3p | 23.62 (22.49, 26.67) | 25.45 (24.01, 28.48) | 0.2 |
| miR-532-5p | 28.2 (26.3, 30.7) | 28.8 (26.2, 30.7) | >0.9 |
| miR-126-3p | 50 (43, 53) | 47 (39, 52) | 0.8 |
| miR-126-5p | 36.7 (35.0, 39.4) | 35.3 (33.9, 40.5) | 0.8 |
| (ID) Age/Gender | Stroke Status | Clinical Characteristics | ICAS | ECAS | № of Aff. Cerebr. Art. |
|---|---|---|---|---|---|
| (1) 58/M | Asymptomatic | Smoking, dyslipidemia, AH | 60–65% (left ICA) | Occlusion (right ICA), 60–65% (left ICA), 20–25% (left VA) | 4 |
| (2) 57/M | Asymptomatic | Smoking, AH | 60–70% (ICA bilaterally) | 50% (left ICA), 25% (right VA), 60–70% (left VA) | 5 |
| (3) 60/M | Asymptomatic | Dyslipidemia, AH | 50% (left ICA) 60% (left MCA), 70% (PCA bilateral), 50% (BA) | 90% (left ICA), 60% (left CCA), 15% (right ICA), 75% (right VA), 65% (left VA) | 10 |
| (4) 66/F | Symptomatic | AH | 70% (right MCA) | 50% (right ICA) | 2 |
| (5) 61/M | Asymptomatic | DM, smoking, AH | 50–55% (ICA bilateral), 95% (left VA), 95% (BA) | 75–80% (right CCA), 45% (right ICA), 25% (left CCA), 50% (left ICA) | 8 |
| (6) 57/M | Symptomatic | DM, smoking, AH | 45–50% (right ICA), 25–30% (left ICA) | 50% (left ICA), 65–75% (right ICA), occlusion (left VA) | 5 |
| (7) 65/M | Asymptomatic | AH | 70% (right MCA) | 90% (right ICA), 70% (left ICA) | 3 |
| (8) 65/F | Symptomatic | AH | 60% (left MCA) | 30–35% (right ICA), 40–45% (left ICA) | 3 |
| (9) 60/M | Symptomatic | AH | 20–25% (left ICA), 80% (right MCA) | 25–30% (left VA), 35% (right ICA), 40% (left ICA) | 5 |
| (10) 69/M | Symptomatic | Smoking, dyslipidemia, AH | 20% (BA), 50% (ICA bilateral), 70–75% (right MCA), 50% (left ACA), 60% (right VA), 30% (left VA) | 65% (right ICA), 50% (left ICA), 45% (right CCA), 50% (left CCA), 35% (right VA), 25% (left VA) | 13 |
| (11) 68/M | Symptomatic | DM, dyslipidemia, AH | 40–45% (ICA bilaterally) | 60% (left ICA), 50% (right ICA), 25% (right CCA), 15% (left CCA) | 6 |
| (12) 70/M | Asymptomatic | AH | 30–40% (ICA bilateraly) | 70% (right ICA), 30–40% (both VA) | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanashyan, M.M.; Raskurazhev, A.A.; Shabalina, A.A.; Mazur, A.S.; Annushkin, V.A.; Kuznetsova, P.I.; Illarioshkin, S.N.; Piradov, M.A. Differential Pattern of Circulating MicroRNA Expression in Patients with Intracranial Atherosclerosis. Biomedicines 2025, 13, 514. https://doi.org/10.3390/biomedicines13020514
Tanashyan MM, Raskurazhev AA, Shabalina AA, Mazur AS, Annushkin VA, Kuznetsova PI, Illarioshkin SN, Piradov MA. Differential Pattern of Circulating MicroRNA Expression in Patients with Intracranial Atherosclerosis. Biomedicines. 2025; 13(2):514. https://doi.org/10.3390/biomedicines13020514
Chicago/Turabian StyleTanashyan, Marine M., Anton A. Raskurazhev, Alla A. Shabalina, Andrey S. Mazur, Vladislav A. Annushkin, Polina I. Kuznetsova, Sergey N. Illarioshkin, and Mikhail A. Piradov. 2025. "Differential Pattern of Circulating MicroRNA Expression in Patients with Intracranial Atherosclerosis" Biomedicines 13, no. 2: 514. https://doi.org/10.3390/biomedicines13020514
APA StyleTanashyan, M. M., Raskurazhev, A. A., Shabalina, A. A., Mazur, A. S., Annushkin, V. A., Kuznetsova, P. I., Illarioshkin, S. N., & Piradov, M. A. (2025). Differential Pattern of Circulating MicroRNA Expression in Patients with Intracranial Atherosclerosis. Biomedicines, 13(2), 514. https://doi.org/10.3390/biomedicines13020514








