Isofraxidin Attenuates Lipopolysaccharide-Induced Cytokine Release in Mice Lung and Liver Tissues via Inhibiting Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
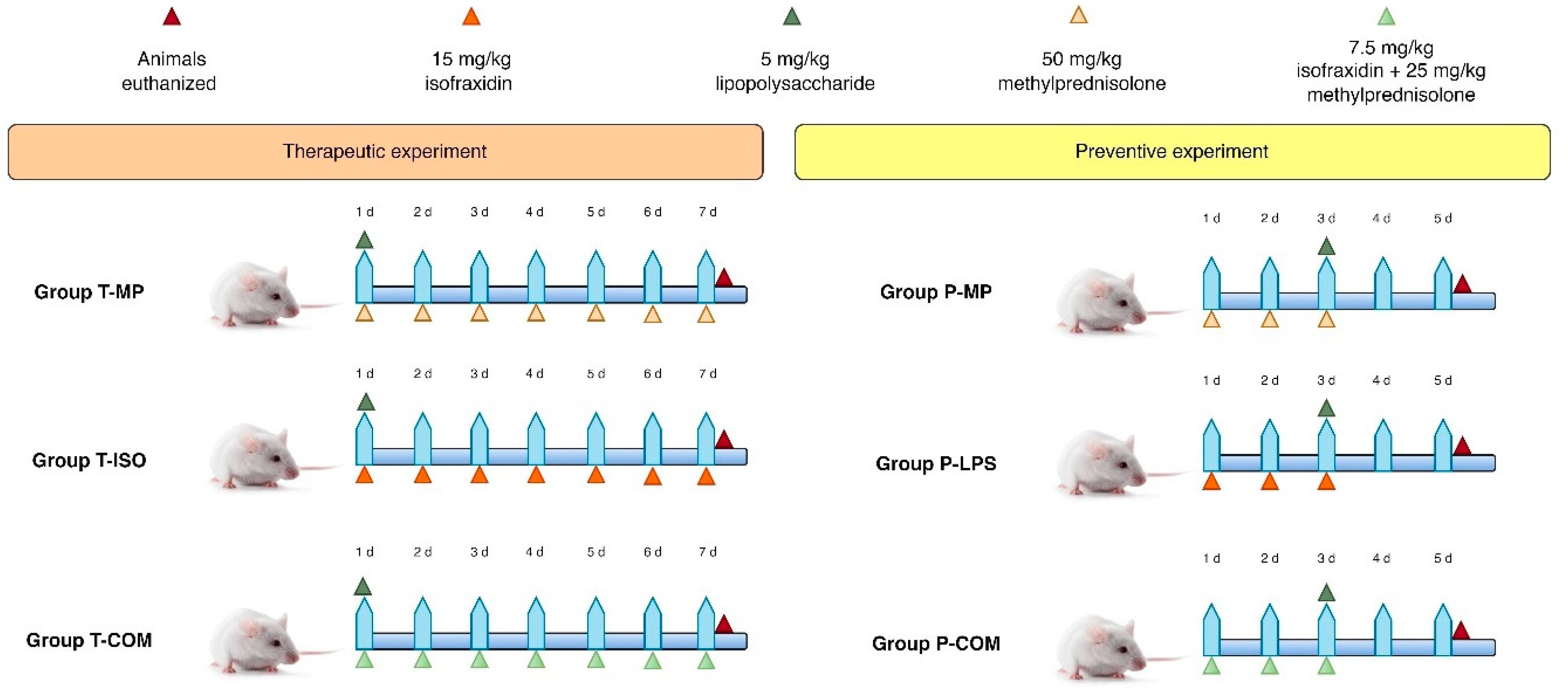
2.2. Study Design
2.3. Clinical Observations and Animal Care
2.4. Laboratory Analysis of Mice Serum
2.5. Histopathology of Lung and Liver Tissue
2.6. Sample Size Calculation and Animal Randomization
2.7. Ethical Consideration
2.8. Statistical Analysis
3. Results
3.1. Prevention of CRS by Isofraxidin With and Without Methylprednisolone
3.2. Therapeutic Effects of Isofraxidin With and Without Methylprednisolone on CRS Inflammatory and Oxidative Stress Markers
3.3. Lung Histopathology
3.4. Liver Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Mihalcik, L.; Bussiere, J.L.; Jawa, V.; Lepherd, M.; Mytych, D.T.; Sharma, A.; Sirivelu, M.P.; Everds, N. 12.21—Immunogenicity and Immune-Related Adverse Drug Reactions. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2018; pp. 498–517. [Google Scholar]
- Bugelski, P.J.; Achuthanandam, R.; Capocasale, R.J.; Treacy, G.; Bouman-Thio, E. Monoclonal antibody-induced cytokine-release syndrome. Expert Rev. Clin. Immunol. 2009, 5, 499–521. [Google Scholar] [CrossRef]
- Haggerty, H.G.; Price, K.D.; Shenton, J.M. 11.37—Immunotoxicology of Biopharmaceutics☆. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2018; pp. 826–851. [Google Scholar]
- Gribble, E.J.; Sivakumar, P.V.; Ponce, R.A.; Hughes, S.D. Toxicity as a result of immunostimulation by biologics. Expert Opin. Drug Metab. Toxicol. 2007, 3, 209–234. [Google Scholar] [CrossRef]
- Heink, S.; Yogev, N.; Garbers, C.; Herwerth, M.; Aly, L.; Gasperi, C.; Husterer, V.; Croxford, A.L.; Möller-Hackbarth, K.; Bartsch, H.S.; et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat. Immunol. 2017, 18, 74–85. [Google Scholar] [CrossRef]
- Chen, P.; Tang, Y.; He, W.; Yang, R.; Lan, Z.; Chen, R.; Zhang, P. Potential Pathophysiological Mechanisms Underlying Multiple Organ Dysfunction in Cytokine Release Syndrome. Mediat. Inflamm. 2022, 2022, 7137900. [Google Scholar] [CrossRef]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The Role and Therapeutic Potential of NF-κB Pathway in Severe COVID-19 Patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Kiyani, N.; Stage, K.; Mohammadi, P.; Gravandi, M.M.; Farzaei, M.H.; Echeverría, J. Phytochemicals: Potential Therapeutic Interventions Against Coronavirus-Associated Lung Injury. Front. Pharmacol. 2020, 11, 588467. [Google Scholar] [CrossRef]
- Niu, X.; Xing, W.; Li, W.; Fan, T.; Hu, H.; Li, Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int. Immunopharmacol. 2012, 14, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, X.; Qi, W.; Yan, Y.; Chen, K.; Xue, X.; Xu, X.; Feng, Z.; Pan, X. Isofraxidin inhibits interleukin-1β induced inflammatory response in human osteoarthritis chondrocytes. Int. Immunopharmacol. 2018, 64, 238–245. [Google Scholar] [CrossRef]
- Lu, W.; Cui, Y.; Zhang, L. Isofraxidin exerts anti-diabetic, antilipidemic, and antioxidant effects and protects renal tissues via inhibition of NF-ĸB in Streptozotocin-induced diabetic rats. Mol. Cell. Toxicol. 2022. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 1 December 2024).
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Al-Naimi, M.S.; Abu-Raghif, A.R.; Fawzi, H.A. Novel therapeutic effects of rifaximin in combination with methylprednisolone for LPS-induced oxidative stress and inflammation in mice: An in vivo study. Toxicol. Rep. 2024, 13, 101808. [Google Scholar] [CrossRef]
- Aal-Aaboda, M.; Abu Raghif, A.R.; Hadi, N.R. Effect of Lipopolysaccharide from Rhodobacter sphaeroides on Inflammatory Pathway and Oxidative Stress in Renal Ischemia/Reperfusion Injury in Male Rats. Arch. Razi Inst. 2021, 76, 911–922. [Google Scholar] [CrossRef]
- Aal-Aaboda, M.S.; Abu Raghif, A.R.; Hadi, N.R. Renoprotective Potential of the Ultra-Pure Lipopolysaccharide from Rhodobacter Sphaeroides on Acutely Injured Kidneys in an Animal Model. Arch. Razi Inst. 2021, 76, 1755–1764. [Google Scholar] [CrossRef]
- Abed Mansoor, A.F.; Abu Raghif, A.R. Attenuated effects of rivastigmine in induced cytokine storm in mice. J. Emerg. Med. Trauma Acute Care 2022, 2022, 12. [Google Scholar] [CrossRef]
- Liu, L.; Mu, Q.; Li, W.; Xing, W.; Zhang, H.; Fan, T.; Yao, H.; He, L. Isofraxidin protects mice from LPS challenge by inhibiting pro-inflammatory cytokines and alleviating histopathological changes. Immunobiology 2015, 220, 406–413. [Google Scholar] [CrossRef]
- Mansoor, A.F.A.; Raghif, A.R.A.; Al-Sudani, I.M.; Aldabagh, M.A. Therapeutic Effects of Rivastigmine in induced Cytokine Storm in Mice: Dose Standardization. J. Carcinog. 2022, 21, 20–28. [Google Scholar]
- Al-Naimi, M.S.; Abu-Raghif, A.R. Potential therapeutic and ameliorative effects of ramipril alone and in combination with methylprednisolone for the cytokine releasing syndrome in mice: An in vivo study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Khafaji, A.W.; Al-Zubaidy, A.A.; Farhood, I.G.; Fawzi, H.A. Effects of topical isoxsuprine ointment on imiquimod-induced psoriasiform skin inflammation in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 1545–1556. [Google Scholar] [CrossRef]
- Obaid, K.A.; Fawzi, H.A. Evaluation of empagliflozin efficacy as a promising anti-aging treatment in mice: In-vivo study. Pharmacia 2024, 71, 1–9. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Kadhim, H.M. The hepatoprotective effects of the polyphenol-enriched n-butanol fraction of Cnicus benedictus against carbon tetrachloride-induced liver fibrosis in rats: In vivo study. Toxicol. Rep. 2025, 14, 101850. [Google Scholar] [CrossRef]
- Ramírez, K.; Quesada-Yamasaki, D.; Jaime, F.-T.C. A Protocol to Perform Systemic Lipopolysacharide (LPS) Challenge in Rats. Odovtos 2019, 21, 53–66. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Kadhim, H.M. Effects of a crude extract of Echinops mosulensis on an induced Parkinson’s disease model in mice. Pharmacia 2024, 71, 1–14. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Ito, Y.; Kikuchi, M.; Imamura, Y.; Hayashi, T. Validation studies on blood collection from the jugular vein of conscious mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 345–351. [Google Scholar]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Singh, H.; Bishen, K.A.; Garg, D.; Sukhija, H.; Sharma, D.; Tomar, U. Fixation and fixatives: Roles and functions—A short review. Dent. J. Adv. Stud. 2019, 7, 051–055. [Google Scholar] [CrossRef]
- Malkoç, M.; Patan, H.; Yaman, S.Ö.; Türedi, S.; Kerimoğlu, G.; Kural, B.V.; Örem, A. l-theanine alleviates liver and kidney dysfunction in septic rats induced by cecal ligation and puncture. Life Sci. 2020, 249, 117502. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Y.; Li, W.; Mu, Q.; Li, H.; Yao, H.; Zhang, H. Protective effects of Isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015, 24, 432–439. [Google Scholar] [CrossRef]
- Chen, G.; Song, X.; Lin, D.; Xu, P. Isofraxidin Alleviates Myocardial Infarction Through NLRP3 Inflammasome Inhibition. Inflammation 2020, 43, 712–721. [Google Scholar] [CrossRef]
- He, S.; Zhang, T.; Wang, Y.Y.; Yuan, W.; Li, L.; Li, J.; Yang, Y.Y.; Wu, D.M.; Xu, Y. Isofraxidin attenuates dextran sulfate sodium-induced ulcerative colitis through inhibiting pyroptosis by upregulating Nrf2 and reducing reactive oxidative species. Int. Immunopharmacol. 2024, 128, 111570. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Q.L.; Wu, L.H.; Jawaid, P.; Jiao, Y.F.; Kadowaki, M.; Kondo, T. Isofraxidin, a potent reactive oxygen species (ROS) scavenger, protects human leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in p53-independent manner. Apoptosis Int. J. Program. Cell Death 2014, 19, 1043–1053. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; Halawi, M.; Alamer, E. Investigation of Antistress and Antidepressant Activities of Synthetic Curcumin Analogues: Behavioral and Biomarker Approach. Biomedicines 2022, 10, 2385. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Rahman, S.U.; Ahmad, M.; Almehmadi, M.; Abdulaziz, O.; Allahyani, M.; Alsaiari, A.A.; et al. Synthetic Mono-Carbonyl Curcumin Analogues Attenuate Oxidative Stress in Mouse Models. Biomedicines 2022, 10, 2597. [Google Scholar] [CrossRef]
- Alarabei, A.A.; Abd Aziz, N.A.L.; Ab Razak, N.I.; Abas, R.; Bahari, H.; Abdullah, M.A.; Hussain, M.K.; Abdul Majid, A.M.S.; Basir, R. Immunomodulating Phytochemicals: An Insight Into Their Potential Use in Cytokine Storm Situations. Adv. Pharm. Bull. 2024, 14, 105–119. [Google Scholar] [CrossRef]
- Singh, M.; Lo, S.H.; Dubey, R.; Kumar, S.; Chaubey, K.K.; Kumar, S. Plant-Derived Natural Compounds as an Emerging Antiviral in Combating COVID-19. Indian J. Microbiol. 2023, 63, 429–446. [Google Scholar] [CrossRef]
- Cao, H.J.; Tan, R.R.; He, R.R.; Tang, L.P.; Wang, X.L.; Yao, N.; Duan, W.J.; Hu, Y.A.; Yao, X.S.; Kurihara, H. Sarcandra glabra Extract Reduces the Susceptibility and Severity of Influenza in Restraint-Stressed Mice. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 236539. [Google Scholar] [CrossRef]
- Jin, L.; Ying, Z.H.; Yu, C.H.; Zhang, H.H.; Yu, W.Y.; Wu, X.N. Isofraxidin ameliorated influenza viral inflammation in rodents via inhibiting platelet aggregation. Int. Immunopharmacol. 2020, 84, 106521. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, Z.; Zhang, L.; Liu, Y.; Ding, H.; Yin, S. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017, 8, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Kong, Y.Y.; Ren, J.J.; Huang, T.J.; Wang, Y.Q.; Liu, L.X. Kidney and lung tissue modifications after BDL-induced liver injury in mice are associated with increased expression of IGFBPrP1 and activation of the NF-κB inflammation pathway. Int. J. Clin. Exp. Pathol. 2020, 13, 192–202. [Google Scholar] [PubMed]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. How to Increase Cellular Glutathione. Antioxidants 2023, 12, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, P.; Mohamed, H.; Myers, B.; Dobossy, L.; Beyries, K.; Trosan, D.; Krebs, F.C.; Miller, V.; Stapelmann, K. GSH Modification as a Marker for Plasma Source and Biological Response Comparison to Plasma Treatment. Appl. Sci. 2020, 10, 2025. [Google Scholar] [CrossRef]
- Marzbani, C.; Bhimaraj, A. Corticosteroids in Immunosuppression. In Pharmacology of Immunosuppression; Eisen, H.J., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 73–84. [Google Scholar]
- Yarnell, E. Herbs for Rheumatoid Arthritis. Altern. Complement. Ther. 2017, 23, 149–156. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Naimi, M.S.; Abu-Raghif, A.R.; Mansoor, A.F.A.; Fawzi, H.A. Isofraxidin Attenuates Lipopolysaccharide-Induced Cytokine Release in Mice Lung and Liver Tissues via Inhibiting Inflammation and Oxidative Stress. Biomedicines 2025, 13, 653. https://doi.org/10.3390/biomedicines13030653
Al-Naimi MS, Abu-Raghif AR, Mansoor AFA, Fawzi HA. Isofraxidin Attenuates Lipopolysaccharide-Induced Cytokine Release in Mice Lung and Liver Tissues via Inhibiting Inflammation and Oxidative Stress. Biomedicines. 2025; 13(3):653. https://doi.org/10.3390/biomedicines13030653
Chicago/Turabian StyleAl-Naimi, Marwa Salih, Ahmed R. Abu-Raghif, Ahmed F. Abed Mansoor, and Hayder Adnan Fawzi. 2025. "Isofraxidin Attenuates Lipopolysaccharide-Induced Cytokine Release in Mice Lung and Liver Tissues via Inhibiting Inflammation and Oxidative Stress" Biomedicines 13, no. 3: 653. https://doi.org/10.3390/biomedicines13030653
APA StyleAl-Naimi, M. S., Abu-Raghif, A. R., Mansoor, A. F. A., & Fawzi, H. A. (2025). Isofraxidin Attenuates Lipopolysaccharide-Induced Cytokine Release in Mice Lung and Liver Tissues via Inhibiting Inflammation and Oxidative Stress. Biomedicines, 13(3), 653. https://doi.org/10.3390/biomedicines13030653






