Abstract
Background/Objectives: Meningiomas are the most common intracranial tumors. Surgery and radiation therapy are the cornerstones of treatment and no standard of care therapy exists for refractory meningiomas. This manuscript aims to provide a comprehensive review of novel diagnostic and therapeutic approaches against these tumors. Methods: A search for the existing literature on systemic therapies for meningiomas was performed on PubMed and a search for presently accruing clinical trials was performed on ClinicalTrials.gov. Results: Systemic treatments, including chemotherapy, somatostatin analogs, anti-hormone therapy, and anti-angiogenic therapy, have been extensively studied with marginal success. Targeted therapies are actively being studied for the treatment of meningiomas, including focal adhesion kinase (FAK), sonic hedgehog signaling pathway, phosphoinositide-3-kinase (PI3K), and cyclin-dependent kinases (CDK) inhibitors. These driver mutations are present only in a subset of meningiomas. In stark contrast, somatostatin receptor 2 (SSTR2) is ubiquitously expressed in meningiomas and was formerly targeted with somatostatin analogs with modest success. Theranostic SSTR2-targeting via [68Ga]DOTATATE for PET imaging and β-emitting [177Lu]DOTATATE for the treatment of meningiomas are currently under active investigation. Conclusions: A nuanced approach is needed for the treatment of refractory meningiomas. Targeted therapies show promise.
Keywords:
meningioma; 177-Lutetium; PRRT; DOTATATE PET; octreotide; NF2; SMO; AKT; vismodegib; abemaciclib; capivasertib 1. Introduction
Meningiomas are the most common adult tumor entity originating in the central nervous system (CNS). Meningiomas account for 41.7% of CNS tumors, occurring in 10.15 per 100,000 of the population, with an incidence on the rise [1]. Meningiomas of any grade occur more frequently in those of non-Hispanic Black ethnicity compared to non-Hispanic White ethnicity [1]. For non-malignant meningiomas, there is a higher incidence among those of non-Hispanic White ethnicity, while a higher incidence of malignant meningiomas is found in non-Hispanic Asian or Pacific Islanders [1]. Further, meningiomas predominantly occur in females and in those of advanced age >65 years [1,2,3]. Sex-specific molecular differences may exist, with increased aggressive biologic behavior in the meningiomas of female patients with chromosome X loss [4]. Spinal meningiomas account for 40.2% of all spine tumors among patients >20 years of age [1]. Furthermore, anatomic location has apparent molecular underpinnings. NF2 mutant meningiomas typically occur along the convexity, while smoothened (SMO) mutated meningiomas are located within the olfactory groove [5]. TRAF7 and AKT1 alterations are associated with the anterior skull base [5]. The only established acquired risk factor for the development of meningiomas is ionizing radiation, as evidenced in atomic bomb survivors and those who received radiation to the scalp for tinea capitis or intracranial tumors [6,7]. While obesity has also been associated with meningioma development, the risk of meningioma with exogenous hormones remains controversial [7,8,9]. Genetic risk factors include predisposition syndromes associated with driver mutations, including NF2 (NF2-related schwannomatosis), PTCH1 (Gorlin Syndrome), PTEN (Cowden Syndrome), SMARCE1, and BAP1 [10,11].
Meningioma patients present with a wide range of neurological symptoms, and meningiomas are provisionally diagnosed with brain imaging [12]. While computed tomography (CT) may identify calcification that negatively correlates with growth rate, magnetic resonance imaging (MRI) T2 hyperintensity positively correlates with growth rate in meningiomas [13]. In addition, meningiomas may demonstrate peritumoral edema, particularly in the angiomatous, microcystic, and secretory histologic subtypes [14]. Meningiomas are typically homogeneous, contrast-enhancing, and may reveal a characteristic dural tail [14]. CT or MR angiography is often obtained to evaluate the surrounding vasculature and the need for pre-operative embolization to minimize intraoperative blood loss [15].
Preceding the 2021 WHO Classification of CNS Tumors, meningioma grade was solely reliant on histopathological criteria, including mitotic count, sheeting, hypercellularity, small cells, prominent nucleoli, and spontaneous necrosis [16]. The 2021 Classification of CNS Tumors was revised to incorporate molecular criteria into the classification of meningiomas to form the basis of an integrated diagnostic framework. With these refined criteria, molecular alterations in the telomerase reverse transcriptase (TERT) promoter region and homozygous loss of the cyclin-dependent kinase inhibitor (CDKN)2A/2B are now classified with a Grade 3 designation [17,18,19,20]. TERT maintains telomere length and chromosomal stability and portends a worse prognosis when alterations are present in meningiomas [19]. CDKN2A encodes for the tumor suppressor p16(INK4A) and p14(ARF) proteins that inhibit cell growth and division [21]. Mutations in this gene are associated with inferior survival when observed in meningiomas [21,22]. Whereas Grade 1 meningiomas typically exhibit an indolent growth pattern, atypical CNS WHO Grade 2 meningiomas (4.3% of meningiomas) have a higher risk of recurrence [23]. Anaplastic CNS Grade 3 meningiomas account for 1.2% of meningiomas, are biologically aggressive and carry a risk of metastasis within and outside the CNS [2,23]. Grade 3 meningiomas of the papillary histologic subtype can harbor PBRM1 loss [24]. Grade 3 meningiomas with either rhabdoid or papillary morphology can be associated with BAP1 loss, which is often germline in nature [25].
Maximal safe resection is the cornerstone of treatment for tissue diagnosis and to provide symptomatic relief in meningiomas [12]. The extent of resection (EOR) is an integral component of correlative outcomes but may be prohibited by the tumor’s proximity to critical neurovascular structures. In 1957, the Simpson grade was developed to assess recurrence risk, though of late, its relevance in the modern era has been called into question [26,27]. For Grade 1 meningiomas, a gross total resection (GTR) can be curative and may obviate the need for additional treatment in the future. For Grade 2 meningiomas, adjunctive immediate or delayed radiation therapy (RT) may be indicated or safely omitted, but the optimal timing fueled by conflicting results remains and remains under debate [28,29]. To this end, the NRG BN-003 (NCT03180268) and ROAM/EORTC-1308 (ISRCTN71502099) Phase II investigations are currently underway to provide more insight into this unknown aspect [30]. For Grade 3 meningiomas, GTR and upfront RT (60 Gy delivered over 30 fractions) decrease local recurrence [31]. Dose escalation was investigated in the Phase II MARCIE trial for Grade 2 or 3 meningiomas with the addition of a carbon ion boost (18 Gy delivered over six fractions) to IMRT or fractionated stereotactic RT (50.4 Gy over 28 fractions). Although 3-year progression-free survival (PFS) and local control rates were >80%, the study was terminated due to increased toxicity [32].
Beyond surgery and radiation therapy, no effective therapies exist, and the prognosis is unvaryingly poor for refractory meningiomas with 6-month PFS (PFS-6) rates of 26–29% [33,34]. Numerous studies investigating different therapeutic approaches for recurrent meningiomas have been futile. Systemic therapies, including irinotecan, temozolomide, hydroxyurea, trabectedin, anti-hormonal therapy, and anti-angiogenic therapy, among others, have been extensively studied for use in recurrent meningiomas with marginal success [35,36,37,38,39,40,41,42,43,44,45]. One such anti-angiogenic therapy is bevacizumab, a biologic vascular endothelial growth factor (VEGF) inhibitor, which can be considered in the recurrent setting when surgery or radiation therapy is not feasible, with PFS-6 ranging from 43.8% to 87% [43,45,46]. However, anti-angiogenic therapy carries a risk of both non-fatal and fatal intratumoral hemorrhage, which can also be observed with sunitinib, a small-molecule biologic inhibitor [42,45].
Somatostatin receptor type 2 (SSTR), a G-protein-coupled receptor, is ubiquitously expressed in meningioma cells and regulates cell proliferation. For these reasons, SSTR as an actionable target has garnered considerable interest for the treatment of meningiomas (15, 16). SSTR is identifiable by immunohistochemistry or via positron emission tomography using [68Ga]-radiolabeled oxodotreotate (DOTATATE PET) [47,48]. SSTR-targeted therapy includes somatostatin analogs such as octreotide (an injectable somatostatin analog) and β-emitting [177Lu]-armed DOTATATE (Lutathera®) peptide receptor radionuclide therapy (PRRT) [49]. Octreotide activates SHP1 and SHP2 and inhibits the PI3K/Akt pathway, collectively mediating direct antitumor effects [50,51,52]. Octreotide monotherapy showed promise for use in meningiomas in early investigations with 44% PFS-6, but this success was not confirmed in subsequent studies [53,54,55,56,57]. This failure has been attributed to intracellular escape mechanisms [58]. To overcome these challenges, the addition of everolimus, a mammalian target of the rapamycin (mTOR) small-molecule inhibitor, to octreotide was studied in the CEVOREM trial [51]. PI3K/Akt/mTOR pathway targeting was justified by its putative role in the tumorigenesis of meningiomas that may stem from NF2 inactivation [59,60,61,62,63,64,65]. Although the use of this combinatorial therapy suggested growth rate reductions, results from this trial were somewhat disappointing, with PFS-6 reaching only 55% [51]. Notably, none of the 14 enrolled patients with available analyzed tissue carried mutations in the Pi3K or AKT pathway [51].
A short follow-up of PFS-6 has been adopted as the current benchmark endpoint for refractory meningioma treatment trials [33,66,67]. However, the reliability of PFS-6 as a predictor of outcomes and biological behavior is debatable, given the frequent insidious growth rates observed in meningiomas [68]. Accordingly, PFS-6 as a point of reference shows modest outcomes, and the Response Assessment in Neuro-Oncology (RANO) Group recently advised that new benchmarks are warranted to inform of future trial success [34]. A three-dimensional volume growth rate (3DVGR) may represent a better predictor of outcome [52,69,70,71].
In this review, we aim to describe novel diagnostic tools and therapeutics for meningiomas. To identify relevant publications investigating innovative modalities and detect meningiomas and potential treatments for these tumors, searches of the literature were conducted in the PubMed database and on clinicaltrials.gov. The last search was completed on 26 February 2025. The search strategy adopted for this review using PubMed included the term “meningioma”. The search strategy adopted for this review using clinicaltrials.gov included the term “meningioma” with an “active” status. The references for accepted publications were analyzed for additional articles not identified in the initial search.
2. Novel Diagnostics
2.1. Grading Criteria
Meningioma grading and classification will continue to be enhanced with molecular features to offset the subjectivity of histopathologic interpretation. Additional guidance from cIMPACT-NOW released in the most recent version 8 addresses the subset of CNS WHO Grade 1 meningiomas that do not follow the natural course of a benign tumor and the subset of CNS WHO Grade 2 or 3 meningiomas that are not biologically aggressive [16,72]. The latest cIMPACT-NOW suggests that histomorphologic CNS WHO Grade 1 meningiomas harboring chromosomal aberrations with 1p deletion and 22q deletion and/or NF2 oncogenic variants should be assigned as Grade 2 meningioma [16,73]. As we cultivate our understanding of these molecular underpinnings, the reliability of outcome predictions for meningiomas will also improve.
To date, there are no reliable predictors of response to radiation therapy. For this reason, improvements to meningioma risk stratification are warranted [74]. Chen et al. developed a 34-gene expression prognostic biomarker using unsupervised analysis to uncover patterns that alter risk in a discovery cohort of meningiomas [75]. Their results suggest that treatment decision-making, including response to radiation therapy, can be enhanced for ~30% of patients [75]. Their 34-gene expression biomarker improved risk stratification for Grade 2 meningiomas and could inform of those Grade 2 meningiomas that are suitable for close observation versus those that carry a risk of recurrence and may benefit from upfront radiation [75]. While this biomarker tool was validated on both retro- and prospective clinical samples, real-world decision-making based on this approach has yet to be investigated [75].
DNA methylation profiling attempts to overcome the limitations of the existing inter-rater variability in grading meningiomas. It is an objective approach to delineating meningiomas into discrete molecular groups with distinct biological behavior more reliably than the existing WHO grading criteria [76,77,78]. A DNA methylation-based classification model developed by Landry et al. was found to be superior to existing WHO 2021 grading criteria for predicting 5-year PFS in meningioma patients [79]. When integrated with EOR, DNA methylation profiling shows promise in predicting a subset of benign meningiomas that may recur [72]. Molecular modeling with DNA methylation, RNA expression, and copy number alterations may additionally advise on meningiomas that are likely to be radioresistant, including meningiomas within the proliferative molecular group or the NF2 loss of function during hypoxia [78,80]. In stark contrast, meningiomas categorized into the NF2 wildtype and immunogenic molecular groups appear to benefit from adjuvant radiation therapy [78]. This integrated model was recently validated and is available for public use [81]. However, a major disadvantage to DNA methylation profiling is its limited availability and protracted timing for results [76].
2.2. Imaging Modalities
[68Ga]DOTATATE PET has emerged as a formidable diagnostic tool for use in meningiomas. DOTATATE PET produces a high-imaging contrast due to high uptake in SSTR2-positive lesional tissue compared to the low background uptake in the brain and calvarium [82,83,84]. While it has yet to receive FDA approval for use in meningiomas, DOTATATE PET is approved and widely used for neuroendocrine tumors (NETs) in clinical practice [82,83,85]. Early investigations show that compared to MRI, DOTATATE PET has higher sensitivity and specificity for detecting meningiomas in both newly diagnosed and recurrent settings [86,87,88]. It excels at detecting viable tumor otherwise unrecognized or indistinguishable from dural scar on conventional MRI [89,90,91,92]. Moreover, cases of false positive detection of tumors with MRI can occur and lead to unnecessary treatment with radiation and unwarranted toxicity.
In addition to surveillance, DOTATATE PET may optimize the surgical planning approach in estimating the EOR, specifically in cases of intraosseous extension [93,94]. Moreover, it may offer benefits within radiation planning with more accurate target delineation volumes over conventional MRI that may overestimate lesional tissue in anatomically difficult locations or areas affected by post-operative scars [89,95,96,97]. Consequently, the added precision offered by DOTATATE PET can reduce the risk of unnecessary radiation exposure to normal tissue, including alopecia, optic neuropathy, and radiation necrosis, among others. Lastly, DOTATATE PET may provide predictive information for somatostatin-directed therapies and, when coupled with therapeutic radionuclides, can be harnessed for theranostics [58,98]. Based on these encouraging data, both the RANO Working Group and the National Comprehensive Cancer Network [99] guidelines have incorporated the consideration of DOTATATE PET for surveillance and surgical or radiation planning [100,101,102]. Advanced diagnostics for meningiomas are ongoing in active clinical trials (Table 1).

Table 1.
Current active diagnostic clinical trials for meningiomas in the United States.
3. Novel Therapeutics
3.1. Small-Molecule Inhibitors
Advances in molecular diagnostics have led to investigations for the subset of meningiomas with actionable targets. The European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets (ESCAT) assigns a score from I to V, which is used to describe the level of evidence for targeted therapy against meningioma [102]. ESCAT I is assigned to “ready for routine use” target–drug combinations with prospectively established clinical activity in meningioma. ESCAT II represents “investigational drugs” with known anti-meningioma activity but with an unknown magnitude of benefits, whereas ESCAT III and IV represent the efficacy of “hypothetical targets” in either other tumors (III) or in preclinical models (IV) [102]. To date, there are no molecular targets for meningiomas that have achieved a level of ESCAT I. At best, mTOR pathway activation and NF2 alterations have attained an ESCAT II designation [102]. The Phase II Alliance A071401 (NCT02523014) trial is currently underway for recurrent/progressive meningiomas with driver mutations [103]. In this trial, meningioma patients harboring actionable targets are treated with small-molecule inhibitors, including neurofibromatosis-2 (NF2) mutations with GSK2256098, which is a focal adhesion kinase (FAK) inhibitor; SMO (ESCAT III) or PTCH1 mutations with vismodegib; CDK (ESCAT IV) or NF2 alterations with abemaciclib; and AKT (ESCAT III), PI3K, or PTEN mutations with capivasertib (Figure 1) [102,103,104].
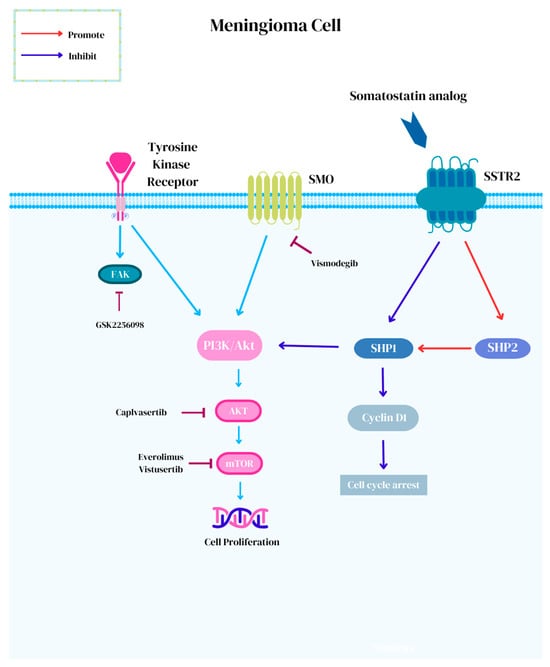
Figure 1.
Novel small-molecule targeted therapies for the treatment of meningiomas.
NF2 is a tumor suppressor on chromosome 22q12 encoding for the Merlin protein that plays an inhibitory role in the FAK, MAPK, and PI3K/Akt/mTOR signaling pathways that regulate cellular activity [59,64,65,102,105,106]. Approximately 50–60% of meningiomas harbor NF2 alterations resulting in a two-hit complete loss of function and are implicated in the tumorigenesis of sporadic meningiomas [107,108,109]. The presence of NF2 alterations can be present across meningioma of all grades (Grade 1= 37%, Grade 2 = 60%, and Grade 3 = 69%) and show genomic instability [110,111]. Early results for treatment with GSK2256098 in the NF2 arm of the Phase II Alliance A071401 (NCT02523014) trial showed partial response in 3% (1/36) and stable disease in 67% (24/36), with PFS-6 reaching 83% (10/12) in Grade 1 meningiomas and 33% (8/24) in Grade 2 and 3 meningiomas [103]. The most common toxicity associated with GSK2256098 is gastrointestinal adverse events [112].
Approximately 5% of meningiomas harbor SMO mutations [107,111]. SMO is a G-coupled protein receptor that encodes for a receptor that activates the sonic hedgehog signaling (SHH) pathway and results in subsequent differentiation and proliferation [102,107,111]. Meningiomas with SMO mutations generally follow a benign course [111]. Vismodegib, a small-molecule inhibitor of SMO, is efficacious for the treatment of basal cell carcinoma and CNS tumors, including SHH medulloblastomas [113,114,115,116,117]. Common adverse events include muscle spasms, alopecia, dysgeusia, diarrhea, fatigue, nausea, weight, and appetite loss [113,118]. In the NCI-MATCH ECOG-ACRIN Trial, meaningful responses were reported in meningioma patients with SMOPro641Ala and PTCHGlu947Ter alterations [119]. Resistance mechanisms to vismodegib most commonly seen among meningiomas, include neurotrophin signaling, downstream signaling changes, a lack of transduction of the SHH pathway, and the SMOL412F mutation [111,118,120,121]. The suppressor of fused homolog (SUFU) is a negative regulator in the SHH pathway downstream to SMO [122]. As such, the upstream inhibition of SMO is therefore ineffective against SUFU mutant tumors [115,123].
Cyclin-dependent kinases (CDKs) regulate the cell cycle and apoptosis [22]. The homozygous deletion of CDKN2A/2B is now accepted as diagnostic criteria to classify meningiomas as WHO Grade 3, irrespective of histologic findings [17,22,68]. Abemaciclib is a brain-penetrating CDK4/6 inhibitor and has shown benefits in preclinical investigations [102,124,125]. The Phase II (NCT03071874) study investigating the use of vistusertib, the oral dual mTORC1/mTORC2 inhibitor in NF2-related schwannomatosis patients with either progressive or symptomatic meningiomas failed to meet the primary endpoint of a 20% volume decrease [126]. Six percent (1/8) achieved partial response and ninety percent (52/59) of patients achieved stable disease but this was poorly tolerated at 125 mg twice daily [126].
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), AKT serine/threonine kinase 1 (AKT1), and phosphatase and TENsin homolog-deleted (PTEN) mutations were found to be capable of being targeted with capivasertib, an AKT inhibitor, in the NCI-MATCH trial. Meningiomas with AKT1 mutations typically show chromosomal stability and are biologically benign [111]. The AKT1 p.E17K mutation accounts for 10% of meningiomas and induces the constitutive activation of downstream oncogenic cellular effects by localizing from the cytoplasm to the plasma membrane [107,111,127]. Capivasertib has shown efficacy in hormone receptor-positive breast cancer and early investigations demonstrate response for the treatment of meningiomas [128,129,130]. Lastly, clear cell meningiomas harbor a loss of SMARCE1 [131]. SMARCE1 is associated with SWI/SNF chromatin remodeling and may be amenable to small-molecule inhibitors [131,132].
Driver mutations vary across meningiomas and may be lacking entirely. Accordingly, a universal targeted therapy such as SSTR2 would provide the advantage of treating virtually the entire cohort of progressive and high-risk meningiomas.
3.2. Somatostatin Analogs
Somatostatin analogs carrying high affinity to SSTR2, are an attractive treatment option for meningiomas and maintain a targeted yet generalizable and scalable approach to treatment [49]. Octreotide is an injectable somatostatin analog that activates SHP1 and SHP2 and inhibits the PI3K/Akt pathway, which collectively mediates direct antitumor effects [50,51,52]. Octreotide showed modest success for use in meningiomas in early investigations with 44% PFS-6, but this success was not reproducible and not substantiated in confirmatory studies [53,54]. This failure can potentially be attributed to intracellular escape mechanisms [58]. A nuanced approach with SSTR2-targeted theranostics has gained traction for use in meningiomas. [177Lu]DOTATATE (Lutathera ®) is a β-emitting radionuclide somatostatin analog that forms reactive oxygen species that trigger single-strand breaks within tumor DNA and ultimately, lethality [94,133]. Akin to antibody–drug conjugates, bystander, and crossfire effects may play a role in radionuclide therapy [134]. Following binding to the SSTR receptor and endocytosis, cytotoxicity affects adjacent tumor cells, but the restricted range can limit the crossfire effect and reduce the impact on neighboring normal brain tissue [49,134,135]. Additionally, the γ-photon-emitting capacity of [177Lu] can be leveraged for the pharmacokinetic imaging of the radiopharmaceutical in vivo [136]. [177Lu]DOTATATE achieved regulatory approval for use in advanced midgut NETs after showing prolonged PFS in the NETTER-1 Phase 3 trial [137]. Preliminary evidence demonstrates efficacy against meningiomas, which varies by grade with PFS-6 rates of 94%, 48%, and 0% in Grade 1, 2, and 3 SSTR2-positive meningiomas, respectively [49,135]. On this basis, PRRT is currently under investigational use in meningiomas and is jointly recommended within the EANM/EANO/RANO/SNMMI practice guidelines for consideration in cases of recurrence following standards of care given its low toxicity profile and proven efficacy in other cancers [58,135,138,139].
3.3. Brachytherapy
Brachytherapy is a form of implantable radiation therapy placed intraoperatively. Both iodine-125 (I-125) and cesium 131 (Cs-131) have been investigated for use in meningiomas and have shown a survival advantage [140,141,142]. However, the adoption of brachytherapy in clinical practice has been hindered by adverse events, including radiation necrosis, infection, wound dehiscence, and seed migration with high rates of reoperation [141,142,143]. Cs-131 carries the advantage of a reduced half-life compared to I-125 and the reduced risk of radiation necrosis [143]. GammaTile® is a form of Cs-131 brachytherapy comprised of bioresorbable collagen tiles that conform to the surgical cavity to deliver uniform radiation without direct contact with normal brain parenchyma [144]. Results from a study investigating the use of GammaTile® in meningioma patients showed that median overall survival was 26 months with 10.5% of patients experiencing radiation necrosis [145].
3.4. Systemic Radionuclide Therapy
With respect to ongoing trials, interim analysis from a Phase II study investigating [177Lu]DOTATATE in recurrent intracranial meningiomas showed encouraging findings, with 14% (2/14) of patients experiencing a > 25% reduction in tumor volume and 50% (7/14) achieving PFS-6 with stable disease as the best response (Table 2) [58]. The European Organization for Research and Treatment of Cancer (EORTC) is initiating the LUMEN-1 trial (NCT06326190), the first randomized study to compare [177Lu]DOTATATE versus the investigator’s choice (e.g., octreotide, everolimus, bevacizumab, sunitinib, hydroxyurea, or observation) in SSTR2-positive meningiomas. Braat et al. suggested that an intra-arterial route of administration for PRRT may saturate SSTR2 in meningiomas, resulting in increased tumor uptake and radiation dose absorption [49,146]. In Grade 1 or Grade 2 meningiomas, there is an apparent 6-month lag in antitumoral activity, but responses can persevere for up to 18 months following treatment initiation [147]. Myelosuppression is the most common treatment-related toxicity [135]. Other toxicities include fatigue, anemia, alopecia, and lymphopenia, which correlate with the number of prior systemic lines of therapy [47].

Table 2.
Current active therapeutic clinical trials for meningiomas in the United States.
3.5. Immunotherapy
Immunotherapy has shown success and meaningful benefits in solid tumors, including melanoma and non-small-cell lung cancer, and is actively being studied in recurrent meningioma [148,149]. Though the programmed death-ligand 1 (PD-L1) is upregulated in meningiomas with higher expression levels rising with meningioma grade, the predictive role of PD-L1 expression remains notional [102,150,151]. The efficacy of immunotherapy may be limited by low-tumor mutational burden and the immunosuppressive tumor microenvironment [150,152]. Accordingly, immunotherapy has shown varied success in treating meningiomas [150,151]. In a Phase 2 study (NCT03279692), a PFS-6 rate of 48% and median PFS of 7.6 months was achieved in Grade 2 and 3 patients with recurrent meningioma treated with pembrolizumab [151]. Similarly, in another Phase 2 trial, prolonged survival was observed in 8% (2/25) of patients with recurrent Grade 2 and 3 meningioma with high mutational burden, though the primary endpoint was not met with a PFS-6 rate of 42.4%. [150].
4. Conclusions
To date, there are no effective therapies for refractory meningiomas. Recent advances in genetic alterations have gained traction in the grading of meningiomas and carry prognostic relevance. Newly uncovered molecular alterations, including TERT mutations and the homozygous loss of CDKN2A/2B, have improved upon the prior histopathologic grading schema. Growing evidence shows that chromosomal aberrations will further refine the grading schema for meningiomas. Additionally, the identification of driver mutations may reveal new therapeutic potential with small-molecule inhibitors. Further investigations with therapeutic targeted therapy, including [177Lu]DOTATATE and immunotherapy, may yield promise for future success in treating these tumors.
5. Future Directions
Artificial intelligence (AI) using radiomics may inform on recurrence risk [153]. Further development within the realm of radiomics, along with gene expression biomarkers, may also refine existing risk stratification models [75]. Currently, assertations regarding the use of AI in the diagnosis and management of meningiomas remain theoretical and speculative in nature but will certainly remain an area of active investigation in the future.
Author Contributions
C.A.Y.—study concept, data collection, analysis, interpretation, manuscript drafting, revision and final approval. M.Z.—data collection and final approval. M.A.S.-G.—data collection and final approval. D.O.K.—manuscript drafting, revision, and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Definition |
| AI | Artificial intelligence |
| AKT1 | AKT serine/threonine kinase 1 |
| BAP1 | BRCA1 associated protein 1 |
| CDK | Cyclin-dependent kinase |
| CDKN | Cyclin-dependent kinase inhibitor |
| CNS | Central nervous system |
| CT | Computed tomography |
| EOR | Extent of resection |
| EORTC | European Organization for Research and Treatment of Cancer |
| ESCAT | European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets |
| FAK | Focal adhesion kinase |
| GEP | Gastroenteropancreatic |
| GTR | Gross total resection |
| IMRT | Intensity-modulated radiation therapy |
| MRI | Magnetic resonance imaging |
| NF2 | Neurofibromatosis type 2 |
| NCT | National Clinical Trial |
| NCCN | National Comprehensive Cancer Network |
| NET | Neuroendocrine tumor |
| OS | Overall survival |
| PBRM1 | Polybromo-1 |
| PD-L1 | Programmed death-ligand 1 |
| DOTATATE PET | Positron emission tomography with [68Ga]DOTATATE |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide 3-kinase |
| PRRT | Peptide receptor radionuclide therapy |
| PTEN | Phosphatase and TENsin |
| RANO | Response Assessment in Neuro-Oncology |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RT | Radiation therapy |
| SHH | Sonic hedgehog signaling |
| SMARCE1 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 |
| SMO | Smoothened |
| SUFU | Suppressor of fused homolog |
| 3DVGR | Three-dimensional volume growth rate |
| TERT | Telomerase reverse transcriptase |
| WHO | World Health Organization |
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro-Oncology 2024, 26, vi1–vi85. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.D.; Lin, J.L.; Deng, X.Y.; Li, W.; Li, D.D.; Yin, B.; Lin, J.; Zhang, N.; Sheng, H.S. Trends in intracranial meningioma incidence in the United States, 2004–2015. Cancer Med. 2019, 8, 6458–6467. [Google Scholar] [CrossRef]
- Walsh, K.M.; Price, M.; Neff, C.; Komisarow, J.M.; Wimberly, C.E.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. The joint impacts of sex and race/ethnicity on incidence of grade 1 versus grades 2-3 meningioma across the lifespan. Neuro-Oncol. Adv. 2023, 5, i5–i12. [Google Scholar] [CrossRef]
- Berghaus, N.; Hielscher, T.; Savran, D.; Schrimpf, D.; Maas, S.L.N.; Preusser, M.; Weller, M.; Acker, T.; Herold-Mende, C.; Wick, W.; et al. Meningiomas: Sex-Specific Differences and Prognostic Implications of a Chromosome X Loss. Neuro Oncol. 2024, 27, noae239. [Google Scholar] [CrossRef]
- Boetto, J.; Plu, I.; Ducos, Y.; Blouin, A.; Teranishi, Y.; Brainbank Neuro, C.E.B.N.N.; Bizzotto, S.; Kalamarides, M.; Peyre, M. Normal meninges harbor oncogenic somatic mutations in meningioma-driver genes. Acta Neuropathol. 2023, 146, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Behrens, G.; Schmid, D.; Schlecht, I.; Fischer, B.; Leitzmann, M.F. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology 2015, 85, 1342–1350. [Google Scholar] [CrossRef]
- Hijiya, N.; Hudson, M.M.; Lensing, S.; Zacher, M.; Onciu, M.; Behm, F.G.; Razzouk, B.I.; Ribeiro, R.C.; Rubnitz, J.E.; Sandlund, J.T.; et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007, 297, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Hoisnard, L.; Laanani, M.; Passeri, T.; Duranteau, L.; Coste, J.; Zureik, M.; Froelich, S.; Weill, A. Risk of intracranial meningioma with three potent progestogens: A population-based case-control study. Eur. J. Neurol. 2022, 29, 2801–2809. [Google Scholar] [CrossRef]
- Dresser, L.; Yuen, C.A.; Wilmington, A.; Walker, M.; Vogel, T.J.; Merrell, R.T.; Kamson, D.O. Estrogen hormone replacement therapy in incidental intracranial meningioma: A growth-rate analysis. Sci. Rep. 2020, 10, 17960. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef]
- Kerr, K.; Qualmann, K.; Esquenazi, Y.; Hagan, J.; Kim, D.H. Familial Syndromes Involving Meningiomas Provide Mechanistic Insight Into Sporadic Disease. Neurosurgery 2018, 83, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Zeng, L.; Liang, P.; Jiao, J.; Chen, J.; Lei, T. Will an Asymptomatic Meningioma Grow or Not Grow? A Meta-analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2015, 76, 341–347. [Google Scholar] [CrossRef] [PubMed]
- El-Abtah, M.E.; Murayi, R.; Lee, J.; Recinos, P.F.; Kshettry, V.R. Radiological Differentiation Between Intracranial Meningioma and Solitary Fibrous Tumor/Hemangiopericytoma: A Systematic Literature Review. World Neurosurg. 2023, 170, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, H.G.; Richter, J.K.; Sahm, F.; Morel, C.; Krayenbuehl, N.; Rushing, E.J.; von Deimling, A.; Valavanis, A.; Weller, M. Post-operative cardiovascular complications and time to recurrence in meningioma patients treated with versus without pre-operative embolization: A retrospective cohort study of 741 patients. J. Neurooncol 2018, 140, 659–667. [Google Scholar] [CrossRef]
- Sahm, F.; Aldape, K.D.; Brastianos, P.K.; Brat, D.J.; Dahiya, S.; von Deimling, A.; Giannini, C.; Gilbert, M.R.; Louis, D.N.; Raleigh, D.R.; et al. cIMPACT-NOW Update 8: Clarifications on molecular risk parameters and recommendations for WHO grading of meningiomas. Neuro Oncol. 2024, 27, 319–330. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Lee, A.; Jentoft, M.; Quinones-Hinojosa, A.; Chaichana, K.L. The prognostic significance of TERT promoter mutations in meningioma: A systematic review and meta-analysis. J. Neuro-Oncol. 2019, 142, 1–10. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv370. [Google Scholar] [CrossRef]
- Spiegl-Kreinecker, S.; Lotsch, D.; Neumayer, K.; Kastler, L.; Gojo, J.; Pirker, C.; Pichler, J.; Weis, S.; Kumar, R.; Webersinke, G.; et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018, 20, 1584–1593. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Patil, V.; Liu, J.; Dogan, H.; Tabatabai, G.; Yefet, L.S.; Behling, F.; Hoffman, E.; Bunda, S.; Yakubov, R.; et al. Increased mRNA expression of CDKN2A is a transcriptomic marker of clinically aggressive meningiomas. Acta Neuropathol. 2023, 146, 145–162. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef]
- Williams, E.A.; Wakimoto, H.; Shankar, G.M.; Barker, F.G., 2nd; Brastianos, P.K.; Santagata, S.; Sokol, E.S.; Pavlick, D.C.; Shah, N.; Reddy, A.; et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. 2020, 140, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Santagata, S. BAP1 mutations in high-grade meningioma: Implications for patient care. Neuro Oncol. 2017, 19, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Bir, S.C.; Maiti, T.K.; Konar, S.K.; Missios, S.; Guthikonda, B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J. Neurosurg. 2017, 126, 201–211. [Google Scholar] [CrossRef]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Sievers, P.; Weber, D.C.; Weller, M.; van den Bent, M.J.; Mair, M.J.; Kros, J.M.; Carparrotti, F.; von Deimling, A.; Salvador, V.F.; et al. Independent prognostic impact of DNA methylation class and chromosome 1p loss in WHO grade 2 and 3 meningioma undergoing adjuvant high-dose radiotherapy: Comprehensive molecular analysis of EORTC 22042-26042. Acta Neuropathol. 2023, 146, 837–840. [Google Scholar] [CrossRef]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018, 129, 35–47. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Javadpour, M.; Haylock, B.J.; Young, B.; Gillard, H.; Vinten, J.; Bulbeck, H.; Das, K.; Farrell, M.; Looby, S.; et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: Study protocol for a randomised controlled trial. Trials 2015, 16, 519. [Google Scholar] [CrossRef]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int. J. Radiat Oncol. Biol. Phys. 2020, 106, 790–799. [Google Scholar] [CrossRef]
- Deng, M.Y.; Maas, S.L.N.; Hinz, F.; Karger, C.P.; Sievers, P.; Eichkorn, T.; Meixner, E.; Hoegen-Sassmannshausen, P.; Horner-Rieber, J.; Lischalk, J.W.; et al. Efficacy and toxicity of bimodal radiotherapy in WHO grade 2 meningiomas following subtotal resection with carbon ion boost: Prospective phase 2 MARCIE trial. Neuro Oncol. 2024, 26, 701–712. [Google Scholar] [CrossRef]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro Oncol. 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Kotecha, R.; Akdemir, E.Y.; Kutuk, T.; Ilgin, C.; Ahluwalia, M.S.; Bi, W.L.; Blakeley, J.; Dixit, K.S.; Dunn, I.F.; Galanis, E.; et al. Benchmarking the Efficacy of Salvage Systemic Therapies for Recurrent Meningioma: A RANO Group Systematic Review and Meta-analysis to Guide Clinical Trial Design. Neuro Oncol. 2025, 27, noaf009. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.P.; Gentili, F.; Macdonald, D.R.; Hariharan, S.; Cruz, C.R.; Abrey, L.E. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J. Neurosurg. 2002, 97, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Loven, D.; Hardoff, R.; Sever, Z.B.; Steinmetz, A.P.; Gornish, M.; Rappaport, Z.H.; Fenig, E.; Ram, Z.; Sulkes, A. Non-resectable slow-growing meningiomas treated by hydroxyurea. J. Neuro-Oncol. 2004, 67, 221–226. [Google Scholar] [CrossRef]
- Chamberlain, M.C. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J. Neuro-Oncol. 2012, 107, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Schrell, U.M.; Rittig, M.G.; Anders, M.; Koch, U.H.; Marschalek, R.; Kiesewetter, F.; Fahlbusch, R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J. Neurosurg. 1997, 86, 840–844. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J. Neurooncol 2006, 78, 271–276. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Temozolomide for treatment-resistant recurrent meningioma. Neurology 2004, 62, 1210–1212. [Google Scholar] [CrossRef]
- Preusser, M.; Silvani, A.; Le Rhun, E.; Soffietti, R.; Lombardi, G.; Sepulveda, J.M.; Brandal, P.; Brazil, L.; Bonneville-Levard, A.; Lorgis, V.; et al. Trabectedin for recurrent WHO grade 2 or 3 meningioma: A randomized phase II study of the EORTC Brain Tumor Group (EORTC-1320-BTG). Neuro Oncol. 2022, 24, 755–767. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015, 17, 116–121. [Google Scholar] [CrossRef]
- Kumthekar, P.; Grimm, S.A.; Aleman, R.T.; Chamberlain, M.C.; Schiff, D.; Wen, P.Y.; Iwamoto, F.M.; Gursel, D.B.; Reardon, D.A.; Purow, B.; et al. A multi-institutional phase II trial of bevacizumab for recurrent and refractory meningioma. Neuro-Oncol. Adv. 2022, 4, vdac123. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, K.; Katiyar, V.; Tandon, V.; Agarwal, D.; Singh, M.; Chandra, S.P.; Suri, A.; Kale, S.S.; Mahapatra, A.K. The role of mifepristone in the management of meningiomas: A systematic review of literature. Neurol. India 2019, 67, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Iwamoto, F.M.; Rudnick, J.D.; Norden, A.D.; Lee, E.Q.; Drappatz, J.; Omuro, A.; Kaley, T.J. Atypical and anaplastic meningiomas treated with bevacizumab. J. Neuro-Oncol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B., 3rd; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A., 3rd; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neuro-Oncol. 2016, 129, 281–288. [Google Scholar] [CrossRef]
- Seystahl, K.; Stoecklein, V.; Schuller, U.; Rushing, E.; Nicolas, G.; Schafer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.C.; et al. Somatostatin receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro Oncol. 2016, 18, 1538–1547. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Y.; Wang, Y.; Liu, L.; Lou, J.; Deng, Y.; Zhao, P.; Shao, A. Clinical Significance of Somatostatin Receptor (SSTR) 2 in Meningioma. Front. Oncol. 2020, 10, 1633. [Google Scholar] [CrossRef] [PubMed]
- Severi, S.; Grassi, I.; Bongiovanni, A.; Nicolini, S.; Marini, I.; Arpa, D.; Ranallo, N.; Azzali, I.; Di Iorio, V.; Sarnelli, A.; et al. Peptide Receptor Radionuclide Therapy in Advanced Refractory Meningiomas: Efficacy and Toxicity in a Long Follow-up. J. Nucl. Med. 2024, 65, 1409–1415. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Zhang, J.; Laupheimer, S.; Paez-Pereda, M.; Erneux, C.; Florio, T.; Pagotto, U.; Stalla, G.K. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006, 66, 1576–1582. [Google Scholar] [CrossRef]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyriere, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef]
- Graillon, T.; Defilles, C.; Mohamed, A.; Lisbonis, C.; Germanetti, A.L.; Chinot, O.; Figarella-Branger, D.; Roche, P.H.; Adetchessi, T.; Fuentes, S.; et al. Combined treatment by octreotide and everolimus: Octreotide enhances inhibitory effect of everolimus in aggressive meningiomas. J. Neuro-Oncol. 2015, 124, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Simo, M.; Argyriou, A.A.; Macia, M.; Plans, G.; Majos, C.; Vidal, N.; Gil, M.; Bruna, J. Recurrent high-grade meningioma: A phase II trial with somatostatin analogue therapy. Cancer Chemother. Pharmacol. 2014, 73, 919–923. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Glantz, M.J.; Fadul, C.E. Recurrent meningioma: Salvage therapy with long-acting somatostatin analogue. Neurology 2007, 69, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Kimmel, D.W.; Burch, P.A.; Cascino, T.L.; Giannini, C.; Wu, W.; Buckner, J.C. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol. 2011, 13, 530–535. [Google Scholar] [CrossRef]
- Norden, A.D.; Ligon, K.L.; Hammond, S.N.; Muzikansky, A.; Reardon, D.A.; Kaley, T.J.; Batchelor, T.T.; Plotkin, S.R.; Raizer, J.J.; Wong, E.T.; et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 2015, 84, 280–286. [Google Scholar] [CrossRef]
- Jensen, L.R.; Maier, A.D.; Lomstein, A.; Graillon, T.; Hrachova, M.; Bota, D.; Ruiz-Patino, A.; Arrieta, O.; Cardona, A.F.; Ruda, R.; et al. Somatostatin analogues in treatment-refractory meningioma: A systematic review with meta-analysis of individual patient data. Neurosurg. Rev. 2022, 45, 3067–3081. [Google Scholar] [CrossRef] [PubMed]
- Kurz, S.C.; Zan, E.; Cordova, C.; Troxel, A.B.; Barbaro, M.; Silverman, J.S.; Snuderl, M.; Zagzag, D.; Kondziolka, D.; Golfinos, J.G.; et al. Evaluation of the SSTR2-targeted Radiopharmaceutical 177Lu-DOTATATE and SSTR2-specific 68Ga-DOTATATE PET as Imaging Biomarker in Patients with Intracranial Meningioma. Clin. Cancer Res. 2024, 30, 680–686. [Google Scholar] [CrossRef]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. mTORC1 inhibitors suppress meningioma growth in mouse models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef]
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol. Cell Biol. 2009, 29, 4250–4261. [Google Scholar] [CrossRef]
- Johnson, M.D.; Okedli, E.; Woodard, A.; Toms, S.A.; Allen, G.S. Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells. J. Neurosurg. 2002, 97, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Wick, W.; Gutmann, D.H.; Mawrin, C. The mTOR signaling pathway as a treatment target for intracranial neoplasms. Neuro Oncol. 2015, 17, 189–199. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Stivison, E.; Beauchamp, R.; Han, S.; Li, H.; Wallace, M.R.; Gusella, J.F.; Stemmer-Rachamimov, A.O.; Ramesh, V. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol. Cancer Res. 2012, 10, 649–659. [Google Scholar] [CrossRef]
- Graillon, T.; Tabouret, E.; Salgues, B.; Horowitz, T.; Padovani, L.; Appay, R.; Farah, K.; Dufour, H.; Regis, J.; Guedj, E.; et al. Innovative treatments for meningiomas. Rev. Neurol. 2023, 179, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Pinker, B.; Barciszewska, A.M. mTOR Signaling and Potential Therapeutic Targeting in Meningioma. Int. J. Mol. Sci. 2022, 23, 1978. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2019, 21, 26–36. [Google Scholar] [CrossRef]
- Huang, R.Y.; Unadkat, P.; Bi, W.L.; George, E.; Preusser, M.; McCracken, J.D.; Keen, J.R.; Read, W.L.; Olson, J.J.; Seystahl, K.; et al. Response assessment of meningioma: 1D, 2D, and volumetric criteria for treatment response and tumor progression. Neuro Oncol. 2019, 21, 234–241. [Google Scholar] [CrossRef]
- Wang, J.Z.; Landry, A.P.; Raleigh, D.R.; Sahm, F.; Walsh, K.M.; Goldbrunner, R.; Yefet, L.S.; Tonn, J.C.; Gui, C.; Ostrom, Q.T.; et al. Meningioma: International Consortium on Meningiomas consensus review on scientific advances and treatment paradigms for clinicians, researchers, and patients. Neuro Oncol. 2024, 26, 1742–1780. [Google Scholar] [CrossRef]
- Graillon, T.; Ferrer, L.; Siffre, J.; Sanson, M.; Peyre, M.; Peyriere, H.; Mougel, G.; Autran, D.; Tabouret, E.; Figarella-Branger, D.; et al. Role of 3D volume growth rate for drug activity evaluation in meningioma clinical trials: The example of the CEVOREM study. Neuro Oncol. 2021, 23, 1139–1147. [Google Scholar] [CrossRef]
- Evers, S.; Verbaan, D.; Sanchez, E.; Peerdeman, S. 3D Volumetric Measurement of Neurofibromatosis Type 2-Associated Meningiomas: Association Between Tumor Location and Growth Rate. World Neurosurg. 2015, 84, 1062–1069. [Google Scholar] [CrossRef]
- Tabouret, E.; Furtner, J.; Graillon, T.; Silvani, A.; Le Rhun, E.; Soffietti, R.; Lombardi, G.; Sepulveda-Sanchez, J.M.; Brandal, P.; Bendszus, M.; et al. 3D volume growth rate evaluation in the EORTC-BTG-1320 clinical trial for recurrent WHO grade 2 and 3 meningiomas. Neuro Oncol. 2024, 26, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.P.; Morshed, R.A.; Youngblood, M.W.; Perlow, H.K.; Lucas, C.G.; Patel, A.J.; Palmer, J.D.; Horbinski, C.M.; Magill, S.T.; Chen, W.C.; et al. A targeted gene expression biomarker predicts clinic low-risk meningioma recurrence. Neuro Oncol. 2025, 27, 445–454. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Preusser, M. A 34-gene expression biomarker predicts meningioma outcomes and radiotherapy responses. Neuro Oncol. 2024, 26, 207–208. [Google Scholar] [CrossRef]
- Chen, W.C.; Choudhury, A.; Youngblood, M.W.; Polley, M.C.; Lucas, C.G.; Mirchia, K.; Maas, S.L.N.; Suwala, A.K.; Won, M.; Bayley, J.C.; et al. Targeted gene expression profiling predicts meningioma outcomes and radiotherapy responses. Nat. Med. 2023, 29, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Nowosielski, M.; Galldiks, N.; Iglseder, S.; Kickingereder, P.; von Deimling, A.; Bendszus, M.; Wick, W.; Sahm, F. Diagnostic challenges in meningioma. Neuro Oncol. 2017, 19, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Patil, V.; Landry, A.P.; Gui, C.; Ajisebutu, A.; Liu, J.; Saarela, O.; Pugh, S.L.; Won, M.; Patel, Z.; et al. Molecular classification to refine surgical and radiotherapeutic decision-making in meningioma. Nat. Med. 2024, 30, 3173–3183. [Google Scholar] [CrossRef]
- Landry, A.P.; Wang, J.Z.; Patil, V.; Gui, C.; Yasin, M.; Patel, Z.; Yakubov, R.; Kaloti, R.; Habibi, P.; Wilson, M.; et al. Validation and next-generation update of a DNA methylation-based recurrence predictor for meningioma: A multicenter prospective study. Neuro Oncol. 2024, 27, noae236. [Google Scholar] [CrossRef]
- Patel, B.; Pugazenthi, S.; English, C.W.; Nitturi, V.; Pari, S.S.; Mahlokozera, T.; Leidig, W.A.; Lu, H.C.; Yang, A.; Roberts, K.; et al. NF2 loss-of-function and hypoxia drive radiation resistance in grade 2 meningiomas. J. Natl. Cancer Inst. 2025, 117, djaf022. [Google Scholar] [CrossRef]
- Landry, A.P.; Wang, J.Z.; Liu, J.; Patil, V.; Gui, C.; Patel, Z.; Ajisebutu, A.; Ellenbogen, Y.; Wei, Q.; Singh, O.; et al. Development and validation of a molecular classifier of meningiomas. Neuro Oncol. 2025, 27, noae242. [Google Scholar] [CrossRef] [PubMed]
- Dutour, A.; Kumar, U.; Panetta, R.; Ouafik, L.; Fina, F.; Sasi, R.; Patel, Y.C. Expression of somatostatin receptor subtypes in human brain tumors. Int. J. Cancer 1998, 76, 620–627. [Google Scholar] [CrossRef]
- Dijkstra, B.M.; Motekallemi, A.; den Dunnen, W.F.A.; Jeltema, J.R.; van Dam, G.M.; Kruyt, F.A.E.; Groen, R.J.M. SSTR-2 as a potential tumour-specific marker for fluorescence-guided meningioma surgery. Acta Neurochir. 2018, 160, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Agopiantz, M.; Carnot, M.; Denis, C.; Martin, E.; Gauchotte, G. Hormone Receptor Expression in Meningiomas: A Systematic Review. Cancers 2023, 15, 980. [Google Scholar] [CrossRef]
- Hennrich, U.; Benesova, M. [(68)Ga]Ga-DOTA-TOC: The First FDA-Approved (68)Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef]
- Bashir, A.; Larsen, V.A.; Ziebell, M.; Fugleholm, K.; Law, I. Improved Detection of Postoperative Residual Meningioma with [(68)Ga]Ga-DOTA-TOC PET Imaging Using a High-resolution Research Tomograph PET Scanner. Clin. Cancer Res. 2021, 27, 2216–2225. [Google Scholar] [CrossRef]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Poschl, J.; Schuller, U.; Schichor, C.; Thon, N.; Tonn, J.C. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef]
- Henze, M.; Schuhmacher, J.; Hipp, P.; Kowalski, J.; Becker, D.W.; Doll, J.; Macke, H.R.; Hofmann, M.; Debus, J.; Haberkorn, U. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: First results in patients with meningiomas. J. Nucl. Med. 2001, 42, 1053–1056. [Google Scholar]
- Afshar-Oromieh, A.; Wolf, M.B.; Kratochwil, C.; Giesel, F.L.; Combs, S.E.; Dimitrakopoulou-Strauss, A.; Gnirs, R.; Roethke, M.C.; Schlemmer, H.P.; Haberkorn, U. Comparison of (6)(8)Ga-DOTATOC-PET/CT and PET/MRI hybrid systems in patients with cranial meningioma: Initial results. Neuro Oncol. 2015, 17, 312–319. [Google Scholar] [CrossRef]
- Perlow, H.K.; Nalin, A.P.; Handley, D.; Gokun, Y.; Blakaj, D.M.; Beyer, S.J.; Thomas, E.M.; Raval, R.R.; Boulter, D.; Kleefisch, C.; et al. A Prospective Registry Study of (68)Ga-DOTATATE PET/CT Incorporation Into Treatment Planning of Intracranial Meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 979–985. [Google Scholar] [CrossRef]
- Toner, Y.C.; Ghotbi, A.A.; Naidu, S.; Sakurai, K.; van Leent, M.M.T.; Jordan, S.; Ordikhani, F.; Amadori, L.; Sofias, A.M.; Fisher, E.L.; et al. Systematically evaluating DOTATATE and FDG as PET immuno-imaging tracers of cardiovascular inflammation. Sci. Rep. 2022, 12, 6185. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.R.; Raleigh, D.R.; Gown, A.M.; Thomas, S.; Perry, A.; Tihan, T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015, 130, 441–443. [Google Scholar] [CrossRef]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using (68)Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Tabouret, E.; Johnson, D.R.; Sulman, E.P.; Wen, P.Y.; Preusser, M.; Albert, N.L. Radioligand therapies in meningioma: Evidence and future directions. Neuro Oncol. 2024, 26, S215–S228. [Google Scholar] [CrossRef] [PubMed]
- Ivanidze, J.; Chang, S.J.; Haghdel, A.; Kim, J.T.; RoyChoudhury, A.; Wu, A.; Ramakrishna, R.; Schwartz, T.H.; Cisse, B.; Stieg, P.; et al. [Ga68] Dotatate PET/MRI-Guided Radiosurgical Treatment Planning and Response Assessment in Meningiomas. Neuro Oncol. 2024, 26, 1526–1535. [Google Scholar] [CrossRef]
- Ivanidze, J.; Roytman, M.; Lin, E.; Magge, R.S.; Pisapia, D.J.; Liechty, B.; Karakatsanis, N.; Ramakrishna, R.; Knisely, J.; Schwartz, T.H.; et al. Gallium-68 DOTATATE PET in the Evaluation of Intracranial Meningiomas. J. Neuroimaging 2019, 29, 650–656. [Google Scholar] [CrossRef]
- Mahase, S.S.; Roth O’Brien, D.A.; No, D.; Roytman, M.; Skafida, M.E.; Lin, E.; Karakatsanis, N.A.; Osborne, J.R.; Brandmaier, A.; Pannullo, S.C.; et al. [(68)Ga]-DOTATATE PET/MRI as an adjunct imaging modality for radiation treatment planning of meningiomas. Neuro-Oncol. Adv. 2021, 3, vdab012. [Google Scholar] [CrossRef]
- Akhavanallaf, A.; Joshi, S.; Mohan, A.; Worden, F.P.; Krauss, J.C.; Zaidi, H.; Frey, K.; Suresh, K.; Dewaraja, Y.K.; Wong, K.K. Enhancing precision: A predictive model for (177)Lu-DOTATATE treatment response in neuroendocrine tumors using quantitative (68)Ga-DOTATATE PET and clinicopathological biomarkers. Theranostics 2024, 14, 3708–3718. [Google Scholar] [CrossRef]
- Neuroendocrine and Adrenal Tumors; Version 2.2024; National Comprehensive Cancer Network: Philadelphia, PA, USA, 2024.
- Wen, P.Y.; van den Bent, M.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, M.A.; Zadeh, G.; et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017, 19, 1576–1587. [Google Scholar] [CrossRef]
- Sahm, F.; Bertero, L.; Brandner, S.; Capper, D.; Goldbrunner, R.; Jenkinson, M.D.; Kalamarides, M.; Lamszus, K.; Albert, N.L.; Mair, M.J.; et al. EANO guideline on molecular testing of meningiomas for targeted therapy selection. Neuro Oncol. 2024, 27, noae253. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Twohy, E.L.; Gerstner, E.R.; Kaufmann, T.J.; Iafrate, A.J.; Lennerz, J.; Jeyapalan, S.; Piccioni, D.E.; Monga, V.; Fadul, C.E.; et al. Alliance A071401: Phase II Trial of Focal Adhesion Kinase Inhibition in Meningiomas With Somatic NF2 Mutations. J. Clin. Oncol. 2023, 41, 618–628. [Google Scholar] [CrossRef]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Papi, L.; De Vitis, L.R.; Vitelli, F.; Ammannati, F.; Mennonna, P.; Montali, E.; Bigozzi, U. Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum. Genet. 1995, 95, 347–351. [Google Scholar] [CrossRef]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjold, M.; Collins, V.P.; et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat. Genet. 1994, 6, 180–184. [Google Scholar] [CrossRef]
- Williams, E.A.; Santagata, S.; Wakimoto, H.; Shankar, G.M.; Barker, F.G., 2nd; Sharaf, R.; Reddy, A.; Spear, P.; Alexander, B.M.; Ross, J.S.; et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol. Commun. 2020, 8, 171. [Google Scholar] [CrossRef]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avsar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef]
- Soria, J.C.; Gan, H.K.; Blagden, S.P.; Plummer, R.; Arkenau, H.T.; Ranson, M.; Evans, T.R.; Zalcman, G.; Bahleda, R.; Hollebecque, A.; et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 2016, 27, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Laterra, J.; Yauch, R.L.; Callahan, C.A.; Fu, L.; Holcomb, T.; Stinson, J.; Gould, S.E.; Coleman, B.; et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009, 361, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef]
- Kresbach, C.; Holst, L.; Schoof, M.; Leven, T.; Gobel, C.; Neyazi, S.; Tischendorf, J.; Loose, C.; Wrzeszcz, A.; Yorgan, T.; et al. Intraventricular SHH inhibition proves efficient in SHH medulloblastoma mouse model and prevents systemic side effects. Neuro Oncol. 2024, 26, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, A.; Migden, M.R.; Lewis, K.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; Marmur, E.; Rudin, C.M.; et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J. Am. Acad. Dermatol. 2015, 72, 1021–1026.e1028. [Google Scholar] [CrossRef]
- Findakly, S.; Choudhury, A.; Daggubati, V.; Pekmezci, M.; Lang, U.E.; Raleigh, D.R. Meningioma cells express primary cilia but do not transduce ciliary Hedgehog signals. Acta Neuropathol. Commun. 2020, 8, 114. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Gray, R.J.; Flaherty, K.T.; Chen, A.P.; Li, S.; Wang, V.; McShane, L.M.; Patton, D.R.; Tricoli, J.V.; Williams, P.M.; et al. The NCI-MATCH trial: Lessons for precision oncology. Nat. Med. 2023, 29, 1349–1357. [Google Scholar] [CrossRef]
- Roesler, R.; de Farias, C.B.; Brunetto, A.T.; Gregianin, L.; Jaeger, M.; Nor, C.; Thomaz, A. Possible mechanisms and biomarkers of resistance to vismodegib in SHH medulloblastoma. Neuro Oncol. 2022, 24, 1210–1211. [Google Scholar] [CrossRef]
- Boetto, J.; Bielle, F.; Sanson, M.; Peyre, M.; Kalamarides, M. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro Oncol. 2017, 19, 345–351. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef]
- Kool, M.; Jones, D.T.; Jager, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef]
- Giordano, S.H.; Franzoi, M.A.B.; Temin, S.; Anders, C.K.; Chandarlapaty, S.; Crews, J.R.; Kirshner, J.J.; Krop, I.E.; Lin, N.U.; Morikawa, A.; et al. Systemic Therapy for Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 2612–2635. [Google Scholar] [CrossRef]
- Ni, J.; Kabraji, S.; Xie, S.; Wang, Y.; Pan, P.; He, X.; Liu, Z.; Leone, J.P.; Long, H.W.; Brown, M.A.; et al. p16(INK4A)-deficiency predicts response to combined HER2 and CDK4/6 inhibition in HER2+ breast cancer brain metastases. Nat. Commun. 2022, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Orr, C.C.; Thalheimer, R.D.; Cambillo, J.V.; Beauchamp, R.L.; Shaikh, G.; Muzikansky, A.; Stemmer-Rachamimov, A.; Giovannini, M.; Kalamarides, M.; et al. Prospective phase II trial of the dual mTORC1/2 inhibitor vistusertib for progressive or symptomatic meningiomas in persons with neurofibromatosis 2. Neurooncol Adv. 2023, 5, vdad041. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Bohmer, K.; Lass, U.; Gock, T.; Kalis, K.; et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol. 2013, 126, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Roth, P.; Sahm, F.; Burghardt, I.; Schuknecht, B.; Rushing, E.J.; Regli, L.; Lindemann, J.P.; von Deimling, A. Durable Control of Metastatic AKT1-Mutant WHO Grade 1 Meningothelial Meningioma by the AKT Inhibitor, AZD5363. J. Natl. Cancer Inst. 2017, 109, 1–4. [Google Scholar] [CrossRef]
- John, P.; Waldt, N.; Liebich, J.; Kesseler, C.; Schnabel, S.; Angenstein, F.; Sandalcioglu, I.E.; Scherlach, C.; Sahm, F.; Kirches, E.; et al. AKT1(E17K) -mutated meningioma cell lines respond to treatment with the AKT inhibitor AZD5363. Neuropathol. Appl. Neurobiol. 2022, 48, e12780. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. A plain language summary of the CAPItello-291 study: Capivasertib in hormone receptor-positive advanced breast cancer. Future Oncol. 2024, 20, 2901–2913. [Google Scholar] [CrossRef]
- Sievers, P.; Sill, M.; Blume, C.; Tauziede-Espariat, A.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Dogan, H.; Hartmann, C.; Mawrin, C.; et al. Clear cell meningiomas are defined by a highly distinct DNA methylation profile and mutations in SMARCE1. Acta Neuropathol. 2021, 141, 281–290. [Google Scholar] [CrossRef]
- St Pierre, R.; Collings, C.K.; Same Guerra, D.D.; Widmer, C.J.; Bolonduro, O.; Mashtalir, N.; Sankar, A.; Liang, Y.; Bi, W.L.; Gerkes, E.H.; et al. SMARCE1 deficiency generates a targetable mSWI/SNF dependency in clear cell meningioma. Nat. Genet. 2022, 54, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Delbart, W.; Marin, G.; Stamatopoulos, B.; de Wind, R.; Sirtaine, N.; Demetter, P.; Vercruyssen, M.; Woff, E.; Karfis, I.; Ghanem, G.E.; et al. Disturbing the Redox Balance Using Buthionine Sulfoximine Radiosensitized Somatostatin Receptor-2 Expressing Pre-Clinical Models to Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE. Cancers 2023, 15, 2332. [Google Scholar] [CrossRef]
- Xue, L.Y.; Butler, N.J.; Makrigiorgos, G.M.; Adelstein, S.J.; Kassis, A.I. Bystander effect produced by radiolabeled tumor cells in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Mirian, C.; Duun-Henriksen, A.K.; Maier, A.; Pedersen, M.M.; Jensen, L.R.; Bashir, A.; Graillon, T.; Hrachova, M.; Bota, D.; van Essen, M.; et al. Somatostatin Receptor-Targeted Radiopeptide Therapy in Treatment-Refractory Meningioma: Individual Patient Data Meta-analysis. J. Nucl. Med. 2021, 62, 507–513. [Google Scholar] [CrossRef]
- Frost, S.H.; Frayo, S.L.; Miller, B.W.; Orozco, J.J.; Booth, G.C.; Hylarides, M.D.; Lin, Y.; Green, D.J.; Gopal, A.K.; Pagel, J.M.; et al. Comparative efficacy of 177Lu and 90Y for anti-CD20 pretargeted radioimmunotherapy in murine lymphoma xenograft models. PLoS ONE 2015, 10, e0120561. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177)Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Bailey, D.L.; Willowson, K.P.; Harris, M.; Biggin, C.; Aslani, A.; Lengkeek, N.A.; Stoner, J.; Eslick, M.E.; Marquis, H.; Parker, M.; et al. (64)Cu Treatment Planning and (67)Cu Therapy with Radiolabeled [(64)Cu/(67)Cu]MeCOSar-Octreotate in Subjects with Unresectable Multifocal Meningioma: Initial Results for Human Imaging, Safety, Biodistribution, and Radiation Dosimetry. J. Nucl. Med. 2023, 64, 704–710. [Google Scholar] [CrossRef]
- Albert, N.L.; Preusser, M.; Traub-Weidinger, T.; Tolboom, N.; Law, I.; Palmer, J.D.; Guedj, E.; Furtner, J.; Fraioli, F.; Huang, R.Y.; et al. Joint EANM/EANO/RANO/SNMMI practice guideline/procedure standards for diagnostics and therapy (theranostics) of meningiomas using radiolabeled somatostatin receptor ligands: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3662–3679. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Trichter, S.; Nedialkova, L.; Sabbas, A.; Christos, P.; Ramakrishna, R.; et al. Cesium-131 brachytherapy for recurrent brain metastases: Durable salvage treatment for previously irradiated metastatic disease. J. Neurosurg. 2017, 126, 1212–1219. [Google Scholar] [CrossRef]
- Magill, S.T.; Lau, D.; Raleigh, D.R.; Sneed, P.K.; Fogh, S.E.; McDermott, M.W. Surgical Resection and Interstitial Iodine-125 Brachytherapy for High-Grade Meningiomas: A 25-Year Series. Neurosurgery 2017, 80, 409–416. [Google Scholar] [CrossRef]
- Koch, M.J.; Agarwalla, P.K.; Royce, T.J.; Shih, H.A.; Oh, K.; Niemierko, A.; Mauceri, T.C.; Curry, W.T.; Barker, F.G.; Loeffler, J.S. Brachytherapy as an Adjuvant for Recurrent Atypical and Malignant Meningiomas. Neurosurgery 2019, 85, E910–E916. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Turner, A.; Brachman, D.G. The role of GammaTile in the treatment of brain tumors: A technical and clinical overview. J. Neuro-Oncol. 2024, 166, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Odia, Y.; Gutierrez, A.N.; Kotecha, R. Surgically targeted radiation therapy (STaRT) trials for brain neoplasms: A comprehensive review. Neuro Oncol. 2022, 24, S16–S24. [Google Scholar] [CrossRef] [PubMed]
- Brachman, D.G.; Youssef, E.; Dardis, C.J.; Sanai, N.; Zabramski, J.M.; Smith, K.A.; Little, A.S.; Shetter, A.G.; Thomas, T.; McBride, H.L.; et al. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: Results in 20 recurrent, previously irradiated meningiomas. J. Neurosurg. 2019, 131, 1819–1828. [Google Scholar] [CrossRef]
- Braat, A.; Snijders, T.J.; Seute, T.; Vonken, E.P.A. Will (177)Lu-DOTATATE Treatment Become More Effective in Salvage Meningioma Patients, When Boosting Somatostatin Receptor Saturation? A Promising Case on Intra-arterial Administration. Cardiovasc. Intervent Radiol. 2019, 42, 1649–1652. [Google Scholar] [CrossRef]
- Graillon, T.; Salgues, B.; Horowitz, T.; Padovani, L.; Appay, R.; Tabouret, E.; Guedj, E.; Chinot, O. Peptide radionuclide radiation therapy with Lutathera in multirecurrent nonanaplastic meningiomas: Antitumoral activity study by growth rate analysis. J. Neuro-Oncol. 2024, 167, 427–436. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Borch, T.H.; van den Berg, J.H.; Met, O.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhoj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Bi, W.L.; Nayak, L.; Meredith, D.M.; Driver, J.; Du, Z.; Hoffman, S.; Li, Y.; Lee, E.Q.; Beroukhim, R.; Rinne, M.; et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro Oncol. 2022, 24, 101–113. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Wang, N.; Eichler, A.F.; Chukwueke, U.; Forst, D.A.; Arrillaga-Romany, I.C.; Dietrich, J.; et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 2022, 13, 1325. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 2015, 6, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- Kertels, O.; Delbridge, C.; Sahm, F.; Ehret, F.; Acker, G.; Capper, D.; Peeken, J.C.; Diehl, C.; Griessmair, M.; Metz, M.C.; et al. Imaging meningioma biology: Machine learning predicts integrated risk score in WHO grade 2/3 meningioma. Neuro-Oncol. Adv. 2024, 6, vdae080. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).