Abstract
After it has metastasized, bladder cancer, the malignant transformation of the bladder urothelium, continues to be a common cause of death after maximal use of all currently available standard treatments. To address this problem in 2025, the drug repurposing movement within oncology aims to identify medicines in common general medical care use that have data indicating that they can interfere or inhibit a growth driving element that has been identified in bladder cancer. This paper now outlines extensive preclinical data showing that four drugs from general medical practice meet these criteria—the melatonergic drug ramelteon, the antidepressant fluoxetine, the antibiotic dapsone, and the analgesic drug celecoxib. This is the UBC4 regimen, meant as a possible adjunct added to standard treatments of metastatic bladder cancer. Three factors justify a clinical pilot trial of UBC4: (1) the UBC4 drugs are usually well tolerated and carry a low risk of harm, (2) the commonly fatal outcome of bladder cancer once it has widely metastasized, plus (3) the strong preclinical database showing UBC growth inhibition by each of the individual UBC4 drugs as outlined in this paper.
1. Introduction
This paper presents the details and rationale behind UBC4, a proposed new adjunctive drug regimen designed to be used alongside current standard treatments for urothelial bladder cancer (UBC). The current standard treatment of metastatic UBC includes platinum based chemotherapy, immune checkpoint focused inhibition, and anti nestin 1 monoclonal antibody [1A].
A section of the oncology community, physicians, and researchers, believe that while there is no silver bullet, a multidrug approach will be required for the long-term control or cure of the common metastatic cancers [1,2,3,4,5,6,7,8,9,10,11,12]. A multidrug approach is required, because like a well-designed complex machine, catastrophic malfunction usually does not arise from just one or two failed components. Several elements and redundant systems must simultaneously fail for the machine to fail. In aviation, this is called “the Swiss cheese model” in that the holes of each slice must align, and each slice must be moved for the hole to be complete through many slices.
Fitting the analogy to cancer, even in the case of a specific mutation creating a central driver, that mutation is not by itself sufficient for malignancy. EGFR positive non-small cell lung cancer, for example, is where initially, EGFR inhibitors allow tumor regression, but eventually, the cancer grows independently from EGFR. By the time the common deadly metastatic cancers are clinically recognized, multiple checks against metastasis and poorly unrestrained growth have been abrogated. It is the current dogma among those calling for multidrug approaches that many of these nonfunctioning checks must be pharmacologically blocked, taking the place of the cancer driving faulty or absent checks [1,2,3,4,5,6,7,8,9,10,11,12]. Cells, normal or malignant, have many work-arounds, should one or two be blocked.
UBC is a tumor resulting from the malignant transformation of urothelium. It is a common cause of death. Tobacco use, exposure to VOCs (volatile organic compounds), alcohol use, processed meat consumption, and exposure to industrial metals and dyes are common and well-known risk factors for UBC [13]. Common sites of UBC metastases are the bone, lung, and lymph nodes [14]. UBC4 is designed to be UBC subclass agnostic.
The UBC4 regimen uses the melatonergic drug ramelteon, the antidepressant fluoxetine, the antibiotic dapsone, and the analgesic drug celecoxib, as adjuncts, not replacements, to standard treatments of metastatic bladder cancer. Each of these drugs has a robust preclinical research database showing that they can inhibit UBC’s growth. Most of the preclinical work showing that is in vitro work with all the entailing caveats. Some of these drugs’ mechanisms of action in UBC growth inhibition are known and recounted here. Much of the preclinical work of UBC growth inhibition has been empirical.
Table 1 shows the suggested target doses for the UBC4 medicines. These doses are the average doses used in the respective drug’s day-to-day use in non-oncology general medical practice.

Table 1.
Target daily doses for the UBC4 regimen. Except for celecoxib, these doses are at or near the usual doses used in general medical practice. For celecoxib, the listed dose is about twice the usual dose used to treat pain. See references on celecoxib in Section 2.3 below for the rationale behind that difference.
The UBC4 drugs were chosen by study of all repurposed drugs that have any published evidence for growth inhibition in UBC. Then, four drugs were selected for inclusion in UBC4 based (1) first on safety. Drugs without low side effect risks were not considered further. (2) The second criterion was the strength of preclinical evidence for UBC growth inhibition. Drugs with weak evidence were not considered further. (3) Having a strong and clear physiological rationale was the third selection criterion. (4) Then, among the few remaining drugs, clinical experience with a given drug favored its inclusion and little clinical experience with a drug favored exclusion. The four drugs remaining constitute UBC4; details follow.
2. The Drugs: Ramelteon, Fluoxetine, Celecoxib, Dapsone
2.1. Melatonin and Ramelteon
Melatonin is a simple, low-molecular-weight signaling molecule (Figure 1) with pleiotropic functions in all major body systems (pleiotropic—having “exceptional multiplicity of actions”) [15]. It is traditionally thought of as a hormone produced by the pineal gland, and indeed the pineal gland is responsible for most of the circulating blood melatonin; but melatonin is actually synthesized in and used for paracrine signaling by most body organs and tissues, where both primary receptors, M1 and M2, are also present [15,16,17,18,19,20]. Melatonin, traditionally thought of as one of the entraining elements directing diurnal rhythms, also has immune system effects [21,22,23]. Melatonin is available without a prescription in many jurisdictions worldwide. Many melatonin preparations have variable, poorly controlled contents [24]. It is, therefore, important to use only USP certified preparations.
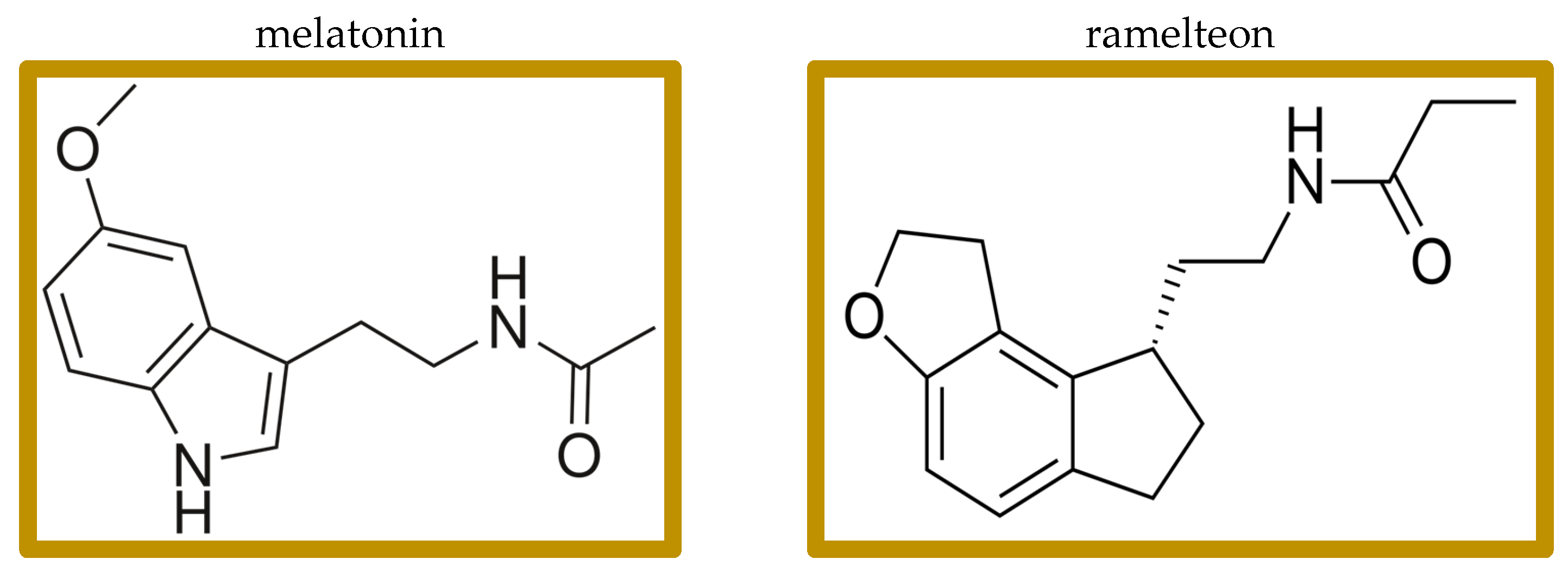
Figure 1.
Structures of melatonin and ramelteon.
The brain location of M1 differs from the M2 brain locations. Mice lacking MT1 have increased non-REM sleep but decreased REM sleep. Mice lacking MT2 have decreased in non-REM. In humans, structured dreams tend to be temporally associated with periods of REM, while illogical, bizarre dreams tend to occur in NREM. M1 and M2 heterodimers and M2 heterodimers with serotonin 5-HT2C receptors can form. Serotonergic agonism at 5-HT2C results in M2 signaling in absence of any melatonergic agonist [25,26].
Of uncertain physiological significance, melatonin also binds to several of the retinoic acid nuclear transcription factors.
Given this pleiotropy of melatonin effects and our lack of clearly definable melatonin actions, the dataset referenced below comes predominantly from empirical studies. A problem in interpreting experimental melatonin results derives from the lack of information on melatonin acting via its receptors, in which case, we can expect ramelteon to have similar effects, or melatonin acting via its physiological effects of the molecule itself, independent from its receptors, in which case, we might not expect ramelteon to have similar effects. Clinical results of melatonin have been inconsistent but do indicate a modest effect in promoting sleep.
A preclinical study showed that melatonin induced growth arrest in UBC cells [27,28]. Over a hundred studies have shown growth inhibition by melatonin in murine experimental tumors and in vitro cancer cell cultures [29,30,31,32,33]. No clinical study has yet replicated these results in humans.
NK cells are a subset of large granular lymphocytes that bear CD56, CD16, CD161, and lack CD3 expression [34]. Unlike most other T cells, their activation and target cell killing does not depend on antigen presentation. They synthesize and secrete IFN-gamma, perforin, granzyme B, and TNF. CD56 and CD16 surface marker expression: CD56 bright CD16 dim/low; these cells are less cytotoxic but secrete proinflammatory cytokines upon stimulation. CD56 dim CD16 high; this highly cytotoxic subset dominates the peripheral blood and plays a major role in NK-mediated tumor cell killing and metastasis prevention. Senescence and other signaling ligands that target cells for destruction by NK cells are usually absent in non-transformed cells but commonly become expressed on cells of a cancer. NK cells tend to be dysfunctional and are poorly activatable in UBC [35,36].
Adding exogenous melatonin increased the number, proliferation, degranulation, and IFN-gamma secretion of NK cells in aging mice [37]. The particularly high melatonin levels in bone marrow do not enter the general circulation in large amounts [17]. Exogenous melatonin increased the number and function of NK cells in old mice, a finding independently confirmed many times [37,38,39,40]. Melatonin induced a similar increased number and function of NK cells in ovariectomized rats [41]. NK cell number increased also in healthy young mice after prolonged oral melatonin supplementation [42]. Three mg oral melatonin lessened NK cell reduction in hemodialysis patients [43].
Oral melatonin slightly but statistically significantly increased baseline IL-2 in children with Down’s syndrome [44]. Increased IL-2 was seen in lupus mice given melatonin [45].
A 2012 meta-analysis of eight randomized controlled clinical trials of adjunctive melatonin treatment of cancer by Wang YM et al. included seven studies from the same single institution, with similar authors. None of the individual authors are answering emails of inquiry. The individual studies in that meta-analysis were of such poor quality that no conclusions of melatonin’s effectiveness can be made other than if it has any effect, it must be minor [46]. The most recent clinical study of melatonin in cancer was a study from 2014 in non-small cell lung cancer that showed no clinical effect on growth [47]. By itself, we should expect the same from adding single agent melatonin.
Ramelteon is an FDA approved generic non-scheduled melatonergic drug marketed to treat insomnia. Ramelteon has affinity for M1 and M2 receptors 3–16 times higher than that of melatonin and has a longer half-life than melatonin [48,49,50]. Clinical results of ramelteon have been inconsistent but do indicate a modest effect in promoting sleep.
Ramelteon increases 100-fold if co-administered with the antidepressant fluvoxamine [51]. Only three studies looked specifically at ramelteon as a melatonin agonist for cancer treatment adjunct. Nanomolar range ramelteon inhibited endometrial cancer cell growth, an effect blocked by a melatonergic receptor antagonist [52]. In vitro colon cancer cell growth was inhibited by ramelteon [53]. A review of the physiology of ramelteon’s potential for the inhibition of glioblastoma was collected and the evidence accrued up until 2015 was discussed [54].
A summary of the data on melatonin and ramelteon: we have evidence for the benefits as cancer treatment adjuncts. The evidence is weak but not zero. Since both melatonin and ramelteon are unlikely to have harms, bothersome side effects, or cancer growth enhancing effects, they are worth a try as an adjunct to other treatments.
2.2. Fluoxetine
Fluoxetine is the first selective serotonin reuptake inhibitor, marketed first in the late 1980s and is still in wide use to treat a depressed mood or anxiety [55,56]. In recent years, a developing research database has prompted interest in using adjunctive fluoxetine as part of a cancer treatment independent of its mood effects [57,58,59,60]. Table 2 lists some representative research evidence for fluoxetine’s inhibition of growth across a variety of different cancers.

Table 2.
Representative studies reporting evidence for fluoxetine’s potential for reducing growth across a variety of different cancers. A common fluoxetine level in these in vitro culture studies was 10 to 40 microM. Fluoxetine concentrates in body tissues at 10 to 15 times greater levels than peak blood levels, typical levels ranging from 1 to 10 microg/mL in brain tissue.
Two studies suggest that UBC inhibition may be a class effect of SSRIs. Other SSRI class drugs, sertraline, and paroxetine also inhibited UBC growth in the 10 micromolar range [104]. A large population epidemiology study showed a decreased risk of developing UBC in those taking SSRIs fluoxetine, paroxetine, or citalopram [61].
Fluoxetine inhibits in vitro UBC growth without inhibiting non-transformed bladder urothelial cells [62]. Fluoxetine inhibited UBC growth with a cytotoxicity that was additive with cisplatin [63].
Several concerns limit the enthusiasm of adding fluoxetine to cancer treatment:
- In vitro studies showing growth inhibition tended to use low micromolar concentrations. That level is higher than ideal. Usually nanomolar growth inhibition marks strong candidate drugs.
- We do not have a unified mechanism of fluoxetine’s action in cancer growth inhibition. Different researchers have documented different cancer growth driving elements inhibited by fluoxetine. Examples: ERK1/2 pathway inhibition, inhibition of c-Myc, drug efflux pump inhibition, inactivating STAT3 driven epithelial to mesenchymal transition, mTOR activation, NK cell increase, and others [64,65,86,93,99,105,106,107].
Two considerations counterbalance these concerns: (a) the excellent tolerability of fluoxetine and low risk of harm, and (b) fluoxetine concentrates in tissue at several times the levels seen in blood [108,109].
2.3. Celecoxib
Celecoxib is a COX-2 selective inhibitor that also inhibits multiple carbonic anhydrase (CA) isoforms [110]. It has been in wide use since the 1990′s as a safe and effective analgesic drug [111].
Interest in celecoxib as an adjuvant during cancer treatment is based on both these attributes [112,113,114,115,116,117].
In bladder urothelial cancer, CA IX and CA XII are elevated; greater elevations shorten survival and are associated with a higher malignancy grade [118,119,120,121,122,123].
Celecoxib inhibits CA isoforms II, IV, IX, and XII [117,124,125,126]. CA-IX and XII are transmembrane and CA-II is soluble.
As an example of celecoxib’s CA inhibition profile compared to the standard CA inhibitor acetazolamide, Sethi et al. [126] found the following:
- acetazolamide...IC50 at CA-II = 12, CA-IX = 25, CA-XII = 6;
- celecoxib…........IC50 at CA-II = 21, CA-IX = 16, CA-XII = 18.
Elevated CA isoforms are characteristic of UBC tissues [127]. CA-II and -IX regulate the pH of the UBC tumor microenvironment and are overexpressed in UBC. As illustrated in Figure 2, CA-II and CA-IX, working together, limit the lowering of intracellular pH that would otherwise result from the UBC hypermetabolic state.
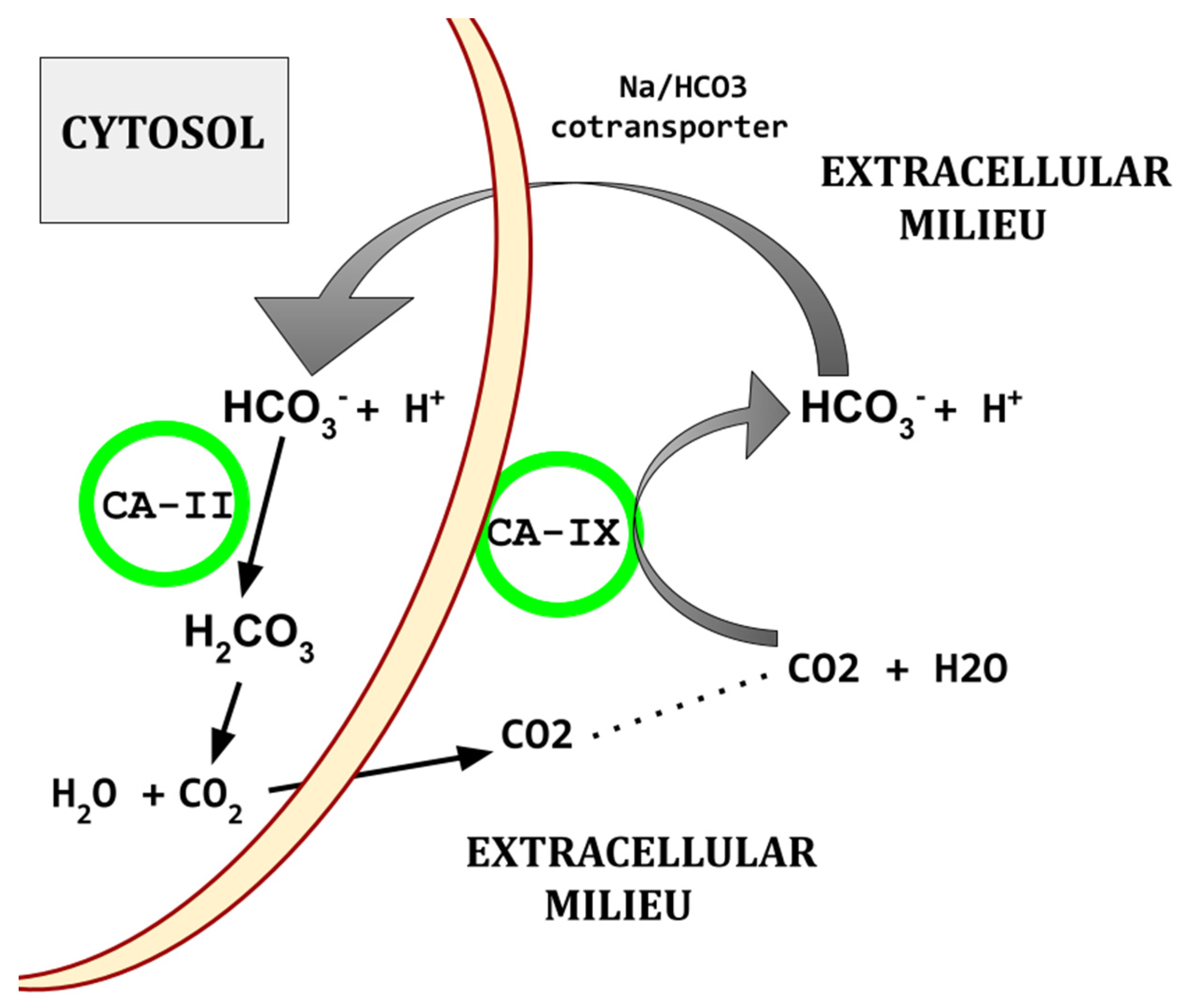
Figure 2.
Diagram of basic CA based acid export. There are other paths by which protons are exported from cells.
Elevated CA isoforms -IX and -XII are a core feature also found across the common cancers [128,129,130,131,132,133]. Figure 2 shows the basic mechanism by which CAs participate in creating the characteristic acidic peritumoral milieu and maintenance of intracellular alkalinization.
Experimental CA-IX inhibitors inhibited UBC growth [134]. CA-IX is elevated in UBC tissue compared to normal urothelium, and the degree of elevation increases with tumor grade [120,135,136,137]. Urine CA-IX is elevated in UBC and correlates with UBC tissue CA-IX elevation [118,119,138]. Higher elevations of CA-IX expression in UBC tissue were associated with shorter survival and predict greater invasiveness and UBC’s metastatic potential [121,122,139]. Others found a UBC tissue elevation of CA-IX but no correlation with survival [140]. Serum carbonic anhydrase activity, subtype not specified, was elevated in UBC cases [141]. Urine exosomal mRNA for CA-IX is also elevated in UBC [142]. The reference pan-carbonic anhydrase inhibitor acetazolamide inhibited UBC growth [143].
As examples, CA-IX and/or CA-XII are elevated, worsen survival, and contribute to malignancy grade in many cancers. Examples: acute myelogenous leukemia [144,145], bladder urothelial cancer [118,119,120,121,122,123], breast [146], esophageal [147,148], gastric [148,149], glioblastoma [150], hepatocellular [151], Hodgkin’s lymphoma [152], laryngeal [153,154], nasopharyngeal [155,156], non-small cell lung [157], oral squamous cell [158], osteosarcoma [159], pancreatic ductal [160,161,162], and thyroid [163].
This dataset leads to the conclusion that CA-IX and or CA-XII tend to be one of the many links in mediating malignant cell behaviors across many of the common cancers. This finding is, however, not universal. Examples of exceptions: in renal cell carcinoma, higher CA-IX expression predicts a better prognosis [163,164,165], and in prostate cancer, CA-IX is absent [166,167].
Celecoxib, in adequate doses, may hobble UBC growth by limiting UBC’s ability to cope with its increased metabolism-related acid production. An indication that this is happening is the finding in glioblastoma, where resistance to temozolomide is overcome by CA-XII inhibition [168,169].
2.4. Celecoxib, IL-6
Of the many cytokines elevated in UBC, IL-6 plays an important if not central driving role. This section presents data, albeit indirect, suggesting that lowering IL-6 will improve UBC prognosis and that celecoxib will mediate that lowering.
Clinical use of celecoxib reduced the elevated circulating IL-6 found in patients with ankylosing spondylitis [170], pancreatitis [171], major depression [172], heavy tobacco smoking [173], frailty of age [174], knee osteoarthritis [175], and inflammatory arthritis [176].
IL-6 is the quintessential pleiotropic cytokine. Its signaling is ubiquitous and central to mammalian physiology. But what it does cannot be simply stated. IL-6 functions as a pro- or anti-inflammation element, depending on the circumstances [177,178]. In cancers generally, and in UBC specifically, IL-6 is predominantly growth promoting.
Excess IL-6 is one of the drivers of UBC growth, invasion, and migration. Oft repeated studies showed that serum or plasma IL-6 increases as UBC progressively invades the muscularis with higher IL-6 levels portending recurrence, metastasis, and shorter survival [179,180,181,182,183,184]. Urine IL-6 levels are elevated in UBC [179,185,186,187]. All clinical UBC biopsy cells expressed the IL-6 receptor [188].
IL-6 enhanced the in vitro growth of UBC cells [189]. In vitro inhibition of IL-6′s receptor inhibited UBC growth [182,190]. IL-6 enhances stem cell characteristics of UBC cells [191,192].
Serum IL-6 increases as UBC progressively invades the myometrium [179]. Urine IL-6 levels are elevated in UBC [179,185,187]. UBC cases with lymph node metastases have higher serum levels of IL-6 than do cases with low IL-6 [193].
The UBC cell subpopulation that exhibits stem cell characteristics of high ALDH expression, high clonogenicity, and low cell numbers needed to transplant the tumor have increased IL-6 receptor proteins compared to cells without these stem attributes [191].
2.5. Celecoxib and Tumor Resident Fibroblasts
Intratumoral, non-transformed fibroblasts of several different subcategories comprise a not insignificant percent of cells within the common cancers, UBC included. Their trophic function derives from providing structural support, and a wealth of growth and angiogenesis promoting factors [194,195,196,197]. Fibroblasts fulfill many crucial physiological functions crucial for the proper homeostatic functioning of all body organs: scaffolding, providing trophic growth signals, recruiting bone marrow cells for angiogenesis, shaping or inhibiting lymphocyte centered immune response, and others.
In UBC specifically, although essentially normal fibroblasts are enlisted to promote cancer growth, these UBC resident fibroblasts are responding normally to their normal activating, directing signaling systems. Their net effect promotes a pathology. Non-transformed, otherwise normal fibroblasts resident within UBC secrete higher levels IL-6 and IL-8 than normal, non-transformed bladder wall fibroblasts [198,199,200]. The trigger or signaling system driving these otherwise normal fibroblasts to secrete abnormal amounts of cancer trophic cytokines is unknown.
In vitro work has shown that celecoxib can diminish cancer tissue resident non-transformed fibroblasts’ trophic function to the malignant cell population [201,202,203]. IL-6 is one of several UBC resident fibroblasts’ growth enhancing products [204,205]. The COX-2 blocking function of celecoxib also contributes to the angiogenesis and fibroblast tumor trophic functions [206].
2.6. Dapsone, Neutrophils, and IL-8
Dapsone is one of the first of modern antibiotics, introduced to clinical practice in the 1940s, and is still in wide use. Dapsone is also active in treating or preventing Toxoplasmosis, Plamidia, Leishmania, and other protozoan infections. Dapsone’s use has expanded in the last two decades to include treatment of the neutrophilic dermatoses, bullous pemphigoid, or the EGFR inhibitor-induced rash as examples [207,208,209,210].
On that basis, dapsone’s use was further extended to other diseases and conditions characterized by the unwanted tissue destructive actions of neutrophil accumulations [210,211,212,213,214,215]. The further repurposing of dapsone on that same basis has potential to reduce the tumor trophic, and angiogenesis promoting functions of neutrophils during cancer treatment [3,4,5,11,216,217,218,219]. Dapsone impedes neutrophil chemotaxis along an IL-8 gradient. That is the basis for dapsone use in the neutrophilic dermatoses and during cancer treatment. By inhibiting neutrophils’ IL-8 chemotaxis, the tumor trophic, immunosuppressive neutrophil accumulations are reduced.
How exactly dapsone inhibits neutrophil chemotaxis along IL-8 gradients during the treatment of the neutrophilic dermatoses is not clear.
Neutrophils are absent in healthy bladder walls, but they heavily infiltrate UBC tissue where they appear to have a largely immunosuppressive role. UBC cells secrete CXCL1, CXCL5, and IL-8—all of which are chemotactic for neutrophils. Although preclinical research can demonstrate some tumor inhibiting properties, the preponderance of actual clinical evidence indicates a tumor trophic, growth enhancing, immunosuppressive role for neutrophils across the common human cancers [220,221,222,223].
Specifically, in UBC, higher tumor neutrophil infiltration portends shorter survival [224]. PGE2 synthesized by neutrophils’ COX-2 drives or contributes to the drive to UBC cells’ synthesis of immunosuppressive indoleamine 2,3-dioxygenase [224].
A metastatic UBC cell line synthesized more IL-8 than a corresponding non-transformed urothelium cell line. When that UBC cell line was treated with IL-8 full-sequence antisense cDNA, it made little IL-8 and lost the ability to metastasize [225]. An orthotopic UBC xenograft grew slower in mice given anti-IL-8 antibody [226]. Autocrine UBC cell IL-8 stimulates UBC cell migration/motility [227].
UBC cells synthesize and secrete IL-8, which is one of the primary drivers of neutrophil accumulations within UBC tissue [228]. UBC with stronger immunohistochemistry staining for IL-8 had more aggressive tumors and shorter survival [229,230]. Urine IL-8 levels are uniformly elevated in UBC and post-treatment elevated urine levels of IL-6 and IL-8 predicted UBC recurrence [118,138,187,230,231,232,233]. Margel et al. found 200 times greater urinary IL-8 in metastatic UBC compared to healthy controls [187]. Elevated serum levels of IL-8 in UBC also predicted shorter survival [230].
It is clear that neutrophils (a) play a predominantly immunosuppressive, tumor trophic role in UBC and (b) that excess IL-6 is one of the drivers of that [181,228,234,235]. Immune checkpoint blockade (pembrolizumab, nivolumab)-treated UBC patients with higher blood levels of IL-6 and IL-8 had poorer responses than did UBC patients with lower levels of these cytokines [236].
In UBC cases, as with other common cancers, a higher NLR correlates with higher IL-6 and IL-8 levels, higher IL-8 levels correlated with greater neutrophil infiltration into the tumor, and greater Treg numbers in UBC [237].
Of concern, and related to the preface of this paper, standard UBC cytotoxic chemotherapy increased the circulating and UBC tissue expression of IL-8 [238]. This makes adjunctive use of dapsone during standard cytotoxic chemotherapy of UBC particularly attractive.
2.7. TICO and the NLR
The ratio of neutrophils to lymphocytes in peripheral blood (NLR) is an interesting measure of prognosis across the common metastatic cancers. Confirmed in over one hundred clinical studies spanning the range of human cancers, a ratio >3:1 predicts poorer prognosis [239,240,241,242,243,244,245]. The remarkable finding of shorter survival in those with higher NLR across our common cancers reflects something profound: a unifying factor in cancers. We do not yet know what that unifying factor might be. Identified malignant tumor enhancing roles for neutrophils have been identified:
- Supplying VEGF and other factors contributing to angiogenesis;
- Inhibition of immune responses, becoming myeloid-derived suppressor cells;
- Contributions to peritumoral tissue destruction and preparation of tumor bed;
- Contributing tumor trophic factors.
There is no other marker or lab finding that is so uniformly, so often confirmed, and constantly seen across the entire range of human cancers than NLR elevation. Specifically in UBC, the NLR becomes elevated and a greater abnormal elevation reflects a more aggressive disease [246,247,248,249,250,251].
The TICO regimen is a four-drug regimen of repurposed drugs designed to lower the NLR [239]. TICO uses tadalafil, a phosphodiesterase 5 inhibitor used to treat pulmonary hypertension, isotretinoin, used to treat acne, colchicine, used to treat gout, and the fish oil omega-3. Unlike most repurposed drug regimens that are based on matching the pharmacology of a drug with the known growth pathways of a cancer, TICO is based on simple empirical observations of NLR lowering by these drugs. TICO has not been proven to lower the NLR in cancer. It is unlikely to harm or give any unpleasant side effects, so could be considered in UBC cases with elevated NLR > 3:1.
As mentioned in the section above on dapsone, it also has potential to lower the NLR [211,213].
3. Discussion
- In using repurposed drugs for the adjunctive treatment of cancer, patients and their doctors commonly underdose them. Repurposed drugs must be coordinated, and used at effective, usually robust doses [1,2,3,4,5]. The preclinical database supporting their use must be sound and many repurposed drugs must be used at the same time to progressively deplete cancer cells’ growth vigor as each individual drug is added [1,2,3,4,5].
- Celecoxib’s inhibition of neutrophils’ PGE2 plus dapsone’s reduction in tumor-resident neutrophils would operate together to decrease neutrophils’ tumor growth promotion.
- Drug levels used in many preclinical in vitro works documenting UBC growth inhibition were higher than levels we usually see during standard clinical use.
- Much—but not all—of the evidence showing UBC growth inhibition by the UBC4 medicines was performed in vitro. We have a long history of in vitro findings being not replicated when tried in the clinic. On the other hand, some of our current effective clinical treatments were originally demonstrated in vitro and they did translate well to the clinic.
- Melatonin products in the USA are poorly controlled. Some contain no melatonin; some contain other, non-listed hypnotic drugs [24]. If melatonin is used, it must be labeled USP.
- Dapsone use will generate some methemoglobinemia, usually <8%, and usually asymptomatic. The histamine receptor 2 inhibitor drug cimetidine will lower dapsone’s methemoglobinemia generation [252,253].
- Cimetidine dosed at 1400 mg q 12 h was well tolerated when treating papilloma verruca in immunosuppressed transplant recipients [254]. Nine studies prior to 2010 in various populations all reported significantly greater verruca resolution with cimetidine [255]. A common dose range for verruca treatment was 40 mg/kg/day.
- Science and medicine must cope with conflicting data. Except for the section on NLR, such is the case for UBC4. Datasets that guide medical practice usually have conflicting or unclear results in the early stages of their development.
- Rarely do clinicians have clear and unequivocal datasets supporting the adoption of a new treatment. Conflicting data in preclinical datasets are common. Fallacious data come about either by overt fabrication or by inadvertent errors of data generation or interpretation.
- Clinicians decide to adopt a treatment based on the preponderance of evidence, and the consideration of balancing risks of treatment versus risks of the target disease. This consideration reflects an old wisdom expressed in the myth of Scylla and Charybdis. As a metaphor, we face these two monsters today when confronting metastatic cancer. The Ionian poet Homer (~800 BCE) advises that we pass by Scylla, losing a few sailors eaten by Scylla rather than lose the entire ship swallowed up by the whirlpool of Charybdis.
Four common modes of failure when embarking on the use of repurposed medicines derive from the following: (a) the treating Oncologist declining to prescribe anything but the current standard chemotherapy for the given cancer; (b) the timidity and reluctance of the monitoring General Practitioner (Family Physician) to add multiple drugs; (c) the inadequate frequency of evaluations or monitoring for tolerability and adherence to a regimen that is unusually complicated; and (d) the use of repurposed drug doses that are too low to adequately inhibit the target system.
4. Conclusions
Much of the data presented here to support these four drugs having growth inhibiting effects in UBC have been performed in vitro. Most drugs with cancer inhibiting effects, in vitro or in rodent graft studies, eventually fail to benefit when tested clinically. Although this may turn out to be the case for UBC4, three considerations may warrant a pilot trial of UBC4: (a) good predicted tolerability and the unlikelihood of harm coming from the UBC4 drugs based on the wide past clinical experience with these drugs in their general medical indications, (b) the sound preclinical evidence of growth inhibition in UBC cells, and (c) the poor prognosis of metastatic UBC as things stand currently in 2025.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kast, R.E. IC Regimen: Delaying Resistance to Lorlatinib in ALK Driven Cancers by Adding Repurposed Itraconazole and Cilostazol. Cells 2024, 13, 1175. [Google Scholar] [CrossRef]
- Duenas-Gonzalez, A.; Gonzalez-Fierro, A.; Bornstein-Quevedo, L.; Gutierrez-Delgado, F.; Kast, R.E.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Candelaria, M.; Romo-Pérez, A.; Correa-Basurto, J.; et al. Multitargeted polypharmacotherapy for cancer treatment. Theoretical concepts and proposals. Expert Rev. Anticancer Ther. 2024, 24, 665–677. [Google Scholar] [CrossRef]
- Kast, R.E. IPIAD- an augmentation regimen added to standard treatment of pancreatic ductal adenocarcinoma using already-marketed repurposed drugs irbesartan, pyrimethamine, itraconazole, azithromycin, and dapsone. Oncoscience 2024, 11, 15–31. [Google Scholar] [CrossRef]
- Kast, R.E. The OSR9 Regimen: A New Augmentation Strategy for Osteosarcoma Treatment Using Nine Older Drugs from General Medicine to Inhibit Growth Drive. Int. J. Mol. Sci. 2023, 24, 15474. [Google Scholar] [CrossRef]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef]
- Halatsch, M.E.; Dwucet, A.; Schmidt, C.J.; Mühlnickel, J.; Heiland, T.; Zeiler, K.; Siegelin, M.D.; Kast, R.E.; Karpel-Massler, G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals 2021, 14, 1241. [Google Scholar] [CrossRef]
- Halatsch, M.E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neurooncol. Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef]
- Kast, R.E.; Halatsch, M.E.; Rosell, R. OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs. Cells 2021, 10, 1148. [Google Scholar] [CrossRef]
- Kast, R.E.; Burns, T.C.; Halatsch, M.E. Short review of SEC, a potential dexamethasone-sparing regimen for glioblastoma: Spironolactone, ecallantide, clotrimazole. Neurochirurgie 2021, 67, 508–515. [Google Scholar] [CrossRef]
- Kast, R.E.; Skuli, N.; Sardi, I.; Capanni, F.; Hessling, M.; Frosina, G.; Kast, A.P.; Karpel-Massler, G.; Halatsch, M.E. Augmentation of 5-Aminolevulinic Acid Treatment of Glioblastoma by Adding Ciprofloxacin, Deferiprone, 5-Fluorouracil and Febuxostat: The CAALA Regimen. Brain Sci. 2018, 8, 203. [Google Scholar] [CrossRef]
- Kast, R.E. Paths for Improving Bevacizumab Available in 2018: The ADZT Regimen for Better Glioblastoma Treatment. Med. Sci. 2018, 6, 84. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Alouini, S. Risk Factors Associated with Urothelial Bladder Cancer. Int. J. Environ. Res. Public Health 2024, 21, 954. [Google Scholar] [CrossRef]
- Dong, F.; Shen, Y.; Gao, F.; Xu, T.; Wang, X.; Zhang, X.; Zhong, S.; Zhang, M.; Chen, S.; Shen, Z. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: A population-based study. Cancer Manag. Res. 2017, 9, 611–626. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Kvetnoy, I. Extrapineal melatonin in pathology: New perspectives for diagnosis, prognosis and treatment of illness. Neuroendocrinol. Lett. 2002, 23 (Suppl. S1), 92–96. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Zhang, M.; Weintraub, S.T.; Cabrera, J.; Sainz, R.M.; Mayo, J.C. Identification of highly elevated levels of melatonin in bone marrow: Its origin and significance. Biochim. Biophys. Acta 1999, 1472, 206–214. [Google Scholar] [CrossRef]
- Markus, R.P.; Sousa, K.S.; da Silveira Cruz-Machado, S.; Fernandes, P.A.; Ferreira, Z.S. Possible Role of Pineal and Extra-Pineal Melatonin in Surveillance, Immunity, and First-Line Defense. Int. J. Mol. Sci. 2021, 22, 12143. [Google Scholar] [CrossRef]
- Damian, E. Extra-pineal gland sources of melatonin. Endocrinologie 1977, 15, 65–66. [Google Scholar] [CrossRef]
- Pires-Lapa, M.A.; Carvalho-Sousa, C.E.; Cecon, E.; Fernandes, P.A.; Markus, R.P. β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes. Int. J. Mol. Sci. 2018, 19, 2182. [Google Scholar] [CrossRef]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef]
- Calvo, J.R.; Maldonado, M.D. Immunoregulatory properties of melatonin in the humoral immune system: A narrative review. Immunol. Lett. 2024, 269, 106901. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Pandi-Perumal, S.R.; Brown, G.M. Melatonin as a Chronobiotic and Cytoprotector in Non-communicable Diseases: More than an Antioxidant. Subcell. Biochem. 2024, 107, 217–244. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.M.; Ianakieva, D. Poor Quality Control of Over-the-Counter Melatonin: What They Say Is Often Not What You Get. J. Clin. Sleep. Med. 2017, 13, 163–165. [Google Scholar] [CrossRef]
- Kamal, M.; Gbahou, F.; Guillaume, J.L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Kalbasi Anaraki, D.; Baroncini, M.; Mannoury la Cour, C.; et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef]
- Comai, S.; Gobbi, G. Melatonin, Melatonin Receptors and Sleep: Moving Beyond Traditional Views. J. Pineal Res. 2024, 76, e13011. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Sung, W.W.; Chang, Y.C.; Yu, C.Y.; Lu, L.Y.; Dong, C.; Lee, T.H.; Chen, S.L. Melatonin induces cell cycle arrest and suppresses tumor invasion in urinary bladder urothelial carcinoma. Aging 2023, 15, 3107–3119. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Z.; Li, H.; Lin, M.; Jiang, Y.; Liang, L.; Ma, Q.; Gou, J.; Ning, L.; Li, X.; et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 2021, 71, e12765. [Google Scholar] [CrossRef]
- Reiter, R.J.; De Almeida Chuffa, L.G.; Simão, V.A.; Martín Giménez, V.M.; De Las Heras, N.; Spandidos, D.A.; Manucha, W. Melatonin and vitamin D as potential synergistic adjuvants for cancer therapy (Review). Int. J. Oncol. 2024, 65, 114. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Tan, D.X.; Huang, G.; de Almeida Chuffa, L.G.; Anderson, G. Melatonin modulates tumor metabolism and mitigates metastasis. Expert. Rev. Endocrinol. Metab. 2023, 18, 321–336. [Google Scholar] [CrossRef]
- Hanikoglu, A.; Kucuksayan, E.; Akduman, R.C.; Ozben, T. A Review on Melatonin’s Effects in Cancer: Potential Mechanisms. Anticancer.. Agents Med. Chem. 2018, 18, 985–992. [Google Scholar] [CrossRef]
- Yi, Y.J.; Tang, H.; Pi, P.L.; Zhang, H.W.; Du, S.Y.; Ge, W.Y.; Dai, Q.; Zhao, Z.Y.; Li, J.; Sun, Z. Melatonin in cancer biology: Pathways, derivatives, and the promise of targeted delivery. Drug Metab. Rev. 2024, 56, 62–79. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef]
- Álvarez-Carrasco, P.; Maldonado-Bernal, C. The innate defenders: A review of natural killer cell immunotherapies in cancer. Front. Immunol. 2024, 15, 1482807. [Google Scholar] [CrossRef]
- Noel, O.D.V.; Hassouneh, Z.; Svatek, R.S.; Mukherjee, N. Innate Lymphoid Cells in Bladder Cancer: From Mechanisms of Action to Immune Therapies. Cancer Immunol. Res. 2024, 12, 149–160. [Google Scholar] [CrossRef]
- Textor, S.; Fiegler, N.; Arnold, A.; Porgador, A.; Hofmann, T.G.; Cerwenka, A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011, 71, 5998–6009. [Google Scholar] [CrossRef]
- Liang, C.; Song, R.; Zhang, J.; Yao, J.; Guan, Z.; Zeng, X. Melatonin enhances NK cell function in aged mice by increasing T-bet expression via the JAK3-STAT5 signaling pathway. Immun. Ageing 2024, 21, 59. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Ostapchuk, Y.O.; Abdolla, N.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Exogenous Melatonin Up-Regulates Expression of CD62L by Lymphocytes in Aged Mice under Inflammatory and Non-Inflammatory Conditions. Immunol. Investig. 2019, 48, 632–643. [Google Scholar] [CrossRef]
- Tian, Y.M.; Zhang, G.Y.; Dai, Y.R. Melatonin rejuvenates degenerated thymus and redresses peripheral immune functions in aged mice. Immunol. Lett. 2003, 88, 101–104. [Google Scholar] [CrossRef]
- Inserra, P.; Zhang, Z.; Ardestani, S.K.; Araghi-Niknam, M.; Liang, B.; Jiang, S.; Shaw, D.; Molitor, M.; Elliott, K.; Watson, R.R. Modulation of cytokine production by dehydroepiandrosterone (DHEA) plus melatonin (MLT) supplementation of old mice. Proc. Soc. Exp. Biol. Med. 1998, 218, 76–82. [Google Scholar] [CrossRef]
- Baeza, I.; Alvarado, C.; Alvarez, P.; Salazar, V.; Castillo, C.; Ariznavarreta, C.; Fdez-Tresguerres, J.A.; De la Fuente, M. Improvement of leucocyte functions in ovariectomised aged rats after treatment with growth hormone, melatonin, oestrogens or phyto-oestrogens. J. Reprod. Immunol. 2009, 80, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Sun, L.Z.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Marzougui, H.; Hammouda, O.; Ben Dhia, I.; Maaloul, R.; Agrebi, I.; Chaker, H.; Kammoun, K.; Ben Hmida, M.; Ayadi, F.; Kallel, C.; et al. Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients. Int. Urol. Nephrol. 2021, 53, 553–562. [Google Scholar] [CrossRef]
- Huggard, D.; Kelly, L.; Worrall, A.; Gallagher, E.; Fallah, L.; Yoo, L.L.; McGrane, F.; Lagan, N.; Roche, E.; Balfe, J.; et al. Melatonin as an immunomodulator in children with Down syndrome. Pediatr. Res. 2022, 91, 1812–1820. [Google Scholar] [CrossRef]
- Zhou, L.L.; Wei, W.; Si, J.F.; Yuan, D.P. Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediat. Inflamm. 2010, 2010, 951210. [Google Scholar] [CrossRef]
- Wang, Y.M.; Jin, B.Z.; Ai, F.; Duan, C.H.; Lu, Y.Z.; Dong, T.F.; Fu, Q.L. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother. Pharmacol. 2012, 69, 1213–1220. [Google Scholar] [CrossRef]
- Sookprasert, A.; Johns, N.P.; Phunmanee, A.; Pongthai, P.; Cheawchanwattana, A.; Johns, J.; Konsil, J.; Plaimee, P.; Porasuphatana, S.; Jitpimolmard, S. Melatonin in patients with cancer receiving chemotherapy: A randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014, 34, 7327–7337. [Google Scholar]
- Greenblatt, D.J.; Harmatz, J.S.; Karim, A. Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J. Clin. Pharmacol. 2007, 47, 485–496. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Spence, D.W.; Moscovitch, A.; Hardeland, R.; Brown, G.M.; Cardinali, D.P. Ramelteon: A review of its therapeutic potential in sleep disorders. Adv. Ther. 2009, 26, 613–626. [Google Scholar] [CrossRef]
- Kato, K.; Hirai, K.; Nishiyama, K.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S.; Kawamata, Y.; Hinuma, S.; Miyamoto, M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 2005, 48, 301–310. [Google Scholar] [CrossRef]
- Obach, R.S.; Ryder, T.F. Metabolism of ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on ramelteon pharmacokinetics. Drug Metab. Dispos. 2010, 38, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Osanai, K.; Kobayashi, Y.; Otsu, M.; Izawa, T.; Sakai, K.; Iwashita, M. Ramelteon, a selective MT1/MT2 receptor agonist, suppresses the proliferation and invasiveness of endometrial cancer cells. Hum. Cell 2017, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Casado, J.; Carazo, A.; Sanjuán, L.; Maté, A.; Muñoz de Rueda, P.; de la Cueva, P.; Quiles, R.; Ruíz, S.; Ruíz-Extremera, A.; et al. Gender-related invasion differences associated with mRNA expression levels of melatonin membrane receptors in colorectal cancer. Mol. Carcinog. 2012, 51, 608–618. [Google Scholar] [CrossRef]
- Kast, R.E. Agomelatine or ramelteon as treatment adjuncts in glioblastoma and other M1- or M2-expressing cancers. Contemp. Oncol. 2015, 19, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cheer, S.M.; Goa, K.L. Fluoxetine: A review of its therapeutic potential in the treatment of depression associated with physical illness. Drugs 2001, 61, 81–110. [Google Scholar] [CrossRef]
- Stokes, P.E.; Holtz, A. Fluoxetine tenth anniversary update: The progress continues. Clin. Ther. 1997, 19, 1135–1250. [Google Scholar] [CrossRef]
- Peer, D.; Margalit, R. Fluoxetine and reversal of multidrug resistance. Cancer Lett. 2006, 237, 180–187. [Google Scholar] [CrossRef]
- Peer, D.; Dekel, Y.; Melikhov, D.; Margalit, R. Fluoxetine inhibits multidrug resistance extrusion pumps and enhances responses to chemotherapy in syngeneic and in human xenograft mouse tumor models. Cancer Res. 2004, 64, 7562–7569. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Association between Fluoxetine Use and Overall Survival among Patients with Cancer Treated with PD-1/L1 Immunotherapy. Pharmaceuticals 2023, 16, 640. [Google Scholar] [CrossRef]
- Kadasah, S.F.; Alqahtani, A.M.S.; Alkhammash, A.; Radwan, M.O. Beyond Psychotropic: Potential Repurposing of Fluoxetine toward Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 6314. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, V.C.; Lu, M.L.; Lee, M.J.; McIntyre, R.S.; Majeed, A.; Lee, Y.; Chen, Y.L. The Association between Selective Serotonin Reuptake Inhibitors (SSRIs) Use and the Risk of Bladder Cancer: A Nationwide Population-Based Cohort Study. Cancers 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Yu, U.B.C.; Chiu, W.T.; Sun, H.Y.; Chien, Y.C.; Su, H.C.; Yen, S.Y.; Lai, H.W.; Bai, C.H.; Young, K.C.; et al. Fluoxetine regulates cell growth inhibition of interferon-α. Int. J. Oncol. 2016, 49, 1746–1754. [Google Scholar] [CrossRef]
- Yang, C.J.; Tan, Z.L.; Yang, J.D.; Hsu, F.T.; Chiang, C.H. Fluoxetine inactivates STAT3/NF-κB signaling and promotes sensitivity to cisplatin in bladder cancer. Biomed. Pharmacother. 2023, 164, 114962. [Google Scholar] [CrossRef]
- Liao, P.A.; Chu, P.Y.; Tan, Z.L.; Hsu, F.T.; Lee, Y.C.; Wu, H.J. STAT3 Inactivation and Induction of Apoptosis Associate with Fluoxetine-inhibited Epithelial-mesenchymal Transition and Growth of Triple-negative Breast Cancer In Vivo. Anticancer Res. 2022, 42, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Duan, J.; Wang, Y.; Chen, X.; Zhou, G.; Wang, R.; Fu, L.; Xu, F. Fluoxetine synergises with anticancer drugs to overcome multidrug resistance in breast cancer cells. Tumour Biol. 2012, 33, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Xu, L.; Zhang, X.; Peng, W.; Tang, Q.; Feng, C. The proliferation effects of fluoxetine and amitriptyline on human breast cancer cells and the underlying molecular mechanisms. Environ. Toxicol. Pharmacol. 2021, 83, 103586. [Google Scholar] [CrossRef] [PubMed]
- Bowie, M.; Pilie, P.; Wulfkuhle, J.; Lem, S.; Hoffman, A.; Desai, S.; Petricoin, E.; Carter, A.; Ambrose, A.; Seewaldt, V.; et al. Fluoxetine induces cytotoxic endoplasmic reticulum stress and autophagy in triple negative breast cancer. World J. Clin. Oncol. 2015, 6, 299–311. [Google Scholar] [CrossRef]
- Chomchoei, C.; Brimson, J.M.; Brimson, S. Repurposing fluoxetine to treat lymphocytic leukemia: Apoptosis induction, sigma-1 receptor upregulation, inhibition of IL-2 cytokine production, and autophagy induction. Expert Opin. Ther. Targets 2022, 26, 1087–1097. [Google Scholar] [CrossRef]
- Argov, M.; Kashi, R.; Peer, D.; Margalit, R. Treatment of resistant human colon cancer xenografts by a fluoxetine-doxorubicin combination enhances therapeutic responses comparable to an aggressive bevacizumab regimen. Cancer Lett. 2009, 274, 118–125. [Google Scholar] [CrossRef]
- Kannen, V.; Garcia, S.B.; Silva, W.A., Jr.; Gasser, M.; Mönch, R.; Alho, E.J.; Heinsen, H.; Scholz, C.J.; Friedrich, M.; Heinze, K.G.; et al. Oncostatic effects of fluoxetine in experimental colon cancer models. Cell Signal. 2015, 27, 1781–1788. [Google Scholar] [CrossRef]
- Jing, Q.; Wan, Q.; Nie, Y.; Luo, J.; Zhang, X.; Zhu, L.; Gui, H.; Li, L.; Wang, C.; Chen, S.; et al. Ansofaxine hydrochloride inhibits tumor growth and enhances Anti-TNFR2 in murine colon cancer model. Front. Pharmacol. 2023, 14, 1286061. [Google Scholar] [CrossRef]
- Marcinkute, M.; Afshinjavid, S.; Fatokun, A.A.; Javid, F.A. Fluoxetine selectively induces p53-independent apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2019, 857, 172441. [Google Scholar] [CrossRef]
- Kang, B.G.; Shende, M.; Inci, G.; Park, S.H.; Jung, J.S.; Kim, S.B.; Kim, J.H.; Mo, Y.W.; Seo, J.H.; Feng, J.H.; et al. Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification. Cancer Biol. Ther. 2023, 24, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Khin, P.P.; Po, W.W.; Thein, W.; Sohn, U.D. Apoptotic effect of fluoxetine through the endoplasmic reticulum stress pathway in the human gastric cancer cell line AGS. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 537–549. [Google Scholar] [CrossRef]
- Khing, T.M.; Po, W.W.; Sohn, U.D. Fluoxetine Enhances Anti-tumor Activity of Paclitaxel in Gastric Adenocarcinoma Cells by Triggering Apoptosis and Necroptosis. Anticancer Res. 2019, 39, 6155–6163. [Google Scholar] [CrossRef] [PubMed]
- Po, W.W.; Thein, W.; Khin, P.P.; Khing, T.M.; Han, K.W.W.; Park, C.H.; Sohn, U.D. Fluoxetine Simultaneously Induces Both Apoptosis and Autophagy in Human Gastric Adenocarcinoma Cells. Biomol. Ther. 2020, 28, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting glioblastoma signaling and metabolism with a re-purposed brain-penetrant drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef]
- Sarker, A.; Aziz, M.A.; Hossen, M.B.; Mollah, M.M.H.; Al-Amin, M.M.N.H. Discovery of key molecular signatures for diagnosis and therapies of glioblastoma by combining supervised and unsupervised learning approaches. Sci. Rep. 2024, 14, 27545. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Najafi, S.H.; Shafiee, F.; Hassanzadeh, S.; Farzipour, S.; Ghasemi, A.; Asgarian-Omran, H. Fluoxetine as an antidepressant medicine improves the effects of ionizing radiation for the treatment of glioma. J. Bioenerg. Biomembr. 2020, 52, 165–174. [Google Scholar] [CrossRef]
- Liu, K.H.; Yang, S.T.; Lin, Y.K.; Lin, J.W.; Lee, Y.H.; Wang, J.Y.; Hu, C.J.; Lin, E.Y.; Chen, S.M.; Then, C.K.; et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget 2015, 6, 5088–5101. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.R.; Chen, W.; Chen, M.H.; Wang, H.; Wang, X.D.; Sun, L.L.; Wang, F.Z.; Wang, D.C. Fluoxetine synergizes with temozolomide to induce the CHOP-dependent endoplasmic reticulum stress-related apoptosis pathway in glioma cells. Oncol. Rep. 2016, 36, 676–684. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Tang, Q.; Bi, F. The selective serotonin reuptake inhibitors enhance the cytotoxicity of sorafenib in hepatocellular carcinoma cells. Anticancer Drugs 2021, 32, 793–801. [Google Scholar] [CrossRef]
- Hsu, L.C.; Tu, H.F.; Hsu, F.T.; Yueh, P.F.; Chiang, I.T. Beneficial effect of fluoxetine on anti-tumor progression on hepatocellular carcinoma and non-small cell lung cancer bearing animal model. Biomed. Pharmacother. 2020, 126, 110054. [Google Scholar] [CrossRef]
- Chen, W.T.; Hsu, F.T.; Liu, Y.C.; Chen, C.H.; Hsu, L.C.; Lin, S.S. Fluoxetine Induces Apoptosis through Extrinsic/Intrinsic Pathways and Inhibits ERK/NF-κB-Modulated Anti-Apoptotic and Invasive Potential in Hepatocellular Carcinoma Cells In Vitro. Int. J. Mol. Sci. 2019, 20, 757. [Google Scholar] [CrossRef]
- Mun, A.R.; Lee, S.J.; Kim, G.B.; Kang, H.S.; Kim, J.S.; Kim, S.J. Fluoxetine-induced apoptosis in hepatocellular carcinoma cells. Anticancer Res. 2013, 33, 3691–3697. [Google Scholar]
- Serafeim, A.; Holder, M.J.; Grafton, G.; Chamba, A.; Drayson, M.T.; Luong, Q.T.; Bunce, C.M.; Gregory, C.D.; Barnes, N.M.; Gordon, J. Selective serotonin reuptake inhibitors directly signal for apoptosis in biopsy-like Burkitt lymphoma cells. Blood 2003, 101, 3212–3219. [Google Scholar] [CrossRef]
- Di Rosso, M.E.; Sterle, H.A.; Cremaschi, G.A.; Genaro, A.M. Beneficial Effect of Fluoxetine and Sertraline on Chronic Stress-Induced Tumor Growth and Cell Dissemination in a Mouse Model of Lymphoma: Crucial Role of Antitumor Immunity. Front. Immunol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Williams, D.C. The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt’s lymphoma. Int. J. Cancer 2011, 128, 1712–1723. [Google Scholar] [CrossRef]
- Frick, L.R.; Palumbo, M.L.; Zappia, M.P.; Brocco, M.A.; Cremaschi, G.A.; Genaro, A.M. Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem. Pharmacol. 2008, 75, 1817–1826. [Google Scholar] [CrossRef]
- Meredith, E.J.; Holder, M.J.; Chamba, A.; Challa, A.; Drake-Lee, A.; Bunce, C.M.; Drayson, M.T.; Pilkington, G.; Blakely, R.D.; Dyer, M.J.; et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: Probing a potential anti-tumor target for psychotropics. FASEB J. 2005, 19, 1187–1189. [Google Scholar] [CrossRef]
- He, A.; Wu, M.; Pu, Y.; Li, R.; Zhang, Y.; He, J.; Xia, Y.; Ma, Y. Fluoxetine as a Potential Therapeutic Agent for Inhibiting Melanoma Brain and Lung Metastasis: Induction of Apoptosis, G0/G1 Cell Cycle Arrest, and Disruption of Autophagy Flux. J. Cancer 2024, 15, 3825–3840. [Google Scholar] [CrossRef]
- Grygier, B.; Arteta, B.; Kubera, M.; Basta-Kaim, A.; Budziszewska, B.; Leśkiewicz, M.; Curzytek, K.; Duda, W.; Lasoń, W.; Maes, M. Inhibitory effect of antidepressants on B16F10 melanoma tumor growth. Pharmacol. Rep. 2013, 65, 672–681. [Google Scholar] [CrossRef]
- Shao, S.; Zhuang, X.; Zhang, L.; Qiao, T. Antidepressants Fluoxetine Mediates Endoplasmic Reticulum Stress and Autophagy of Non-Small Cell Lung Cancer Cells Through the ATF4-AKT-mTOR Signaling Pathway. Front. Pharmacol. 2022, 13, 904701. [Google Scholar] [CrossRef]
- Gui, H.; Nie, Y.; Yuan, H.; Wang, M.; Li, L.; Zhu, L.; Chen, S.; Jing, Q.; Wan, Q.; Lv, H.; et al. Ansofaxine suppressed NSCLC progression by increasing sensitization to combination immunotherapy. Int. Immunopharmacol. 2024, 146, 113918. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lin, S.S.; Hsu, F.T.; Chung, J.G. Fluoxetine Inhibits DNA Repair and NF-ĸB-modulated Metastatic Potential in Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 5201–5210. [Google Scholar] [CrossRef]
- Stepulak, A.; Rzeski, W.; Sifringer, M.; Brocke, K.; Gratopp, A.; Kupisz, K.; Turski, L.; Ikonomidou, C. Fluoxetine inhibits the extracellular signal regulated kinase pathway and suppresses growth of cancer cells. Cancer Biol. Ther. 2008, 7, 1685–1693. [Google Scholar] [CrossRef]
- Chen, W.T.; Tsai, Y.H.; Tan, P.; Hsu, F.T.; Wang, H.D.; Lin, W.C.; Lin, F.H.; Wu, C.T. Fluoxetine Inhibits STAT3-mediated Survival and Invasion of Osteosarcoma Cells. Anticancer Res. 2023, 43, 1193–1199. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, Y.J.; Jang, E.R.; Kim, W.; Myung, S.C. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin. Pharmacol. Toxicol. 2010, 106, 446–453. [Google Scholar] [CrossRef]
- Schneider, M.A.; Heeb, L.; Beffinger, M.M.; Pantelyushin, S.; Linecker, M.; Roth, L.; Lehmann, K.; Ungethüm, U.; Kobold, S.; Graf, R.; et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med. 2021, 13, eabc8188. [Google Scholar] [CrossRef]
- Saponara, E.; Visentin, M.; Baschieri, F.; Seleznik, G.; Martinelli, P.; Esposito, I.; Buschmann, J.; Chen, R.; Parrotta, R.; Borgeaud, N.; et al. Serotonin uptake is required for Rac1 activation in Kras-induced acinar-to-ductal metaplasia in the pancreas. J. Pathol. 2018, 246, 352–365. [Google Scholar] [CrossRef]
- Chen, L.; Ji, Y.; Li, A.; Liu, B.; Shen, K.; Su, R.; Ma, Z.; Zhang, W.; Wang, Q.; Zhu, Y.; et al. High-throughput drug screening identifies fluoxetine as a potential therapeutic agent for neuroendocrine prostate cancer. Front. Oncol. 2023, 13, 1085569. [Google Scholar] [CrossRef]
- Abdul, M.; Logothetis, C.J.; Hoosein, N.M. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J. Urol. 1995, 154, 247–250. [Google Scholar] [CrossRef]
- Lin, K.L.; Chou, C.T.; Cheng, J.S.; Chang, H.T.; Liang, W.Z.; Kuo, C.C.; Chen, I.L.; Tseng, L.L.; Shieh, P.; Wu, R.F.; et al. Effect of fluoxetine on [Ca2+]i and cell viability in OC2 human oral cancer cells. Chin. J. Physiol. 2014, 57, 256–264. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Ribeiro, E.; Vale, N. A Surprising Repurposing of Central Nervous System Drugs against Squamous Cell Carcinoma of the Bladder, UM-UC-5. Pharmaceutics 2024, 16, 212. [Google Scholar] [CrossRef]
- Petrosyan, E.; Fares, J.; Cordero, A.; Rashidi, A.; Arrieta, V.A.; Kanojia, D.; Lesniak, M.S. Repurposing autophagy regulators in brain tumors. Int. J. Cancer 2022, 151, 167–180. [Google Scholar] [CrossRef]
- Stopper, H.; Garcia, S.B.; Waaga-Gasser, A.M.; Kannen, V. Antidepressant fluoxetine and its potential against colon tumors. World J. Gastrointest. Oncol. 2014, 6, 11–21. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Zhao, Y.; Jiang, Y.; Chen, L.; Yu, Y.; Ouyang, L. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018, 51, e12402. [Google Scholar] [CrossRef]
- Johnson, R.D.; Lewis, R.J.; Angier, M.K. The distribution of fluoxetine in human fluids and tissues. J. Anal. Toxicol. 2007, 31, 409–414. [Google Scholar] [CrossRef]
- Karson, C.N.; Newton, J.E.; Livingston, R.; Jolly, J.B.; Cooper, T.B.; Sprigg, J.; Komoroski, R.A. Human brain fluoxetine concentrations. J. Neuropsychiatry Clin. Neurosci. 1993, 5, 322–329. [Google Scholar] [CrossRef]
- Frampton, J.E.; Keating, G.M. Celecoxib: A review of its use in the management of arthritis and acute pain. Drugs 2007, 67, 2433–2472. [Google Scholar] [CrossRef]
- Clemett, D.; Goa, K.L. Celecoxib: A review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Bąk, U.; Krupa, A. Challenges and Opportunities for Celecoxib Repurposing. Pharm. Res. 2023, 40, 2329–2345. [Google Scholar] [CrossRef]
- Kast, R.E. Adding high-dose celecoxib to increase effectiveness of standard glioblastoma chemoirradiation. Ann. Pharm. Fr. 2021, 79, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Wei, Y.T.; Mu, L.L.; Wen, G.R.; Zhao, K. The molecular mechanisms of celecoxib in tumor development. Medicine 2020, 99, e22544. [Google Scholar] [CrossRef]
- Qian, X.; Yang, H.; Ye, Z.; Gao, B.; Qian, Z.; Ding, Y.; Mao, Z.; Du, Y.; Wang, W. Celecoxib Augments Paclitaxel-Induced Immunogenic Cell Death in Triple-Negative Breast Cancer. ACS Nano 2024, 18, 15864–15877. [Google Scholar] [CrossRef] [PubMed]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention—Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Gentili, A.; Mollica, A.; Trisciuoglio, D.; Supuran, C.T. Dual Cyclooxygenase and Carbonic Anhydrase Inhibition by Nonsteroidal Anti-Inflammatory Drugs for the Treatment of Cancer. Curr. Med. Chem. 2015, 22, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Urquidi, V.; Goodison, S.; Kim, J.; Chang, M.; Dai, Y.; Rosser, C.J. Vascular endothelial growth factor, carbonic anhydrase 9, and angiogenin as urinary biomarkers for bladder cancer detection. Urology 2012, 79, e1–e6. [Google Scholar] [CrossRef]
- de Martino, M.; Lucca, I.; Mbeutcha, A.; Wiener, H.G.; Haitel, A.; Susani, M.; Shariat, S.F.; Klatte, T. Carbonic anhydrase IX as a diagnostic urinary marker for urothelial bladder cancer. Eur. Urol. 2015, 68, 552–554. [Google Scholar] [CrossRef]
- Malentacchi, F.; Vinci, S.; Della Melina, A.; Kuncova, J.; Villari, D.; Giannarini, G.; Nesi, G.; Selli, C.; Orlando, C. Splicing variants of carbonic anhydrase IX in bladder cancer and urine sediments. Urol. Oncol. 2012, 30, 278–284. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; Rao, J.Y.; Yu, H.; de Martino, M.; Kawaoka, K.; Wong, S.G.; Belldegrun, A.S.; Pantuck, A.J. Carbonic anhydrase IX in bladder cancer: A diagnostic, prognostic, and therapeutic molecular marker. Cancer 2009, 115, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.P.; Chen, X.N.; Xu, P.F.; Shao, S.H.; Shen, Y.F. Expression and prognostic value of carbonic anhydrase IX (CA-IX) in bladder urothelial carcinoma. BMC Urol. 2022, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; James, N.D. Molecular markers in bladder cancer. Semin. Radiat. Oncol. 2005, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Tille, J.C.; Combescure, C.; Egger, J.F.; Laouiti, M.; Hammad, K.; Granger, P.; Rubbia-Brandt, L.; Miralbell, R. The prognostic value of expression of HIF1α, EGFR and VEGF-A, in localized prostate cancer for intermediate- and high-risk patients treated with radiation therapy with or without androgen deprivation therapy. Radiat. Oncol. 2012, 30, 66. [Google Scholar] [CrossRef]
- Knudsen, J.F.; Carlsson, U.; Hammarström, P.; Sokol, G.H.; Cantilena, L.R. The cyclooxygenase-2 inhibitor celecoxib is a potent inhibitor of human carbonic anhydrase II. Inflammation 2004, 28, 285–290. [Google Scholar] [CrossRef]
- Sethi, K.K.; Vullo, D.; Verma, S.M.; Tanç, M.; Carta, F.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of the human carbonic anhydrase isoforms I, II, VII, IX and XII with benzene sulfonamides incorporating 4,5,6,7-tetrabromophthalimide moiety. Bioorg. Med. Chem. 2013, 21, 5973–5982. [Google Scholar] [CrossRef]
- Aoun, F.; Kourie, H.R.; Artigas, C.; Roumeguère, T. Next revolution in molecular theranostics: Personalized medicine for urologic cancers. Future Oncol. 2015, 11, 2205–2219. [Google Scholar] [CrossRef]
- Swietach, P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Rev. 2019, 38, 5–15. [Google Scholar] [CrossRef]
- Lee, S.H.; McIntyre, D.; Honess, D.; Hulikova, A.; Pacheco-Torres, J.; Cerdán, S.; Swietach, P.; Harris, A.L.; Griffiths, J.R. Carbonic anhydrase IX is a pH-stat that sets an acidic tumour extracellular pH in vivo. Br. J. Cancer 2018, 119, 622–630. [Google Scholar] [CrossRef]
- Becker, H.M. Carbonic anhydrase IX and acid transport in cancer. Br. J. Cancer 2020, 122, 157–167. [Google Scholar] [CrossRef]
- Queen, A.; Bhutto, H.N.; Yousuf, M.; Syed, M.A.; Hassan, M.I. Carbonic anhydrase IX: A tumor acidification switch in heterogeneity and chemokine regulation. Semin. Cancer Biol. 2022, 86 Pt 3, 899–913. [Google Scholar] [CrossRef]
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Dedhar, S. Carbonic anhydrase IX (CAIX) as a mediator of hypoxia-induced stress response in cancer cells. Subcell. Biochem. 2014, 75, 255–269. [Google Scholar] [CrossRef]
- Mussi, S.; Rezzola, S.; Chiodelli, P.; Nocentini, A.; Supuran, C.T.; Ronca, R. Antiproliferative effects of sulphonamide carbonic anhydrase inhibitors C18, SLC-0111 and acetazolamide on bladder, glioblastoma and pancreatic cancer cell lines. J. Enzym. Inhib. Med. Chem. 2022, 37, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, R.; Wang, L.; Chen, G.; Wang, H.; Wang, Z.; Zhao, D.; Pavlov, V.N.; Kabirov, I.; Wang, Z.; et al. Identification of Carbonic Anhydrase IX as a Novel Target for Endoscopic Molecular Imaging of Human Bladder Cancer. Cell Physiol. Biochem. 2018, 47, 1565–1577. [Google Scholar] [CrossRef]
- Furuya, H.; Sakatani, T.; Tanaka, S.; Murakami, K.; Waldron, R.T.; Hogrefe, W.; Rosser, C.J. Bladder cancer risk stratification with the Oncuria 10-plex bead-based urinalysis assay using three different Luminex xMAP instrumentation platforms. J. Transl. Med. 2024, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Todenhöfer, T.; Gibb, E.A.; Seiler, R.; Kamyabi, A.; Hennenlotter, J.; McDonald, P.; Moskalev, I.; Stewart, C.; Gao, J.; Fazli, L.; et al. Evaluation of carbonic anhydrase IX as a potential therapeutic target in urothelial carcinoma. Urol. Oncol. 2021, 39, e1–e498. [Google Scholar] [CrossRef]
- Urquidi, V.; Chang, M.; Dai, Y.; Kim, J.; Wolfson, E.D.; Goodison, S.; Rosser, C.J. IL-8 as a urinary biomarker for the detection of bladder cancer. BMC Urol. 2012, 12, 12. [Google Scholar] [CrossRef]
- Tachibana, H.; Gi, M.; Kato, M.; Yamano, S.; Fujioka, M.; Kakehashi, A.; Hirayama, Y.; Koyama, Y.; Tamada, S.; Nakatani, T.; et al. Carbonic anhydrase 2 is a novel invasion-associated factor in urinary bladder cancers. Cancer Sci. 2017, 108, 331–337. [Google Scholar] [CrossRef]
- Sherwood, B.T.; Colquhoun, A.J.; Richardson, D.; Bowman, K.J.; O’Byrne, K.J.; Kockelbergh, R.C.; Symonds, R.P.; Mellon, J.K.; Jones, G.D. Carbonic anhydrase IX expression and outcome after radiotherapy for muscle-invasive bladder cancer. Clin. Oncol. (R. Coll. Radiol.) 2007, 19, 777–783. [Google Scholar] [CrossRef]
- Pirinççi, N.; Geçit, I.; Güneş, M.; Yüksel, M.B.; Kaba, M.; Tanık, S.; Demir, H.; Aslan, M. Serum adenosine deaminase, catalase and carbonic anhydrase activities in patients with bladder cancer. Clinics 2012, 67, 1443–1446. [Google Scholar] [CrossRef]
- Wen, J.; Yang, T.; Mallouk, N.; Zhang, Y.; Li, H.; Lambert, C.; Li, G. Urinary Exosomal CA9 mRNA as a Novel Liquid Biopsy for Molecular Diagnosis of Bladder Cancer. Int. J. Nanomed. 2021, 16, 4805–4811. [Google Scholar] [CrossRef] [PubMed]
- Matsue, T.; Gi, M.; Shiota, M.; Tachibana, H.; Suzuki, S.; Fujioka, M.; Kakehashi, A.; Yamamoto, T.; Kato, M.; Uchida, J.; et al. The carbonic anhydrase inhibitor acetazolamide inhibits urinary bladder cancers via suppression of β-catenin signaling. Cancer Sci. 2022, 113, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Licarete, E.; Wu, X.; Petrusca, D.; Maguire, C.; Jacobsen, M.; Colter, A.; Sandusky, G.E.; Czader, M.; Capitano, M.L.; et al. Pharmacological inhibition of Carbonic Anhydrase IX and XII to enhance targeting of acute myeloid leukaemia cells under hypoxic conditions. J. Cell Mol. Med. 2021, 25, 11039–11052. [Google Scholar] [CrossRef] [PubMed]
- Alyaqubi, K.J.; Dosh, R.H.; Al-Fatlawi, R.B.; Al-Rehemi, S.M.; Hadi, N.R. Gene expression of carbonic anhydrase 9 (CA9) in de novo acute leukemia as a predictive marker for prognosis. J. Med. Life 2022, 15, 1158–1163. [Google Scholar] [CrossRef]
- Shamis, S.A.K.; Quinn, J.; Al-Badran, S.; McKenzie, M.; Hatthakarnkul, P.; Lynch, G.; Lian, G.Y.; Numprasit, W.; Romics, L., Jr.; Andersen, D.; et al. Elucidation of Dysregulated Pathways Associated with Hypoxia in Oestrogen Receptor-Negative Breast Cancer. Cancer Med. 2024, 13, e70274. [Google Scholar] [CrossRef]
- Ochi, F.; Shiozaki, A.; Ichikawa, D.; Fujiwara, H.; Nakashima, S.; Takemoto, K.; Kosuga, T.; Konishi, H.; Komatsu, S.; Okamoto, K.; et al. Carbonic Anhydrase XII as an Independent Prognostic Factor in Advanced Esophageal Squamous Cell Carcinoma. J. Cancer 2015, 6, 922–929. [Google Scholar] [CrossRef]
- Driessen, A.; Landuyt, W.; Pastorekova, S.; Moons, J.; Goethals, L.; Haustermans, K.; Nafteux, P.; Penninckx, F.; Geboes, K.; Lerut, T.; et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann. Surg. 2006, 243, 334–340. [Google Scholar] [CrossRef]
- Fidan, E.; Mentese, A.; Ozdemir, F.; Deger, O.; Kavgaci, H.; Caner Karahan, S.; Aydin, F. Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Med. Oncol. 2013, 30, 540. [Google Scholar] [CrossRef]
- Li, G.; Chen, T.W.; Nickel, A.C.; Muhammad, S.; Steiger, H.J.; Tzaridis, T.; Hänggi, D.; Zeidler, R.; Zhang, W.; Kahlert, U.D. Carbonic Anhydrase XII is a Clinically Significant, Molecular Tumor-Subtype Specific Therapeutic Target in Glioma with the Potential to Combat Invasion of Brain Tumor Cells. Onco Targets Ther. 2021, 14, 1707–1718. [Google Scholar] [CrossRef]
- Gu, X.F.; Shi, C.B.; Zhao, W. Prognostic value of carbonic anhydrase XII (CA XII) overexpression in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 2173–2183. [Google Scholar] [PubMed]
- Méhes, G.; Matolay, O.; Beke, L.; Czenke, M.; Pórszász, R.; Mikó, E.; Bai, P.; Berényi, E.; Trencsényi, G. Carbonic Anhydrase Inhibitor Acetazolamide Enhances CHOP Treatment Response and Stimulates Effector T-Cell Infiltration in A20/BalbC Murine B-Cell Lymphoma. Int. J. Mol. Sci. 2020, 21, 5001. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.J.; Park, J.J.; Ko, G.H.; Seo, J.H.; Jeong, B.K.; Kang, K.M.; Woo, S.H.; Kim, J.P.; Hwa, J.S.; Carey, T.E. HIF-1α and CA-IX as predictors of locoregional control for determining the optimal treatment modality for early stage laryngeal carcinoma. Head Neck 2015, 37, 505–510. [Google Scholar] [CrossRef]
- Schrijvers, M.L.; van der Laan, B.F.; de Bock, G.H.; Pattje, W.J.; Mastik, M.F.; Menkema, L.; Langendijk, J.A.; Kluin, P.M.; Schuuring, E.; van der Wal, J.E. Overexpression of intrinsic hypoxia markers HIF1alpha and CA-IX predict for local recurrence in stage T1-T2 glottic laryngeal carcinoma treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, H.; Lin, L.; Sun, X.; Wang, D. Association between carbonic anhydrase 9 expression and poor prognosis in sinonasal squamous cell carcinoma. Ann. Diagn. Pathol. 2020, 49, 151643. [Google Scholar] [CrossRef]
- Hui, E.P.; Chan, A.T.; Pezzella, F.; Turley, H.; To, K.F.; Poon, T.C.; Zee, B.; Mo, F.; Teo, P.M.; Huang, D.P.; et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin. Cancer Res. 2002, 8, 2595–2604. [Google Scholar]
- Fecikova, S.; Csaderova, L.; Belvoncikova, P.; Puzderova, B.; Bernatova, K.; Talac, T.; Pastorek, J.; Barathova, M. Can hypoxia marker carbonic anhydrase IX serve as a potential new diagnostic marker and therapeutic target of non-small cell lung cancer? Neoplasma 2024, 71, 123–142. [Google Scholar] [CrossRef]
- Eckert, A.W.; Horter, S.; Bethmann, D.; Kotrba, J.; Kaune, T.; Rot, S.; Bache, M.; Bilkenroth, U.; Reich, W.; Greither, T.; et al. Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 375. [Google Scholar] [CrossRef]
- Nazon, C.; Pierrevelcin, M.; Willaume, T.; Lhermitte, B.; Weingertner, N.; Marco, A.D.; Bund, L.; Vincent, F.; Bierry, G.; Gomez-Brouchet, A.; et al. Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas. Cancers 2022, 14, 1482. [Google Scholar] [CrossRef]
- Yang, J.; Tong, X.; Wang, W.; Yu, X.; Xu, J.; Shi, S. Targeting CA9 restricts pancreatic cancer progression through pH regulation and ROS production. Cell. Oncol. 2024, 47, 2367–2382. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Kou, Q.; Sun, L.; Ma, Y.; Yang, T.; Hu, X. Establishment of a Prognostic Model for Pancreatic Cancer Based on Hypoxia-Related Genes. Technol. Cancer Res. Treat. 2024, 23, 15330338241288687. [Google Scholar] [CrossRef]
- Terai, T.; Nishiwada, S.; Nagai, M.; Nakamura, K.; Kohara, Y.; Yasuda, S.; Matsuo, Y.; Doi, S.; Sakata, T.; Kumada, H.; et al. Clinical impact of carbonic anhydrase 9 expression on neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Pancreatology 2024, 24, 938–946. [Google Scholar] [CrossRef]
- Schmidt, J.; Oppermann, E.; Blaheta, R.A.; Schreckenbach, T.; Lunger, I.; Rieger, M.A.; Bechstein, W.O.; Holzer, K.; Malkomes, P. Carbonic-anhydrase IX expression is increased in thyroid cancer tissue and represents a potential therapeutic target to eradicate thyroid tumor-initiating cells. Mol. Cell Endocrinol. 2021, 535, 111382. [Google Scholar] [CrossRef] [PubMed]
- Elfakharany, H.K.; Ghoraba, H.M.; Gaweesh, K.A.; Eldeen, A.A.S.; Eid, A.M. Immunohistochemical expression of cytochrome P4A11 (CYP4A11), carbonic anhydrase 9 (CAIX) and Ki67 in renal cell carcinoma; diagnostic relevance and relations to clinicopathological parameters. Pathol. Res. Pract. 2024, 253, 155070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, L.; Cui, Y.; Wang, L.; Wu, J.; Wang, J.; Zhao, H.; Liu, C.; Cui, Y.; Zhang, Y.; et al. Prognostic Significance of Membranous Carbonic Anhydrase IX Expression in Patients with Nonmetastatic Clear Cell Renal Cell Carcinoma of Different Tumor Stages. Cancer Biother. Radiopharm. 2022, 37, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.G.; O’Hurley, G.; O’Grady, A.; Fitzpatrick, J.M.; Kay, E.; Watson, R.W. Carbonic anhydrase IX expression in prostate cancer. Prostate Cancer Prostatic Dis. 2010, 13, 178–181. [Google Scholar] [CrossRef]
- Faviana, P.; Belgio, B.; Panichi, M.; Manassero, F.; Selli, C.; Boldrini, L. Intraductal prostate cancer: An aggressive subset of prostate cancers? Immunophenotypic evaluation. Urol. Ann. 2022, 14, 177–182. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.A.; Riganti, C. Carbonic Anhydrase XII Inhibitors Overcome P-Glycoprotein-Mediated Resistance to Temozolomide in Glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef]
- Mujumdar, P.; Kopecka, J.; Bua, S.; Supuran, C.T.; Riganti, C.; Poulsen, S.A. Carbonic Anhydrase XII Inhibitors Overcome Temozolomide Resistance in Glioblastoma. J. Med. Chem. 2019, 62, 4174–4192. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, T.M.; Zhu, C. Effects of nonsteroidal anti-inflammatory drugs on serum proinflammatory cytokines in the treatment of ankylosing spondylitis. Biochem. Cell Biol. 2018, 96, 450–456. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, X.; Jia, X.; Wang, R.; Liu, L.; Zhang, M.; Wan, X.; Tang, C.; Huang, L. Prevention of Severe Acute Pancreatitis with Cyclooxygenase-2 Inhibitors: A Randomized Controlled Clinical Trial. Am. J. Gastroenterol. 2020, 115, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.H.; Hosseini, F.; Modabbernia, A.; Ashrafi, M.; Akhondzadeh, S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. J. Affect. Disord. 2012, 141, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Roth, M.D.; Fishbein, M.C.; Aberle, D.R.; Zhang, Z.F.; Rao, J.Y.; Tashkin, D.P.; Goodglick, L.; Holmes, E.C.; Cameron, R.B.; et al. Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev. Res. 2011, 4, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Mets, T.; Bautmans, I.; Njemini, R.; Lambert, M.; Demanet, C. The influence of celecoxib on muscle fatigue resistance and mobility in elderly patients with inflammation. Am. J. Geriatr. Pharmacother. 2004, 2, 230–238. [Google Scholar] [CrossRef]
- Bianchi, M.; Broggini, M.; Balzarini, P.; Franchi, S.; Sacerdote, P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: Comparison with celecoxib. Int. J. Clin. Pract. 2007, 61, 1270–1277. [Google Scholar] [CrossRef]
- Theodoridou, A.; Gika, H.; Diza, E.; Garyfallos, A.; Settas, L. In vivo study of pro-inflammatory cytokine changes in serum and synovial fluid during treatment with celecoxib and etoricoxib and correlation with VAS pain change and synovial membrane penetration index in patients with inflammatory arthritis. Mediterr. J. Rheumatol. 2017, 28, 33–40. [Google Scholar] [CrossRef]
- Mauer, J.; Denson, J.L.; Brüning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Seguchi, T.; Yokokawa, K.; Sugao, H.; Nakano, E.; Sonoda, T.; Okuyama, A. Interleukin-6 activity in urine and serum in patients with bladder carcinoma. J. Urol. 1992, 148, 791–794. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Sun, Y.; Cai, L.; Zhang, J. The prognostic significance of preoperative platelet-to-lymphocyte ratio and interleukin-6 level in non-muscle invasive bladder cancer. Int. J. Biol. Mark. 2024, 39, 255–264. [Google Scholar] [CrossRef]
- Eruslanov, E.; Neuberger, M.; Daurkin, I.; Perrin, G.Q.; Algood, C.; Dahm, P.; Rosser, C.; Vieweg, J.; Gilbert, S.M.; Kusmartsev, S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int. J. Cancer 2012, 130, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Lin, P.Y.; Wu, C.F.; Chen, W.C.; Wu, C.T. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE 2013, 8, e61901. [Google Scholar] [CrossRef]
- Andrews, B.; Shariat, S.F.; Kim, J.H.; Wheeler, T.M.; Slawin, K.M.; Lerner, S.P. Preoperative plasma levels of interleukin-6 and its soluble receptor predict disease recurrence and survival of patients with bladder cancer. J. Urol. 2002, 167, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Schuettfort, V.M.; Pradere, B.; Trinh, Q.D.; D’Andrea, D.; Quhal, F.; Mostafaei, H.; Laukhtina, E.; Mori, K.; Sari Motlagh, R.; Rink, M.; et al. Impact of preoperative plasma levels of interleukin 6 and interleukin 6 soluble receptor on disease outcomes after radical cystectomy for bladder cancer. Cancer Immunol. Immunother. 2022, 71, 85–95. [Google Scholar] [CrossRef]
- Tonry, C.L.; Evans, R.M.; Ruddock, M.W.; Duggan, B.; McCloskey, O.; Maxwell, A.P.; O’Rourke, D.; Boyd, R.E.; Watt, J.; Reid, C.N.; et al. Clinical features and predictive biomarkers for bladder cancer in patients with type 2 diabetes presenting with haematuria. Diabetes Metab. Res. Rev. 2022, 38, e3546. [Google Scholar] [CrossRef] [PubMed]
- El-Salahy, E.M. Evaluation of cytokeratin-19 & cytokeratin-20 and interleukin-6 in Egyptian bladder cancer patients. Clin. Biochem. 2002, 35, 607–613. [Google Scholar] [CrossRef]
- Margel, D.; Pevsner-Fischer, M.; Baniel, J.; Yossepowitch, O.; Cohen, I.R. Stress proteins and cytokines are urinary biomarkers for diagnosis and staging of bladder cancer. Eur. Urol. 2011, 59, 113–119. [Google Scholar] [CrossRef]
- Meyers, F.J.; Gumerlock, P.H.; Kawasaki, E.S.; Wang, A.M.; deVere White, R.W.; Erlich, H.A. Bladder cancer. Human leukocyte antigen II, interleukin-6, and interleukin-6 receptor expression determined by the polymerase chain reaction. Cancer 1991, 67, 2087–2095. [Google Scholar] [CrossRef]
- Okamoto, M.; Hattori, K.; Oyasu, R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. Int. J. Cancer 1997, 72, 149–154. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Tian, Y.; Weiss, R.M.; Martin, D.T. Synergistic inhibition of GP130 and ERK signaling blocks chemoresistant bladder cancer cell growth. Cell Signal. 2019, 63, 109381. [Google Scholar] [CrossRef]
- Wei, H. Interleukin 6 signaling maintains the stem-like properties of bladder cancer stem cells. Transl. Cancer Res. 2019, 8, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Lin, W.Y.; Chen, W.C.; Chen, M.F. Predictive Value of CD44 in Muscle-Invasive Bladder Cancer and Its Relationship with IL-6 Signaling. Ann. Surg. Oncol. 2018, 25, 3518–3526. [Google Scholar] [CrossRef] [PubMed]
- Warli, S.M.; Prapiska, F.F.; Siregar, D.I.S.; Wijaya, W.S. Association Between Interleukin-6 Levels and Lymph Node Metastasis in Bladder Cancer Patients. World J. Oncol. 2022, 13, 365–369. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Knipper, K.; Lyu, S.I.; Quaas, A.; Bruns, C.J.; Schmidt, T. Cancer Associated Fibroblast Heterogeneity and Its Influence on the Extracellular Matrix and the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 13482. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef]
- Dong, D.; Yao, Y.; Song, J.; Sun, L.; Zhang, G. Cancer-Associated Fibroblasts Regulate Bladder Cancer Invasion and Metabolic Phenotypes through Autophagy. Dis. Mark. 2021, 2021, 6645220. [Google Scholar] [CrossRef]
- Yang, F.; Guo, Z.; He, C.; Qing, L.; Wang, H.; Wu, J.; Lu, X. Cancer associated fibroblasts promote cell proliferation and invasion via paracrine Wnt/IL1β signaling pathway in human bladder cancer. Neoplasma 2021, 68, 79–86. [Google Scholar] [CrossRef]
- Cho, J.; Lee, H.J.; Hwang, S.J.; Min, H.Y.; Kang, H.N.; Park, A.Y.; Hyun, S.Y.; Sim, J.Y.; Lee, H.J.; Jang, H.J.; et al. The Interplay between Slow-Cycling, Chemoresistant Cancer Cells and Fibroblasts Creates a Proinflammatory Niche for Tumor Progression. Cancer Res. 2020, 80, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Peluffo, G.; Chen, H.; Gelman, R.; Schnitt, S.; Polyak, K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc. Natl. Acad. Sci. USA 2009, 106, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.D.; Leigh, R.J.; Tasena, H.; Mellone, M.; Coletta, R.D.; Parkinson, E.K.; Prime, S.S.; Thomas, G.J.; Paterson, I.C.; Zhou, D.; et al. A miR-335/COX-2/PTEN axis regulates the secretory phenotype of senescent cancer-associated fibroblasts. Aging 2016, 8, 1608–1635. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front. Mol. Biosci. 2023, 9, 1070383. [Google Scholar] [CrossRef]
- Gately, S.; Li, W.W. Multiple roles of COX-2 in tumor angiogenesis: A target for antiangiogenic therapy. Semin. Oncol. 2004, 31 (Suppl. S7), 2–11. [Google Scholar] [CrossRef] [PubMed]
- Franceschin, L.; Guidotti, A.; Mazzetto, R.; Tartaglia, J.; Ciolfi, C.; Alaibac, M.; Sernicola, A. Repurposing Historic Drugs for Neutrophil-Mediated Inflammation in Skin Disorders. Biomolecules 2024, 14, 1515. [Google Scholar] [CrossRef]
- Lovell, K.K.; Momin, R.I.; Sangha, H.S.; Feldman, S.R.; Pichardo, R.O. Dapsone Use in Dermatology. Am. J. Clin. Dermatol. 2024, 25, 811–822. [Google Scholar] [CrossRef]
- Ghaoui, N.; Hanna, E.; Abbas, O.; Kibbi, A.G.; Kurban, M. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020, 59, 787–795. [Google Scholar] [CrossRef]
- Kast, R.E. Erlotinib augmentation with dapsone for rash mitigation and increased anti-cancer effectiveness. Springerplus 2015, 4, 638. [Google Scholar] [CrossRef]
- Kanwar, B.; Khattak, A.; Kast, R.E. Dapsone Lowers Neutrophil to Lymphocyte Ratio and Mortality in COVID-19 Patients Admitted to the ICU. Int. J. Mol. Sci. 2022, 23, 15563. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kanwar, B.; Khattak, A.; Balentine, J.; Nguyen, N.H.; Kast, R.E.; Lee, C.J.; Bourbeau, J.; Altschuler, E.L.; Sergi, C.M.; et al. COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs. Int. J. Mol. Sci. 2022, 23, 13260. [Google Scholar] [CrossRef]
- Kanwar, B.A.; Khattak, A.; Balentine, J.; Lee, J.H.; Kast, R.E. Benefits of Using Dapsone in Patients Hospitalized with COVID-19. Vaccines 2022, 10, 195. [Google Scholar] [CrossRef]
- Kast, R.E. Dapsone as treatment adjunct in ARDS. Exp. Lung Res. 2020, 46, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Quagliuolo, L.; Alaia, C.; Grimaldi, A.; Addeo, R.; Nicoletti, G.F.; Kast, R.E.; Caraglia, M. The strange connection between epidermal growth factor receptor tyrosine kinase inhibitors and dapsone: From rash mitigation to the increase in antitumor activity. Curr. Med. Res. Opin. 2016, 32, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Research Supporting a Pilot Study of Metronomic Dapsone during Glioblastoma Chemoirradiation. Med. Sci. 2021, 9, 12. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Kast, R.E.; Siegelin, M.D.; Dwucet, A.; Schneider, E.; Westhoff, M.A.; Wirtz, C.R.; Chen, X.Y.; Halatsch, M.E.; Bolm, C. Anti-glioma Activity of Dapsone and Its Enhancement by Synthetic Chemical Modification. Neurochem. Res. 2017, 42, 3382–3389. [Google Scholar] [CrossRef]
- Mao, P.; Wang, T.; Du, C.W.; Yu, X.; Wang, M.D. CXCL5 promotes tumorigenesis and angiogenesis of glioblastoma via JAK-STAT/NF-κb signaling pathways. Mol. Biol. Rep. 2023, 50, 8015–8023. [Google Scholar] [CrossRef]
- Diaz-Ruiz, A.; Nader-Kawachi, J.; Calderón-Estrella, F.; Mata-Bermudez, A.; Alvarez-Mejia, L.; Ríos, C. Dapsone, More than an Effective Neuro and Cytoprotective Drug. Curr. Neuropharmacol. 2022, 20, 194–210. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Lou, J.; Leng, M.; Yang, Q.; Xu, X.; Zhou, X. CTC-neutrophil interaction: A key driver and therapeutic target of cancer metastasis. Biomed. Pharmacother. 2024, 180, 117474. [Google Scholar] [CrossRef]
- Sabit, H.; Arneth, B.; Abdel-Ghany, S.; Madyan, E.F.; Ghaleb, A.H.; Selvaraj, P.; Shin, D.M.; Bommireddy, R.; Elhashash, A. Beyond Cancer Cells: How the Tumor Microenvironment Drives Cancer Progression. Cells 2024, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Koenderman, L.; Vrisekoop, N. Neutrophils in cancer: From biology to therapy. Cell Mol. Immunol. 2025, 22, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Masui, H.; Kawada, K.; Obama, K. Neutrophil and Colorectal Cancer. Int. J. Mol. Sci. 2024, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zhong, W.; Xu, P.; Wang, B.; Zhang, L.; Yang, M.; Chen, J.; Li, H.; Li, S.; Chen, X.; et al. Tumor-associated neutrophils suppress CD8+ T cell immunity in urothelial bladder carcinoma through the COX-2/PGE2/IDO1 Axis. Br. J. Cancer 2024, 130, 880–891. [Google Scholar] [CrossRef]
- Inoue, K.; Slaton, J.W.; Kim, S.J.; Perrotte, P.; Eve, B.Y.; Bar-Eli, M.; Radinsky, R.; Dinney, C.P. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000, 60, 2290–2299. [Google Scholar] [CrossRef]
- Mian, B.M.; Dinney, C.P.; Bermejo, C.E.; Sweeney, P.; Tellez, C.; Yang, X.D.; Gudas, J.M.; McConkey, D.J.; Bar-Eli, M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 2003, 9, 3167–3175. [Google Scholar]
- Lang, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Signal processing in migrating T24 human bladder carcinoma cells: Role of the autocrine interleukin-8 loop. Int. J. Cancer 2002, 99, 673–680. [Google Scholar] [CrossRef]
- Jing, W.; Wang, G.; Cui, Z.; Li, X.; Zeng, S.; Jiang, X.; Li, W.; Han, B.; Xing, N.; Zhao, Y.; et al. Tumor-neutrophil cross talk orchestrates the tumor microenvironment to determine the bladder cancer progression. Proc. Natl. Acad. Sci. USA 2024, 121, e2312855121. [Google Scholar] [CrossRef]
- Reis, S.T.; Leite, K.R.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Kumari, N.; Agrawal, U.; Mishra, A.K.; Kumar, A.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour Biol. 2017, 39, 1010428317697552. [Google Scholar] [CrossRef]
- Koçak, H.; Oner-Iyidoğan, Y.; Koçak, T.; Oner, P. Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-alpha, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin. Biochem. 2004, 37, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Ali, M.H.; Hassoba, H.M.; Elhadidy, G.S. Serum interleukin-8 and insulin like growth factor-1 in Egyptian bladder cancer patients. Cancer Biomark. 2010, 6, 105–110. [Google Scholar] [CrossRef]
- VandenBussche, C.J.; Heaney, C.D.; Kates, M.; Hooks, J.J.; Baloga, K.; Sokoll, L.; Rosenthal, D.; Detrick, B. Urinary IL-6 and IL-8 as predictive markers in bladder urothelial carcinoma: A pilot study. Cancer Cytopathol. 2024, 132, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, X.; Zhu, Y.; Yao, Z.; Zhao, W.; Zhu, Y.; Sun, F.; Mu, X.; Wang, Y.; He, W.; et al. IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer. BioMed Res. Int. 2021, 2021, 5535578. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shen, W.; Zhang, Y.; Liu, M.; Zhang, L.; Liu, Q.; Lu, H.H.; Bo, J. Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 2017, 8, 38378–38388. [Google Scholar] [CrossRef]
- Alcantara, M.B.; Tang, W.S.; Wang, D.; Kaniowski, D.; Kang, E.; Dizman, N.; Chehrazi-Raffle, A.; Meza, L.; Zengin, Z.; Hall, J.; et al. Targeting STAT3 in tumor-associated antigen-presenting cells as a strategy for kidney and bladder cancer immunotherapy. Front. Immunol. 2024, 14, 1274781. [Google Scholar] [CrossRef]
- Morizawa, Y.; Miyake, M.; Shimada, K.; Hori, S.; Tatsumi, Y.; Nakai, Y.; Onishi, S.; Tanaka, N.; Konishi, N.; Fujimoto, K. Correlation of Immune Cells and Cytokines in the Tumor Microenvironment with Elevated Neutrophil-To-Lymphocyte Ratio in Blood: An Analysis of Muscle Invasive Bladder Cancer. Cancer Investig. 2018, 36, 395–405. [Google Scholar] [CrossRef]
- Kikuchi, H.; Maishi, N.; Annan, D.A.; Alam, M.T.; Dawood, R.I.H.; Sato, M.; Morimoto, M.; Takeda, R.; Ishizuka, K.; Matsumoto, R.; et al. Chemotherapy-Induced IL8 Upregulates MDR1/AUBCB1 in Tumor Blood Vessels and Results in Unfavorable Outcome. Cancer Res. 2020, 80, 2996–3008. [Google Scholar] [CrossRef]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth-Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef]
- Bailey-Whyte, M.; Minas, T.Z.; Dorsey, T.H.; Smith, C.J.; Loffredo, C.A.; Ambs, S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers 2023, 15, 1869. [Google Scholar] [CrossRef]
- Seretis, K.; Sfaelos, K.; Boptsi, E.; Gaitanis, G.; Bassukas, I.D. The Neutrophil-to-Lymphocyte Ratio as a Biomarker in Cutaneous Oncology: A Systematic Review of Evidence beyond Malignant Melanoma. Cancers 2024, 16, 1044. [Google Scholar] [CrossRef] [PubMed]
- Lianos, G.D.; Alexiou, G.A.; Exarchos, C.; Rausei, S.; Mitsis, M.; Voulgaris, S. Prognostic significance of neutrophil-to-lymphocyte ratio in several malignancies: Where do we stand? Biomark. Med. 2020, 14, 169–172. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.; Agrawal, S.; Emond, J.C.; Halazun, K.J. Pretreatment neutrophil-lymphocyte ratio: Useful prognostic biomarker in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2018, 5, 17–28. [Google Scholar] [CrossRef]
- Bhindi, B.; Hermanns, T.; Wei, Y.; Yu, J.; Richard, P.O.; Wettstein, M.S.; Templeton, A.; Li, K.; Sridhar, S.S.; Jewett, M.A.; et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br. J. Cancer 2016, 114, 207–212. [Google Scholar] [CrossRef]
- Viers, B.R.; Boorjian, S.A.; Frank, I.; Tarrell, R.F.; Thapa, P.; Karnes, R.J.; Thompson, R.H.; Tollefson, M.K. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur. Urol. 2014, 66, 1157–1164. [Google Scholar] [CrossRef]
- Hermanns, T.; Bhindi, B.; Wei, Y.; Yu, J.; Noon, A.P.; Richard, P.O.; Bhatt, J.R.; Almatar, A.; Jewett, M.A.; Fleshner, N.E.; et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br. J. Cancer 2014, 111, 444–451. [Google Scholar] [CrossRef]
- D’Andrea, D.; Moschini, M.; Gust, K.M.; Abufaraj, M.; Özsoy, M.; Mathieu, R.; Soria, F.; Briganti, A.; Rouprêt, M.; Karakiewicz, P.I.; et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J. Surg. Oncol. 2017, 115, 455–461. [Google Scholar] [CrossRef]
- Tan, Y.G.; Eu, E.; Lau Kam On, W.; Huang, H.H. Pretreatment neutrophil to lymphocyte ratio predicts worse survival outcomes and advanced tumor staging in patients undergoing radical cystectomy for bladder cancer. Asian J. Urol. 2017, 4, 239–246. [Google Scholar] [CrossRef]
- Ninomiya, S.; Kawahara, T.; Miyoshi, Y.; Yao, M.; Uemura, H. A retrospective study on the possible systematic inflammatory response markers to predict the prognosis of patients with bladder cancer undergoing radical cystectomy. Mol. Clin. Oncol. 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Sahu, R.; Walker, L.A.; Tekwani, B.L. Cytochrome P450-dependent toxicity of dapsone in human erythrocytes. J. Appl. Toxicol. 2010, 30, 271–275. [Google Scholar] [CrossRef]
- Rhodes, L.E.; Tingle, M.D.; Park, B.K.; Chu, P.; Verbov, J.L.; Friedmann, P.S. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br. J. Dermatol. 1995, 132, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Anton, K.; Soares, N.; Riojas, S.; Mcdermott, J.; Knox, L.; Daneman, S.; Puente, B.N. Cimetidine: A Safe Treatment Option for Cutaneous Warts in Pediatric Heart Transplant Recipients. Med. Sci. 2018, 6, 30. [Google Scholar] [CrossRef]
- Chern, E.; Cheng, Y.W. Treatment of recalcitrant periungual warts with cimetidine in pediatrics. J. Dermatol. Treat. 2010, 21, 314–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).