Sodium–Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction
Abstract
1. Introduction
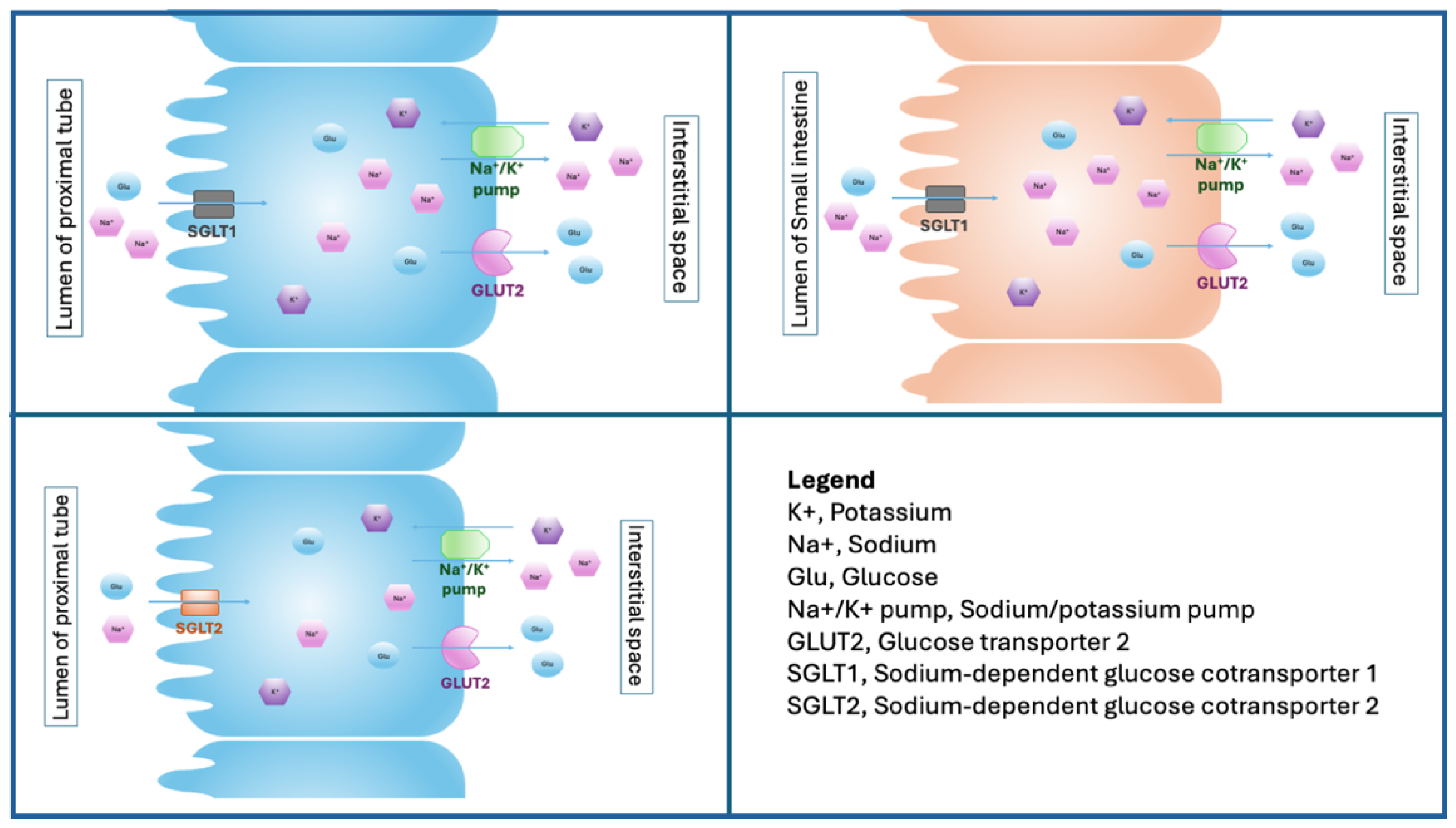
2. Biology of Sodium–Glucose Cotransporter
3. The Effects of SGLT2i on Inflammatory Biomarkers After AMI
4. The Effects of SGLT2i on Cardiac Sympathetic and Parasympathetic Activity
5. Possible Antiarrhythmic Effects of SGLT2i After AMI
6. The Effects of SGLT2i on Heart Failure Occurrence After AMI
| Author | Type of SGLT2i | Timing of SGLT2i Initiation | Sample | Follow-Up | Events | Results | ||
|---|---|---|---|---|---|---|---|---|
| SGLT2i | Controls | SGLT2i | Non-SGLT2i | |||||
| Mao [39] | D | N/A | 231 | 231 | 540 days | 13 (5.6%) | 35 (15.2%) | SGLT2i reduced the risk of heart failure rehospitalization |
| Zhu [41] | D | N/A | 141 | 645 | 23 months | 3 (2.1%) | 67 (10.4%) | SGLT2i reduced the risk of heart failure rehospitalization |
| Adel [42] | E | N/A | 45 | 48 | 6 months | 0 (0.0%) | 0 (0.0%) | No effect |
| von Lewinski [43] | E | Within 72 h from PCI | 237 | 239 | 26 weeks | 3 (1.3%) | 4 (1.7%) | No effect |
| Butler [44] | E | Within 14 days | 3260 | 3262 | 17.9 months | 267 (8.2%) * | 298 (9.1%) * | No effect on composite outcome (heart failure and death from any cause) |
| Hernandez [45] | E | Within 14 days | 3260 | 3262 | 17.9 months | 118 (3.6%) # 148 (4.5%) ## | 153 (4.7%) # 207 (6.3%) ## | SGLT2i reduced the risk of HF in patients with left ventricular dysfunction or congestion after acute myocardial infarction |
| Kwon [40] | N/A | Within 14 days | 938 | 1876 | 2 years | 68 (7.4%) ### | 166 (9.8%) | SGLT2i reduced the risk of hospitalizations for heart failure |
7. The Effects of SGLT2i on Ventricular Remodeling After AMI
| Author | Sample | Follow-Up | Echocardiographic Parameter | Baseline | Post-Treatment | Results | |||
|---|---|---|---|---|---|---|---|---|---|
| SGLT2i | Control | SGLT2i | Control | SGLT2i | Control | ||||
| Wan [48] | 239 | 184 | 6 months | LVEF | 53.53 ± 10.20 | 56.25 ± 9.27 | 2.17 ± 6.66 * | 0.55 ± 9.51 * | SGLT2i protects against LVR |
| LVEDV | 101.92 ± 30.38 | 98.17 ± 21.83 | 1.42±18.10 * | 6.36 ± 14.75 * | |||||
| LVESV | 49.64 ± 26.62 | 44.44 ± 19.02 | −2.16 ± 14.89 * | 2.87 ± 16.02 * | |||||
| LVRI | N/A | N/A | 3.49 ± 19.71 | 7.06 ± 15.15 | |||||
| Dayem [49] | 50 | 50 | 12 weeks | LVEF | 42.70 ± 7.21 | 43.29 ± 5.23 | 50.33 ± 7.49 | 49.84 ± 8.22 | Early SGLT2i administration improves cardiac function |
| LVEDd | 47.62 ± 0.59 | 46.28 ± 0.88 | 47.97 ± 0.82 | 46.85 ± 0.79 | |||||
| LVESd | 35.76 ± 0.66 | 35.56 ± 0.74 | 35.08 ± 0.73 | 34.61 ± 0.49 | |||||
| LV mass | 103.34 ± 18.12 | 101.47 ± 19.50 | 93.60 ± 21.58 | 103.53 ± 17.18 | |||||
| ePASP | 33.4 ± 9.15 | 32.90 ± 10.31 | 31.10 ± 11.54 | 30.71 ± 10.65 | |||||
| Carberry [51] | 51 | 53 | 24 weeks | LVESVI | 65.6 | 62.8 | 57.2 | 55 | SGLT2i had no effect on cardiac volumes and LVEF |
| LVEDVI | 97.8 | 97.6 | 98.3 | 97.3 | |||||
| LVEF | 33.4 | 36 | 42.7 | 44.4 | |||||
| LAVI | 34.3 | 36.2 | 37.3 | 39.2 | |||||
| LVMI | 62.2 | 59.1 | 52.2 | 50.4 | |||||
8. The Possible Protective Role of SGLT2i on Acute Kidney Injury After AMI
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar] [CrossRef]
- Rosas-Guzman, J.; Rosas-Saucedo, J.; Romero-Garcia, A.R.J. SGLT2 Inhibitors in Diabetes Mellitus Treatment. Rev. Recent Clin. Trials 2017, 12, 8–18. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Carvalho, P.E.P.; Veiga, T.M.A.; Simoes, E.S.A.C.; Gewehr, D.M.; Dagostin, C.S.; Fernandes, A.; Nasi, G.; Cardoso, R. Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: A meta-analysis of randomized controlled trials. Clin. Res. Cardiol. 2023, 112, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Profili, N.I.; Castelli, R.; Gidaro, A.; Manetti, R.; Maioli, M.; Delitala, A.P. Sodium-Glucose Cotransporter-2 Inhibitors in Diabetic Patients with Heart Failure: An Update. Pharmaceuticals 2024, 17, 1419. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G.; Pujol-Gimenez, J.; Kanai, Y.; Hediger, M.A. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: From molecular discovery to clinical application. Pflugers Arch. 2020, 472, 1177–1206. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A.; Turk, E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology 2004, 19, 370–376. [Google Scholar] [CrossRef]
- Salvatore, T.; Carbonara, O.; Cozzolino, D.; Torella, R.; Nasti, R.; Lascar, N.; Sasso, F.C. Kidney in diabetes: From organ damage target to therapeutic target. Curr. Drug Metab. 2011, 12, 658–666. [Google Scholar] [CrossRef]
- Cannizzaro, M.; Jarosova, J.; De Paepe, B. Relevance of solute carrier family 5 transporter defects to inherited and acquired human disease. J. Appl. Genet. 2019, 60, 305–317. [Google Scholar] [CrossRef]
- Novikov, A.; Vallon, V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: An update. Curr. Opin. Nephrol. Hypertens. 2016, 25, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Polidori, D.; Zambrowicz, B.; Henry, R.R. Sodium-Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport: From Bench to Bedside. Diabetes Care 2015, 38, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef]
- Benedikt, M.; Mangge, H.; Aziz, F.; Curcic, P.; Pailer, S.; Herrmann, M.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Wallner, M.; et al. Impact of the SGLT2-inhibitor empagliflozin on inflammatory biomarkers after acute myocardial infarction—A post-hoc analysis of the EMMY trial. Cardiovasc. Diabetol. 2023, 22, 166. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Pratt, C.M.; Schwartz, P.J.; Al-Khalidi, H.R.; Spyt, M.J.; Holroyde, M.J.; Karam, R.; Sonnenblick, E.H.; Brum, J.M.; AzimiLide post Infarct surVival Evaluation, I. Mortality in patients after a recent myocardial infarction: A randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation 2004, 109, 990–996. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Sacha, J.; Pluta, W. Different methods of heart rate variability analysis reveal different correlations of heart rate variability spectrum with average heart rate. J. Electrocardiol. 2005, 38, 47–53. [Google Scholar] [CrossRef]
- Shimizu, W.; Kubota, Y.; Hoshika, Y.; Mozawa, K.; Tara, S.; Tokita, Y.; Yodogawa, K.; Iwasaki, Y.K.; Yamamoto, T.; Takano, H.; et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: The EMBODY trial. Cardiovasc. Diabetol. 2020, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Zannad, F. Effects of Sodium-Glucose Cotransporter 2 Inhibitors for the Treatment of Patients With Heart Failure: Proposal of a Novel Mechanism of Action. JAMA Cardiol. 2017, 2, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Yamada, T.; Tsukita, S.; Takahashi, K.; Munakata, Y.; Shirai, Y.; Kodama, S.; Asai, Y.; Sugisawa, T.; Uno, K.; et al. Dapagliflozin, a Sodium-Glucose Co-Transporter 2 Inhibitor, Acutely Reduces Energy Expenditure in BAT via Neural Signals in Mice. PLoS ONE 2016, 11, e0150756. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.H.; Morrison, S.F. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003, 980, 1–10. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Sodium-glucose cotransporter type 2 inhibitors and cardiac arrhythmias. Trends Cardiovasc. Med. 2023, 33, 418–428. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.M.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, W.; Li, J.; Weng, W.; Li, Q. Association of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) with Cardiac Arrhythmias: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Rev. Cardiovasc. Med. 2023, 24, 258. [Google Scholar] [CrossRef]
- Cesaro, A.; Gragnano, F.; Paolisso, P.; Bergamaschi, L.; Gallinoro, E.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; Sansonetti, A.; et al. In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: Insights from the SGLT2-I AMI PROTECT study. Front. Cardiovasc. Med. 2022, 9, 1012220. [Google Scholar] [CrossRef]
- Yang, F.; Meng, R.; Zhu, D.L. Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chronic Dis. Transl. Med. 2020, 6, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, Y.; Tse, G.; Korantzopoulos, P.; Letsas, K.P.; Zhang, Q.; Li, G.; Lip, G.Y.H.; Liu, T. Effect of sodium-glucose cotransporter-2 inhibitors on cardiac remodelling: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2022, 28, 1961–1973. [Google Scholar] [CrossRef]
- Attachaipanich, T.; Chattipakorn, S.C.; Chattipakorn, N. Potential roles of sodium-glucose co-transporter 2 inhibitors in attenuating cardiac arrhythmias in diabetes and heart failure. J. Cell Physiol. 2022, 237, 2404–2419. [Google Scholar] [CrossRef] [PubMed]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef]
- Ismayl, M.; Abbasi, M.A.; Al-Abcha, A.; El-Am, E.; Lundgren, S.; Goldsweig, A.M.; Anavekar, N.S. Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Probl. Cardiol. 2023, 48, 101597. [Google Scholar] [CrossRef]
- Usman, M.S.; Januzzi, J.L.; Anker, S.D.; Salman, A.; Parikh, P.B.; Adamo, M.; Filippatos, G.; Khan, M.S.; Lala, A.; Verma, S.; et al. The effect of sodium-glucose cotransporter 2 inhibitors on left cardiac remodelling in heart failure with reduced ejection fraction: Systematic review and meta-analysis. Eur. J. Heart Fail. 2024, 26, 373–382. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Cai, D.; Chi, B.; Xiao, T.; Zou, A.; Wang, Y.; Chen, Q.; Gu, Q.; Wang, Q.; Ji, Y.; et al. Dapagliflozin reduces risk of heart failure rehospitalization in diabetic acute myocardial infarction patients: A propensity score-matched analysis. Eur. J. Clin. Pharmacol. 2023, 79, 915–926. [Google Scholar] [CrossRef]
- Kwon, O.; Myong, J.P.; Lee, Y.; Choi, Y.J.; Yi, J.E.; Seo, S.M.; Jang, S.W.; Kim, P.J.; Lee, J.M. Sodium-Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction in Patients With Type 2 Diabetes: A Population-Based Investigation. J. Am. Heart Assoc. 2023, 12, e027824. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.L.; Yan, X.J.; Sun, L.; Ji, Y.; Wang, F.F. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2022, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Adel, S.M.H.; Jorfi, F.; Mombeini, H.; Rashidi, H.; Fazeli, S. Effect of a low dose of empagliflozin on short-term outcomes in type 2 diabetics with acute coronary syndrome after percutaneous coronary intervention. Saudi Med. J. 2022, 43, 458–464. [Google Scholar] [CrossRef] [PubMed]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Udell, J.A.; Jones, W.S.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Seide, S.; Zwiener, I.; Amir, O.; et al. Effect of Empagliflozin on Heart Failure Outcomes After Acute Myocardial Infarction: Insights From the EMPACT-MI Trial. Circulation 2024, 149, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Margonato, D.; Galati, G.; Mazzetti, S.; Cannistraci, R.; Perseghin, G.; Margonato, A.; Mortara, A. Renal protection: A leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail. Rev. 2021, 26, 337–345. [Google Scholar] [CrossRef]
- Wan, J.; Xu, F.; Zuo, H.; Jiang, X.; Wang, Y.; Jiang, Y.; Chen, C.; Yin, C.; Cheng, J.; Li, H. Impact of SGLT2 Inhibitors on Left Ventricular Remodeling in Diabetic Patients with Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2024, 29, 10742484241301191. [Google Scholar] [CrossRef]
- Dayem, K.A.; Younis, O.; Zarif, B.; Attia, S.; AbdelSalam, A. Impact of dapagliflozin on cardiac function following anterior myocardial infarction in non-diabetic patients—DACAMI (a randomized controlled clinical trial). Int. J. Cardiol. 2023, 379, 9–14. [Google Scholar] [CrossRef]
- von Lewinski, D.; Kolesnik, E.; Aziz, F.; Benedikt, M.; Tripolt, N.J.; Wallner, M.; Pferschy, P.N.; von Lewinski, F.; Schwegel, N.; Holman, R.R.; et al. Timing of SGLT2i initiation after acute myocardial infarction. Cardiovasc. Diabetol. 2023, 22, 269. [Google Scholar] [CrossRef]
- Carberry, J.; Petrie, M.C.; Lee, M.M.Y.; Stanley, B.; Brooksbank, K.J.M.; Campbell, R.T.; Good, R.; Jhund, P.S.; Kellman, P.; Lang, N.N.; et al. Empagliflozin to prevent worsening of left ventricular volumes and systolic function after myocardial infarction (EMPRESS-MI). Eur. J. Heart Fail. 2024. early view. [Google Scholar] [CrossRef] [PubMed]
- Stougiannou, T.M.; Christodoulou, K.C.; Koufakis, T.; Mitropoulos, F.; Mikroulis, D.; Mazer, C.D.; Karangelis, D. Progenitor Cell Function and Cardiovascular Remodelling Induced by SGLT2 Inhibitors. Front. Biosci. 2024, 29, 145. [Google Scholar] [CrossRef] [PubMed]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits IL-1beta-Mediated Inflammatory Response in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef]
- Kang, S.; Verma, S.; Hassanabad, A.F.; Teng, G.; Belke, D.D.; Dundas, J.A.; Guzzardi, D.G.; Svystonyuk, D.A.; Pattar, S.S.; Park, D.S.J.; et al. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can. J. Cardiol. 2020, 36, 543–553. [Google Scholar] [CrossRef]
- Mather, A.; Pollock, C. Glucose handling by the kidney. Kidney Int. Suppl. 2011, 120, S1–S6. [Google Scholar] [CrossRef]
- Mehran, R.; Owen, R.; Chiarito, M.; Baber, U.; Sartori, S.; Cao, D.; Nicolas, J.; Pivato, C.A.; Nardin, M.; Krishnan, P.; et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: Derivation and validation from an observational registry. Lancet 2021, 398, 1974–1983. [Google Scholar] [CrossRef]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef]
- Cai, D.; Chen, Q.; Mao, L.; Xiao, T.; Wang, Y.; Gu, Q.; Wang, Q.; Ji, Y.; Sun, L. Association of SGLT2 inhibitor dapagliflozin with risks of acute kidney injury and all-cause mortality in acute myocardial infarction patients. Eur. J. Clin. Pharmacol. 2024, 80, 613–620. [Google Scholar] [CrossRef]
- Kultursay, B.; Yilmaz, C.; Guven, B.; Mutlu, D.; Karagoz, A. Potential renoprotective effect of SGLT2 inhibitors against contrast-induced AKI in diabetic STEMI patients undergoing primary PCI. Kardiol. Pol. 2024, 82, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Basutkar, R.S.; Cutinha, R.M.; Sathish, V.; Shahil, A.; Saneen Ck, N. Impact of SGLT2 Inhibitors on Renal Function in Type 2 Diabetic Patients with Coronary Artery Disease Undergoing Percutaneous Intervention: A Systematic Review and Meta-Analysis. Curr. Diabetes Rev. 2024, 21, e030724231535. [Google Scholar] [CrossRef]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, X.; Yan, G.; Zhang, Y.; Yao, Y.; Qiao, Y.; Wang, D.; Chen, G.; Zhang, W.; Tang, C.; et al. Dapagliflozin Attenuates Contrast-induced Acute Kidney Injury by Regulating the HIF-1alpha/HE4/NF-kappaB Pathway. J. Cardiovasc. Pharmacol. 2022, 79, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Matoba, K.; Takeda, Y.; Nagai, Y.; Akamine, T.; Yokota, T.; Sango, K.; Utsunomiya, K. SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int. J. Mol. Sci. 2017, 18, 1083. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Profili, N.I.; Castelli, R.; Manetti, R.; Sircana, M.C.; Pagni, M.; Sechi, G.L.; Gidaro, A.; Cossu, C.; Bella, F.; Delitala, A.P. Sodium–Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction. Biomedicines 2025, 13, 720. https://doi.org/10.3390/biomedicines13030720
Profili NI, Castelli R, Manetti R, Sircana MC, Pagni M, Sechi GL, Gidaro A, Cossu C, Bella F, Delitala AP. Sodium–Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction. Biomedicines. 2025; 13(3):720. https://doi.org/10.3390/biomedicines13030720
Chicago/Turabian StyleProfili, Nicia I., Roberto Castelli, Roberto Manetti, Marta C. Sircana, Michela Pagni, Gemma Lisa Sechi, Antonio Gidaro, Costantino Cossu, Francesco Bella, and Alessandro P. Delitala. 2025. "Sodium–Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction" Biomedicines 13, no. 3: 720. https://doi.org/10.3390/biomedicines13030720
APA StyleProfili, N. I., Castelli, R., Manetti, R., Sircana, M. C., Pagni, M., Sechi, G. L., Gidaro, A., Cossu, C., Bella, F., & Delitala, A. P. (2025). Sodium–Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction. Biomedicines, 13(3), 720. https://doi.org/10.3390/biomedicines13030720










