5-Aminolaevulinic Acid-Mediated Photodynamic Therapy Combined with Tirapazamine Enhances Efficacy in Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Chemicals and PDT Treatment
2.3. Cell Counting Kit-8 Assay
2.4. Animals and Tumours
2.5. In Vivo Fluorescence Imaging
2.6. Haematoxylin and Eosin (HE) Staining
2.7. Immunohistochemical Analysis
2.8. Data Analysis
3. Results
3.1. TPZ Enhanced PDT Cytotoxicity Under Hypoxic Conditions
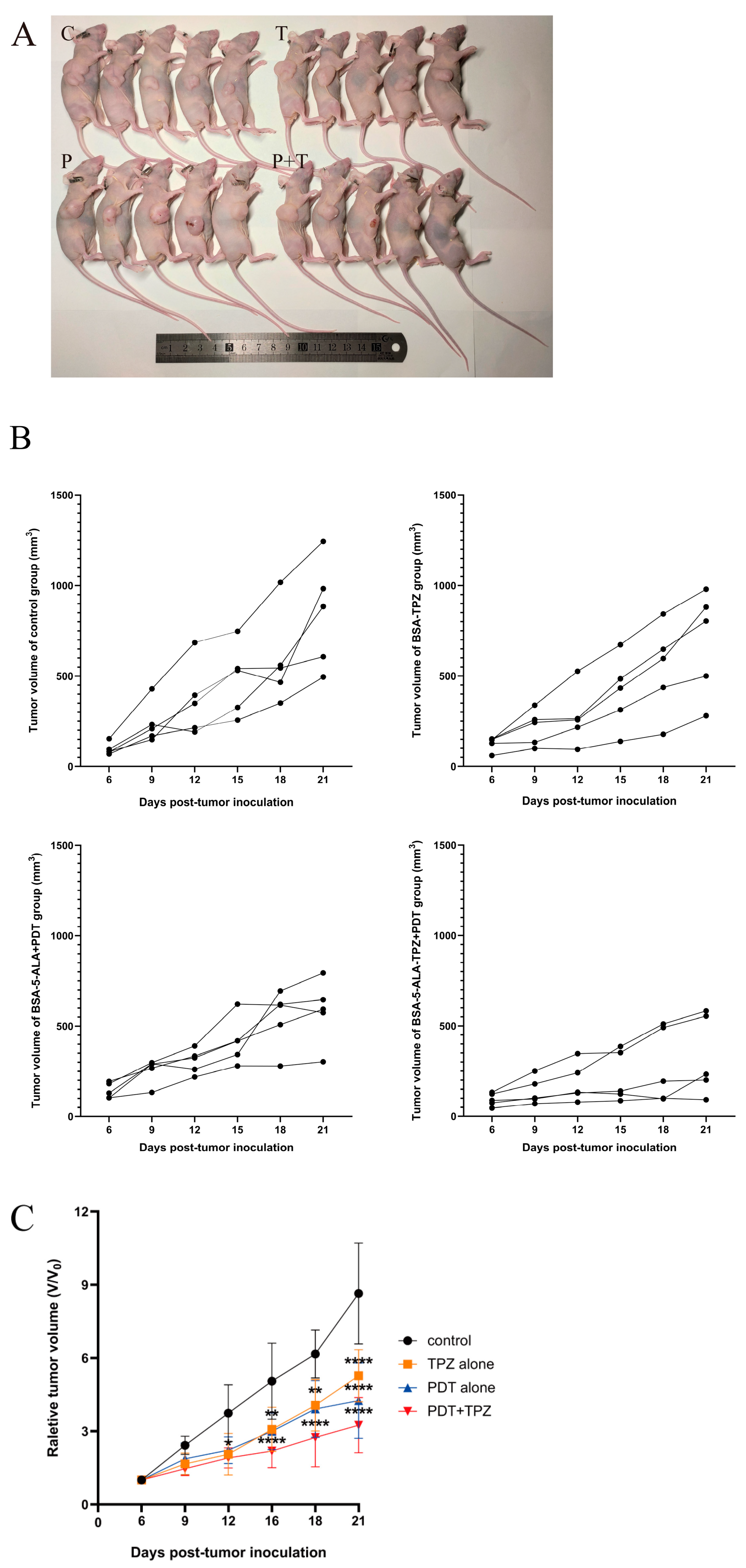
3.2. Combined Treatment Slowed Tumour Growth
3.3. Combined Effect of 5-ALA-Mediated PDT and TPZ on Tumour Volume in Mice with Human Ovarian Cancer
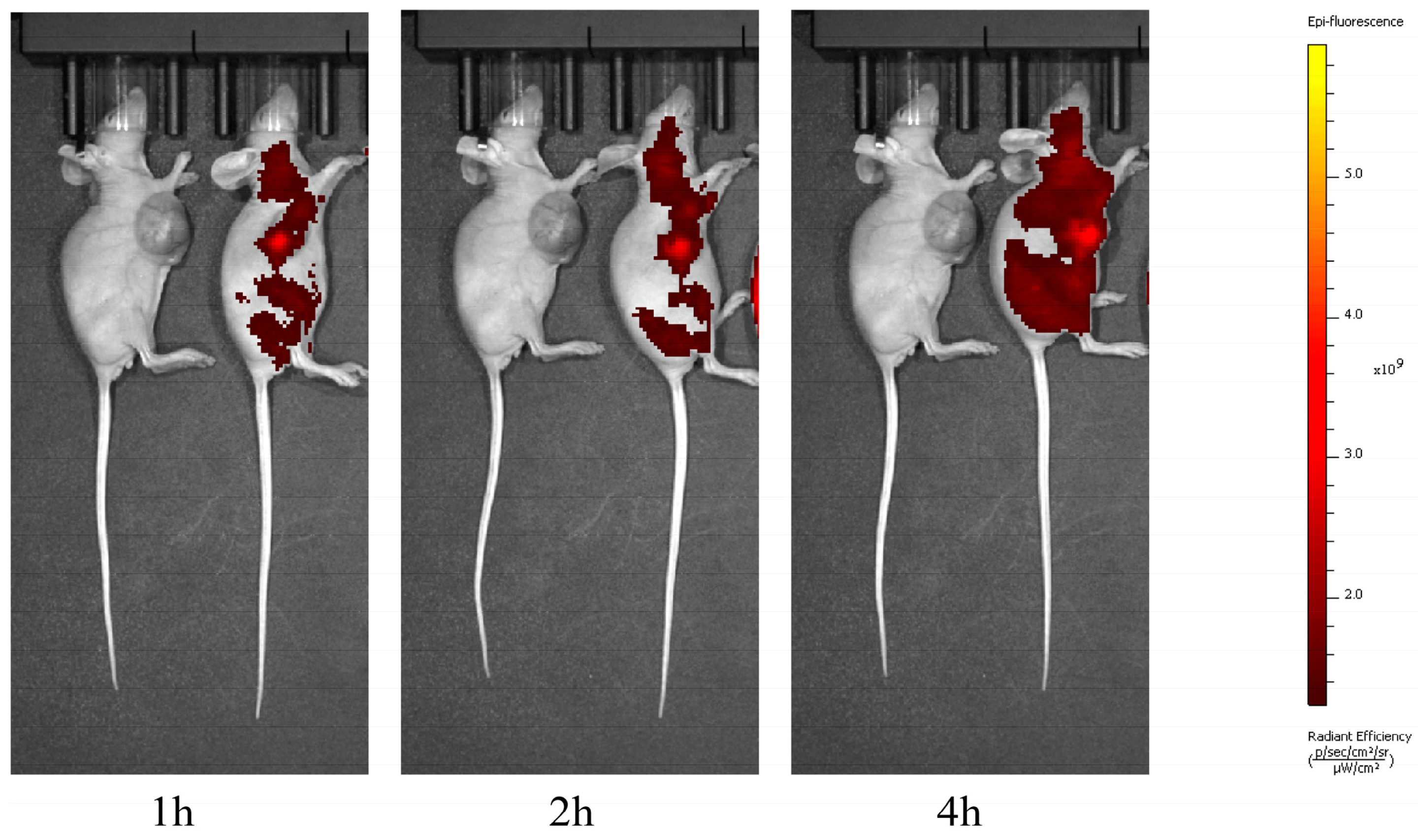
3.4. PpIX Can Reach Systemic Distribution but Maintains High Concentrations Within the Tumour
3.5. HE Staining Revealed That the Combination Treatment Significantly Increased the Extent of Necrosis
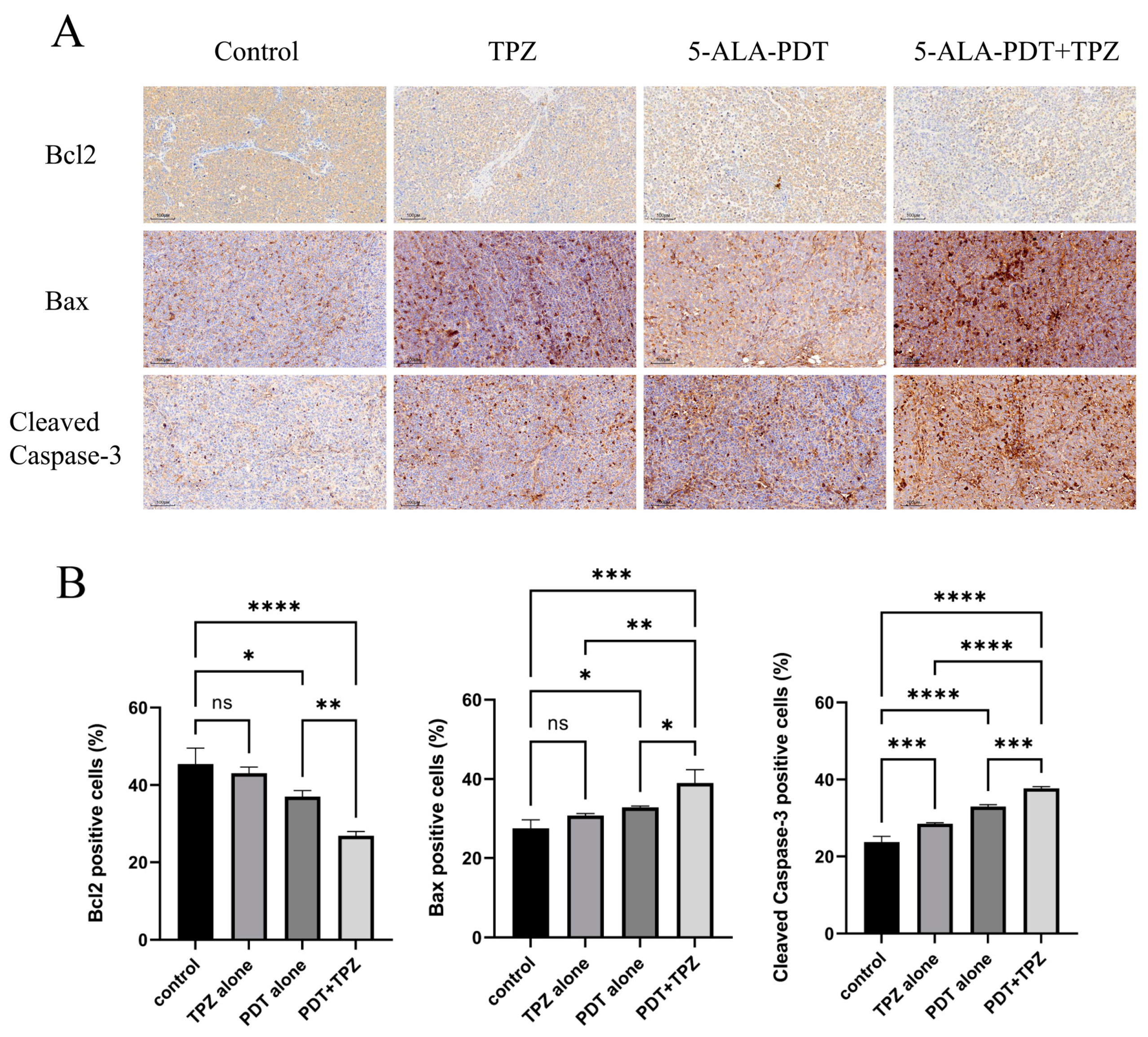
3.6. Changes in the Expression Levels of Apoptosis-Related Proteins Indicate That Combination Therapy Enhanced Apoptosis
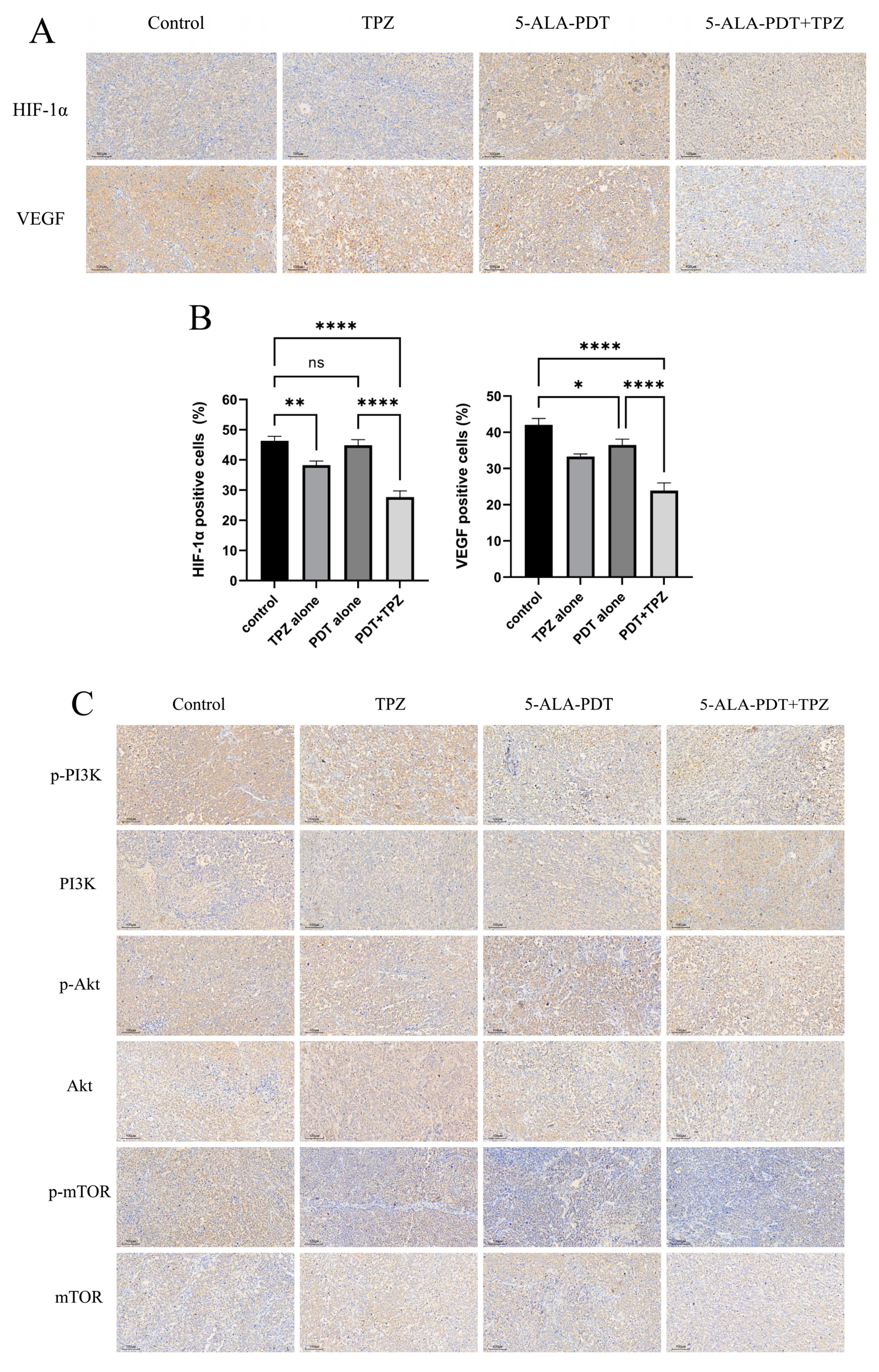
3.7. Expression Levels of HIF-1α/VEGF Axis-Related Proteins and Phosphorylated Proteins in the PI3K/Akt/mTOR Pathway Were Reduced in Tumours After Combination Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.E.; Farland, L.V.; Yan, B.; Wang, J.; Trabert, B.; Doherty, J.A.; Meeks, H.D.; Madsen, M.; Guinto, E.; Collin, L.J.; et al. Endometriosis Typology and Ovarian Cancer Risk. JAMA 2024, 332, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.P.; Gershenson, D.M. Treatment of Rare Epithelial Ovarian Tumors. Hematol. Oncol. Clin. N. Am. 2018, 32, 1011–1024. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Maloth, K.N.; Velpula, N.; Kodangal, S.; Sangmesh, M.; Vellamchetla, K.; Ugrappa, S.; Meka, N. Photodynamic Therapy—A Non-invasive Treatment Modality for Precancerous Lesions. J. Lasers Med. Sci. 2016, 7, 30–36. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaen, P.; Sanz-Rodriguez, F.; Cuevas, J.; Gonzalez, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Kessel, D.; Reiners, J.J. Photodynamic therapy: Autophagy and mitophagy, apoptosis and paraptosis. Autophagy 2020, 16, 2098–2101. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, L.; Song, W.; Yuan, Y.; Yan, S.; Yu, S.; Chen, S. Elsinochrome A induces cell apoptosis and autophagy in photodynamic therapy. J. Cell Biochem. 2023, 124, 1346–1365. [Google Scholar] [CrossRef]
- Wang, Q.; Suo, Y.; Wang, X.; Wang, Y.; Tian, X.; Gao, Y.; Liu, N.; Liu, R. Study on the mechanism of photodynamic therapy mediated by 5-aminoketovalerate in human ovarian cancer cell line. Lasers Med. Sci. 2021, 36, 1873–1881. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Koch, C.J. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003, 195, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Philip, B.; Ito, K.; Moreno-Sanchez, R.; Ralph, S.J. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis 2013, 34, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2021, 268, 120537. [Google Scholar] [CrossRef]
- Harada, R.; Kawamoto, T.; Ueha, T.; Minoda, M.; Toda, M.; Onishi, Y.; Fukase, N.; Hara, H.; Sakai, Y.; Miwa, M.; et al. Reoxygenation using a novel CO2 therapy decreases the metastatic potential of osteosarcoma cells. Exp. Cell Res. 2013, 319, 1988–1997. [Google Scholar] [CrossRef]
- Foster, T.H.; Murant, R.S.; Bryant, R.G.; Knox, R.S.; Gibson, S.L.; Hilf, R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat. Res. 1991, 126, 296–303. [Google Scholar] [CrossRef]
- Reddy, S.B.; Williamson, S.K. Tirapazamine: A novel agent targeting hypoxic tumor cells. Expert Opin. Investig. Drugs 2009, 18, 77–87. [Google Scholar] [CrossRef]
- Guise, C.P.; Mowday, A.M.; Ashoorzadeh, A.; Yuan, R.; Lin, W.H.; Wu, D.H.; Smaill, J.B.; Patterson, A.V.; Ding, K. Bioreductive prodrugs as cancer therapeutics: Targeting tumor hypoxia. Chin. J. Cancer 2014, 33, 80–86. [Google Scholar] [CrossRef]
- Friery, O.P.; Gallagher, R.; Murray, M.M.; Hughes, C.M.; Galligan, E.S.; McIntyre, I.A.; Patterson, L.H.; Hirst, D.G.; McKeown, S.R. Enhancement of the anti-tumour effect of cyclophosphamide by the bioreductive drugs AQ4N and tirapazamine. Br. J. Cancer 2000, 82, 1469–1473. [Google Scholar] [CrossRef]
- Huxham, L.A.; Kyle, A.H.; Baker, J.H.; McNicol, K.L.; Minchinton, A.I. Tirapazamine causes vascular dysfunction in HCT-116 tumour xenografts. Radiother. Oncol. 2006, 78, 138–145. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Hu, Z.; Yang, B.; Li, Q.; Wang, J.; Zheng, J.; Cao, W. 5-Aminolevulinic Acid-Based Sonodynamic Therapy Induces the Apoptosis of Osteosarcoma in Mice. PLoS ONE 2015, 10, e0132074. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Murayama, Y.; Kubo, H.; Matsuo, H.; Morimura, R.; Ikoma, H.; Fujiwara, H.; Okamoto, K.; Tanaka, T.; Otsuji, E. Photodynamic diagnosis of peritoneal metastasis in human pancreatic cancer using 5-aminolevulinic acid during staging laparoscopy. Oncol. Lett. 2018, 16, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Dhanabal, M.; Griffioen, A.W.; Sukhatme, V.P.; Ramakrishnan, S. Synergy between angiostatin and endostatin: Inhibition of ovarian cancer growth. Cancer Res. 2000, 60, 2190–2196. [Google Scholar]
- Zhou, J.R.; Yu, L.; Mai, Z.; Blackburn, G.L. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int. J. Cancer 2003, 108, 8–14. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Cui, J.; Li, X.; Dou, Y.; Su, L.; Chang, J.; Wang, H.; Li, X.; Zhang, B. pH- and NIR light responsive nanocarriers for combination treatment of chemotherapy and photodynamic therapy. Biomater. Sci. 2016, 4, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Tian, J.; Chen, C.; Jiang, D.; Xue, Y.; Wang, C.; Gao, Y.; Zhang, W. An oxygen self-sufficient NIR-responsive nanosystem for enhanced PDT and chemotherapy against hypoxic tumors. Chem. Sci. 2019, 10, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Kiesslich, T.; Oberdanner, C.B.; Krammer, B. Apoptosis following photodynamic tumor therapy: Induction, mechanisms and detection. Curr. Pharm. Des. 2005, 11, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, H.; Jin, W.; Liu, W.; Yin, H.; Li, Y.; Dong, H. Metronomic photodynamic therapy with 5-aminolevulinic acid induces apoptosis and autophagy in human SW837 colorectal cancer cells. J. Photochem. Photobiol. B 2019, 198, 111586. [Google Scholar] [CrossRef]
- Pevna, V.; Horvath, D.; Wagnieres, G.; Huntosova, V. Photobiomodulation and photodynamic therapy-induced switching of autophagy and apoptosis in human dermal fibroblasts. J. Photochem. Photobiol. B 2022, 234, 112539. [Google Scholar] [CrossRef]
- Wang, Q.; Suo, Y.; Shi, R.; Wang, Y. Studies related to the enhanced the effect of 5-aminolevulinic acid-based photodynamic therapy combined with tirapazamine. Photodiagnosis Photodyn. Ther. 2024, 49, 104287. [Google Scholar] [CrossRef]
- Das, V.; Stepankova, J.; Hajduch, M.; Miller, J.H. Role of tumor hypoxia in acquisition of resistance to microtubule-stabilizing drugs. Biochim. Biophys. Acta 2015, 1855, 172–182. [Google Scholar] [CrossRef]
- Denny, W.A.; Wilson, W.R. Tirapazamine: A bioreductive anticancer drug that exploits tumour hypoxia. Expert Opin. Investig. Drugs 2000, 9, 2889–2901. [Google Scholar] [CrossRef]
- Gandara, D.R.; Lara, P.N., Jr.; Goldberg, Z.; Le, Q.T.; Mack, P.C.; Lau, D.H.; Gumerlock, P.H. Tirapazamine: Prototype for a novel class of therapeutic agents targeting tumor hypoxia. Semin. Oncol. 2002, 29, 102–109. [Google Scholar] [CrossRef]
- Lin, P.S.; Ho, K.C.; Yang, S.J. Tirapazamine (SR 4233) interrupts cell cycle progression and induces apoptosis. Cancer Lett. 1996, 105, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Govaert, K.M.; Nijkamp, M.W.; Emmink, B.L.; Steller, E.J.; Minchinton, A.I.; Kranenburg, O.; Borel Rinkes, I.H. Effects of tirapazamine on experimental colorectal liver metastases after radiofrequency ablation. Br. J. Surg. 2012, 99, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, J.; Lv, J.; Dong, B.; Liu, M.; Liu, J.; Sun, L.; Zhang, G.; Zhang, L.; Huang, G.; et al. Ce6-C6-TPZ co-loaded albumin nanoparticles for synergistic combined PDT-chemotherapy of cancer. J. Mater. Chem. B 2019, 7, 5797–5807. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, Y.; Mao, M.; Xiong, Y.; Mu, D. The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1α in the developing rat brain after hypoxia-ischemia. Brain Res. 2008, 1197, 152–158. [Google Scholar] [CrossRef]
- Di, Y.; Chen, X.L. Inhibition of LY294002 in retinal neovascularization via down-regulation the PI3K/AKT-VEGF pathway in vivo and in vitro. Int. J. Ophthalmol. 2018, 11, 1284–1289. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, H.; Hong, C.; Chen, Z.; Deng, X.; Wang, A.; Yang, F.; Yang, L.; Chen, C.; Qin, X. PDGF Promotes the Warburg Effect in Pulmonary Arterial Smooth Muscle Cells via Activation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 1603–1613. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Yang, X.M.; Wang, Y.S.; Zhang, J.; Li, Y.; Xu, J.F.; Zhu, J.; Zhao, W.; Chu, D.K.; Wiedemann, P. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1α and VEGF in laser-induced rat choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1873–1879. [Google Scholar] [CrossRef]
- Karar, J.; Cerniglia, G.J.; Lindsten, T.; Koumenis, C.; Maity, A. Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1α expression by blocking protein translation and increases cell death under hypoxia. Cancer Biol. Ther. 2012, 13, 1102–1111. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef]
- van den Beucken, T.; Koritzinsky, M.; Wouters, B.G. Translational control of gene expression during hypoxia. Cancer Biol. Ther. 2006, 5, 749–755. [Google Scholar] [CrossRef]
- Hudson, C.C.; Liu, M.; Chiang, G.G.; Otterness, D.M.; Loomis, D.C.; Kaper, F.; Giaccia, A.J.; Abraham, R.T. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 2002, 22, 7004–7014. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Laughner, E.; Taghavi, P.; Chiles, K.; Mahon, P.C.; Semenza, G.L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 2001, 21, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Zheng, J.Z.; Aoki, M.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1749–1753. [Google Scholar] [CrossRef]
- Jiang, B.H.; Jiang, G.; Zheng, J.Z.; Lu, Z.; Hunter, T.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2001, 12, 363–369. [Google Scholar]
- Chen, E.Y.; Mazure, N.M.; Cooper, J.A.; Giaccia, A.J. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001, 61, 2429–2433. [Google Scholar]
- Arsham, A.M.; Plas, D.R.; Thompson, C.B.; Simon, M.C. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1α nor sufficient for HIF-1-dependent target gene transcription. J. Biol. Chem. 2002, 277, 15162–15170. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Alvarez-Tejado, M.; Alfranca, A.; Aragonés, J.; Vara, A.; Landázuri, M.O.; del Peso, L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J. Biol. Chem. 2002, 277, 13508–13517. [Google Scholar] [CrossRef]
- Lin, Y.W.; Huang, S.T.; Wu, J.C.; Chu, T.H.; Huang, S.C.; Lee, C.C.; Tai, M.H. Novel HDGF/HIF-1α/VEGF axis in oral cancer impacts disease prognosis. BMC Cancer 2019, 19, 1083. [Google Scholar] [CrossRef] [PubMed]
- Navone, S.E.; Guarnaccia, L.; Cordiglieri, C.; Crisa, F.M.; Caroli, M.; Locatelli, M.; Schisano, L.; Rampini, P.; Miozzo, M.; La Verde, N.; et al. Aspirin Affects Tumor Angiogenesis and Sensitizes Human Glioblastoma Endothelial Cells to Temozolomide, Bevacizumab, and Sunitinib, Impairing Vascular Endothelial Growth Factor-Related Signaling. World Neurosurg. 2018, 120, e380–e391. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.X.; Gao, X.H.; Zhao, L.F.; Du, J.; Wang, T.Y.; Wang, L.; Zhang, J.; Wang, H.Y.; Dong, R.; et al. HIF1A and VEGF regulate each other by competing endogenous RNA mechanism and involve in the pathogenesis of peritoneal fibrosis. Pathol. Res. Pract. 2019, 215, 644–652. [Google Scholar] [CrossRef] [PubMed]



| Fractional Tumour Volume (FTV) Relative to Untreated Controls a | |||||
|---|---|---|---|---|---|
| Combination Treatment | |||||
| Day b | 5-ALA | TPZ | Expected c | Observed | Ratio of Expected FTV/Observed FTV d |
| 15 | 1.00 ± 0.55 | 1.05 ± 0.67 | 1.05 | 0.44 ± 0.17 | 2.42 |
| 18 | 0.97 ± 0.26 | 1.14 ± 0.71 | 1.10 | 0.47 ± 0.34 | 2.33 |
| 21 | 0.71 ± 0.15 | 0.93 ± 0.50 | 0.66 | 0.37 ± 0.16 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Suo, Y.; Tian, X. 5-Aminolaevulinic Acid-Mediated Photodynamic Therapy Combined with Tirapazamine Enhances Efficacy in Ovarian Cancer. Biomedicines 2025, 13, 724. https://doi.org/10.3390/biomedicines13030724
Wang Q, Suo Y, Tian X. 5-Aminolaevulinic Acid-Mediated Photodynamic Therapy Combined with Tirapazamine Enhances Efficacy in Ovarian Cancer. Biomedicines. 2025; 13(3):724. https://doi.org/10.3390/biomedicines13030724
Chicago/Turabian StyleWang, Qian, Yuping Suo, and Xiaojuan Tian. 2025. "5-Aminolaevulinic Acid-Mediated Photodynamic Therapy Combined with Tirapazamine Enhances Efficacy in Ovarian Cancer" Biomedicines 13, no. 3: 724. https://doi.org/10.3390/biomedicines13030724
APA StyleWang, Q., Suo, Y., & Tian, X. (2025). 5-Aminolaevulinic Acid-Mediated Photodynamic Therapy Combined with Tirapazamine Enhances Efficacy in Ovarian Cancer. Biomedicines, 13(3), 724. https://doi.org/10.3390/biomedicines13030724





