Recent Advancements Towards the Use of Vitamin D Isoforms and the Development of Their Synthetic Analogues as New Therapeutics
Abstract
1. Introduction
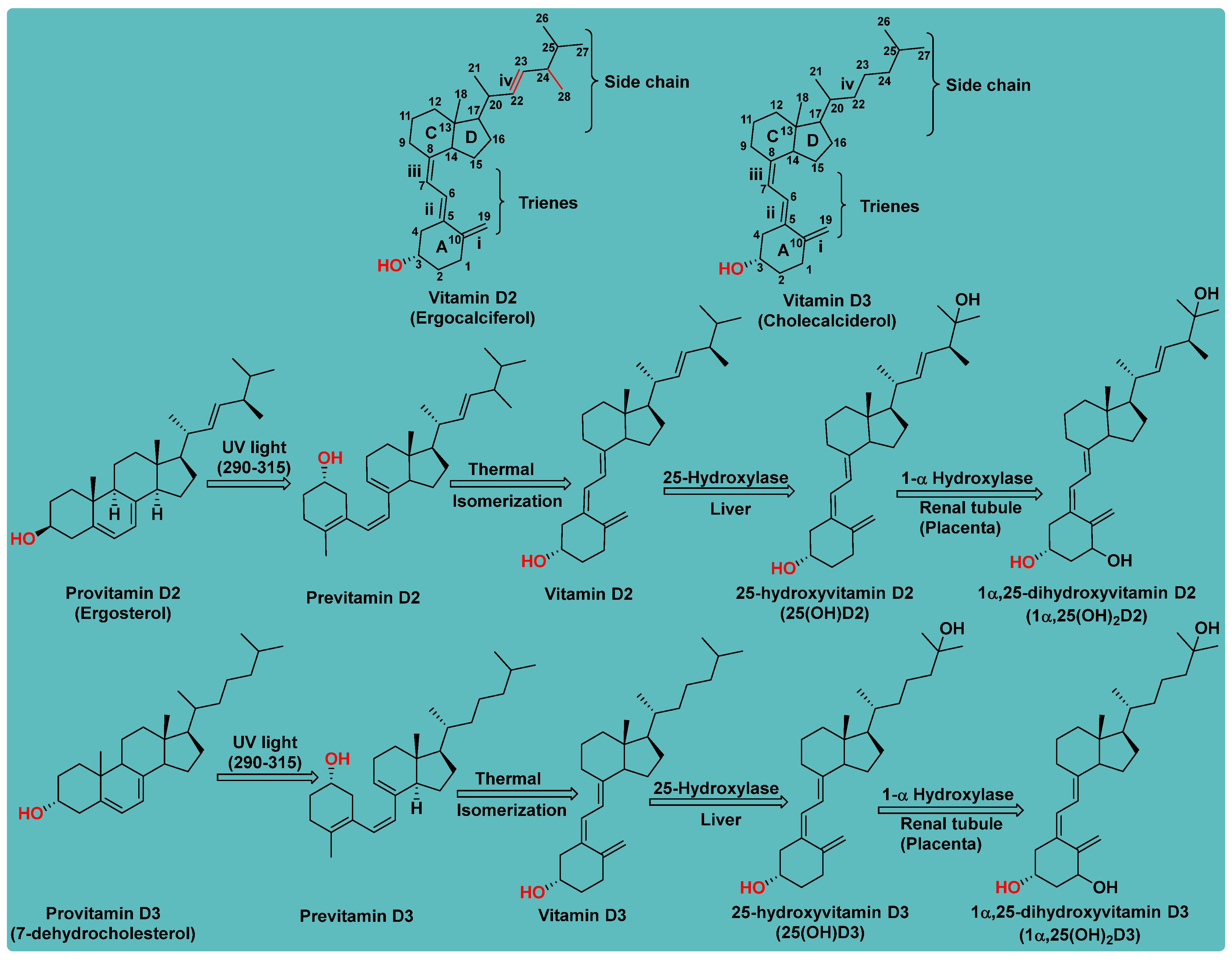
1.1. Chemistry of Vitamin D
1.2. Receptor of Vitamin D
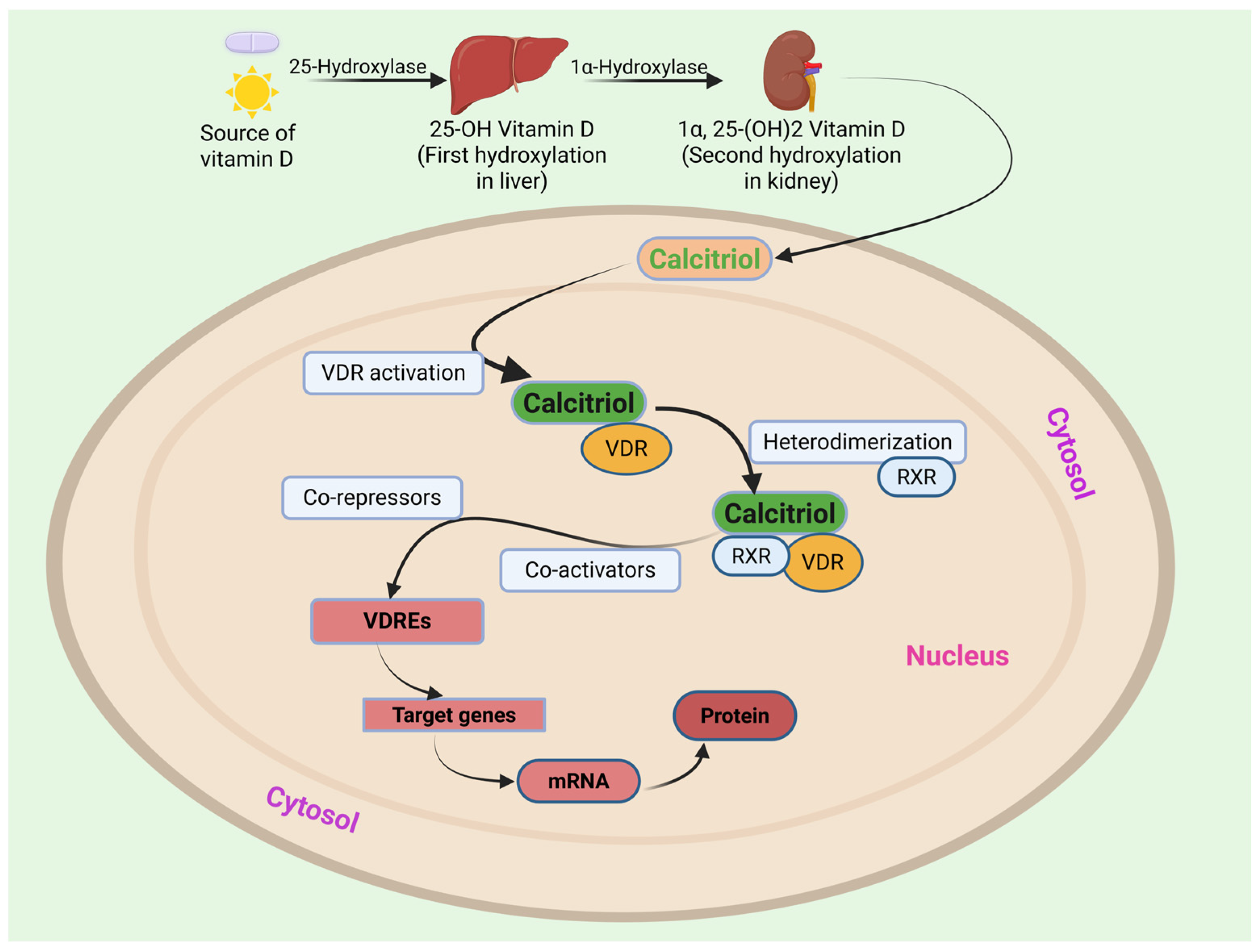
1.3. Mechanism of Action of Vitamin D
1.4. Overview of Vitamin D and Its Modified Forms in Clinical Use
2. Pharmacological Activities of Vitamin D and Its Derivatives
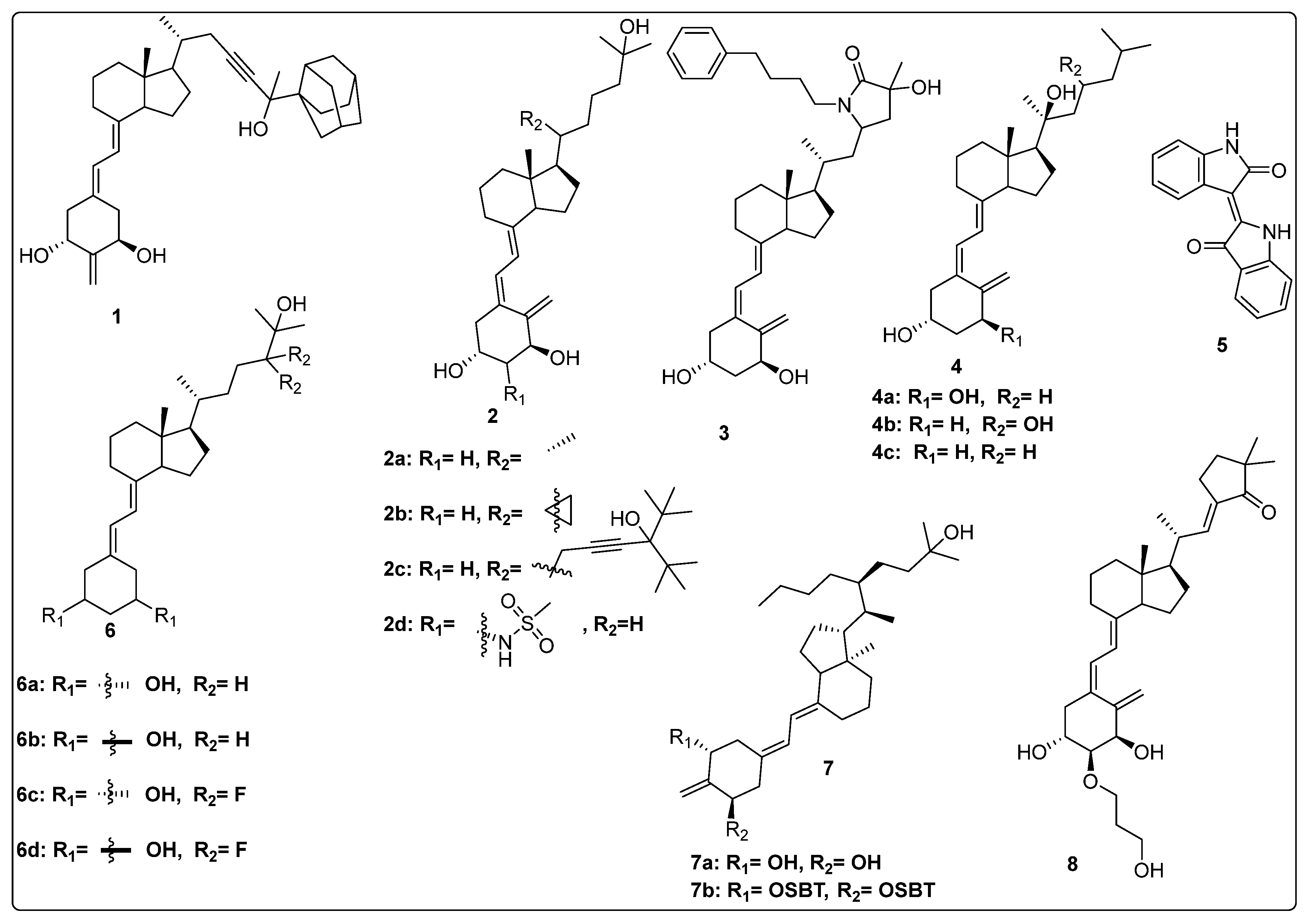
2.1. Vitamin D Derivatives as Modulator of VDR
2.2. Vitamin D Derivatives as Anticancer Agents
2.3. Anti-Inflammatory Activity of Vitamin D Analogues
2.4. Dual Activities (Binding Affinity and Anti-Cancer) of Vitamin D Derivatives
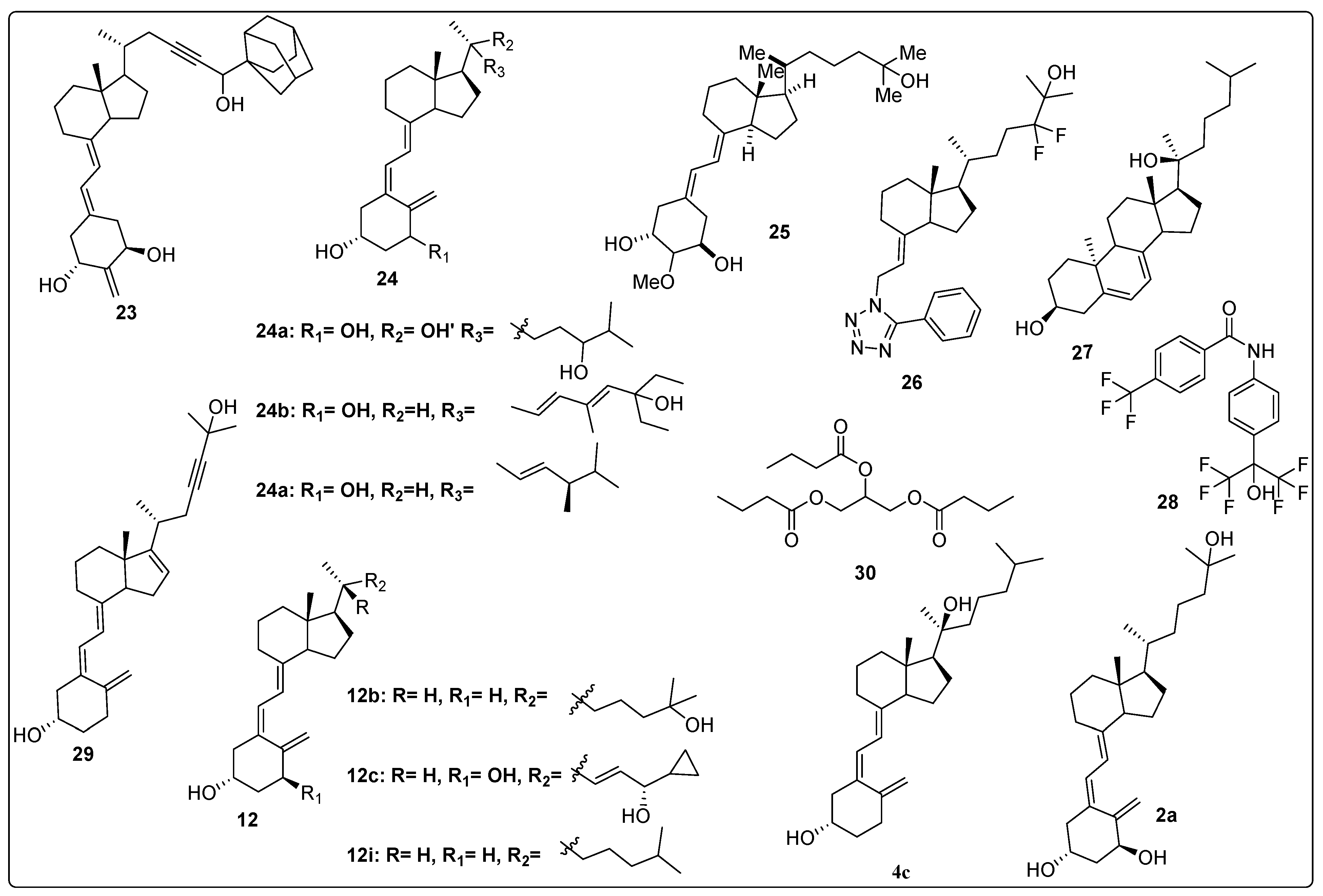
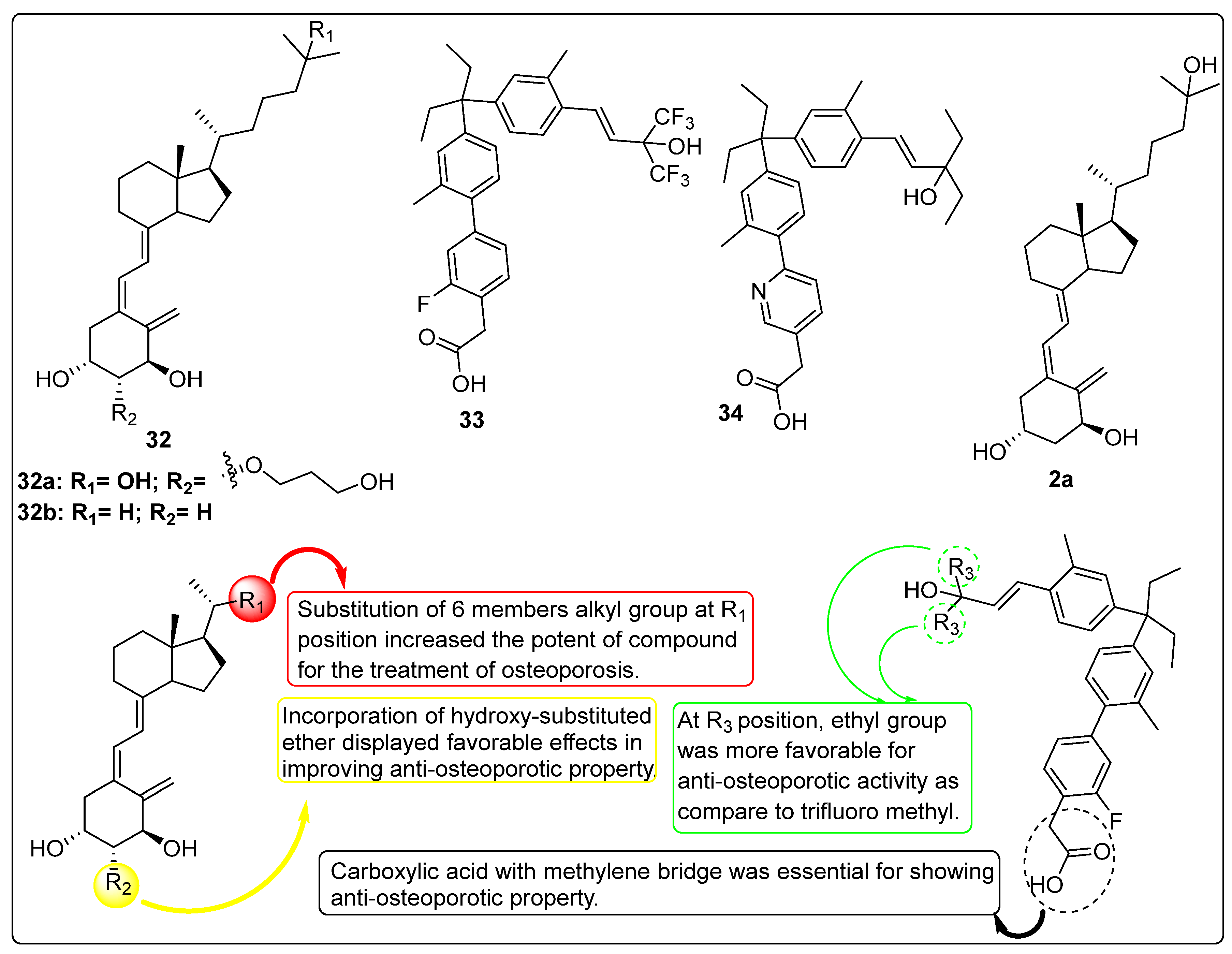
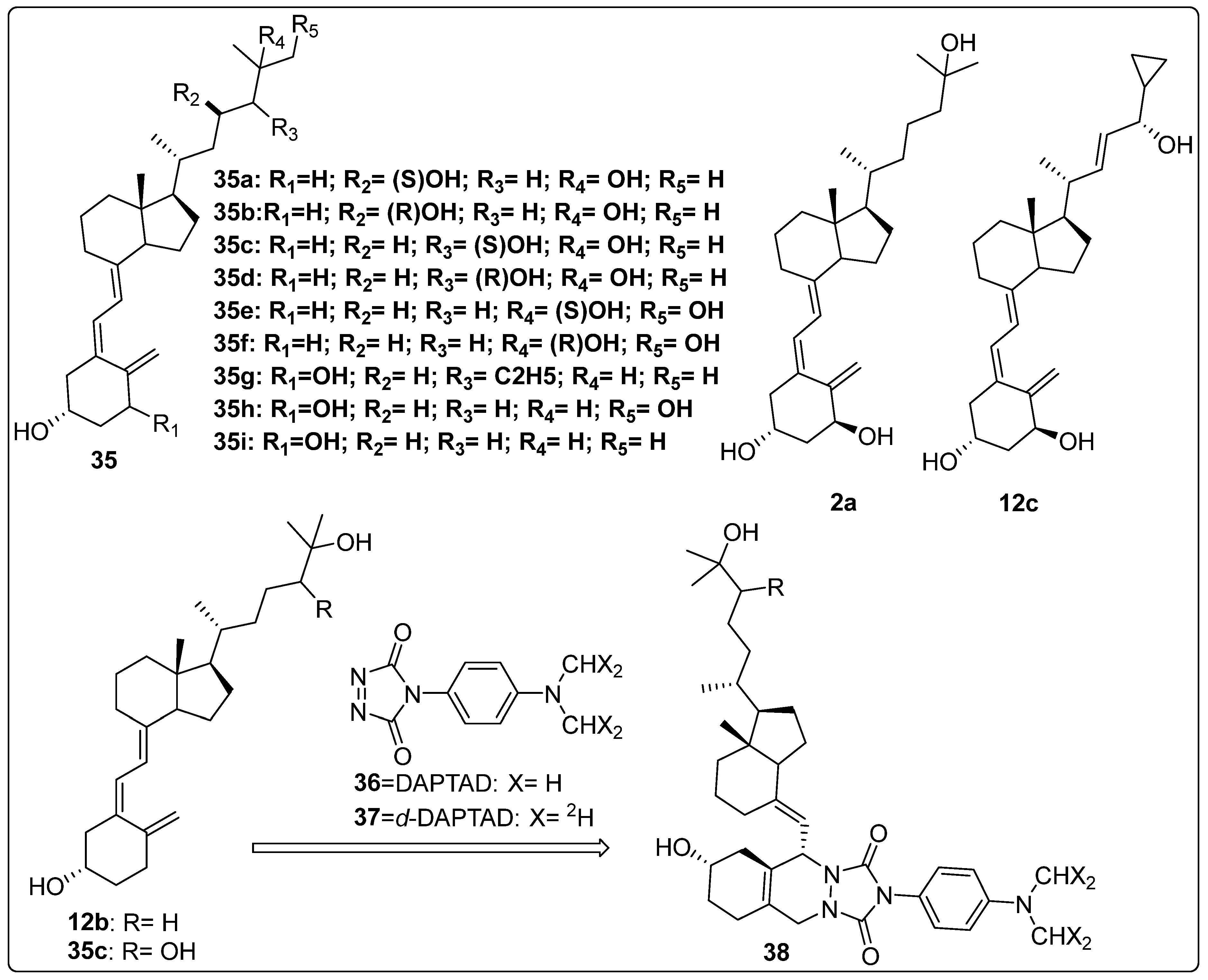
2.5. Vitamin D Analogues as Potential Anti-Osteoporotic Agents
2.6. Metabolic Studies of Vitamin D Derivatives
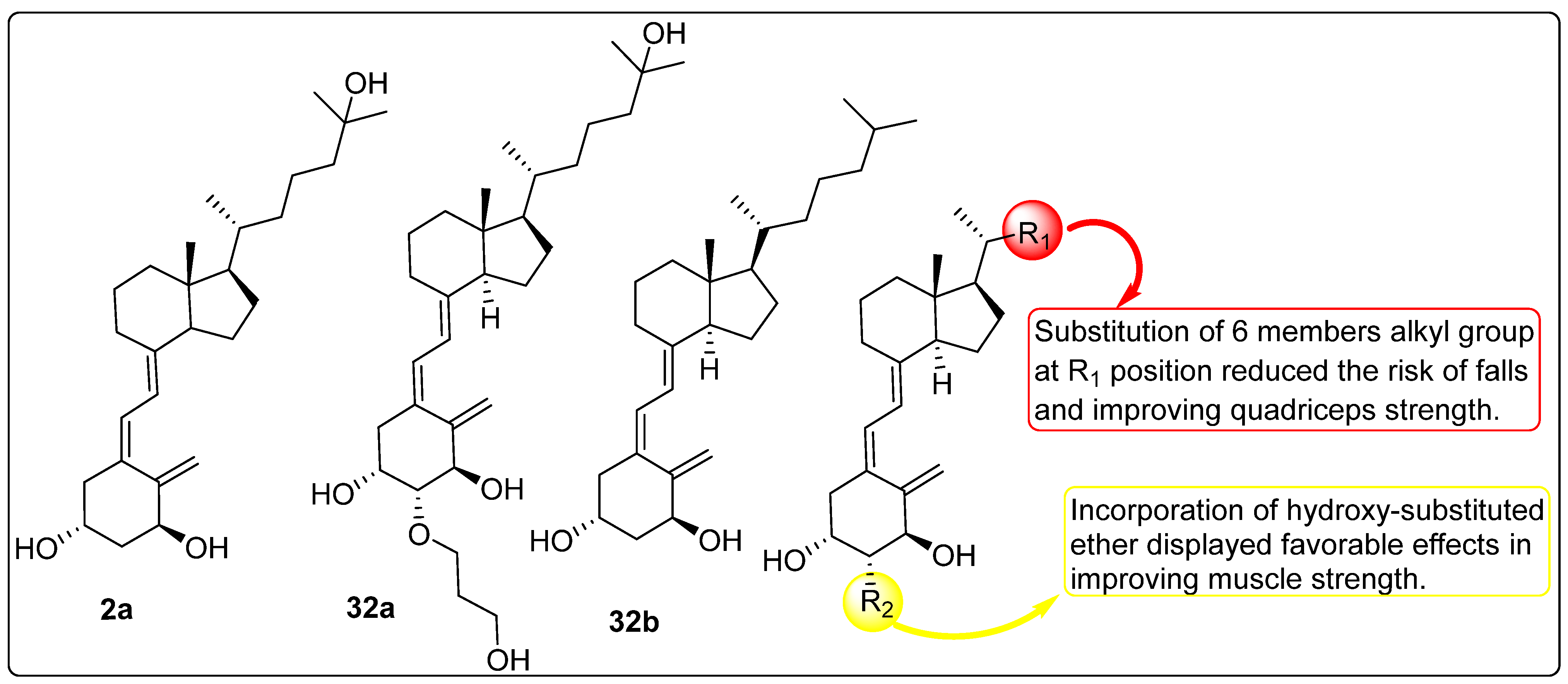
2.7. Effect of Vitamin D Analogues on Muscular Strength
2.8. Activity of Vitamin D Analogues Against Liver Cirrhosis
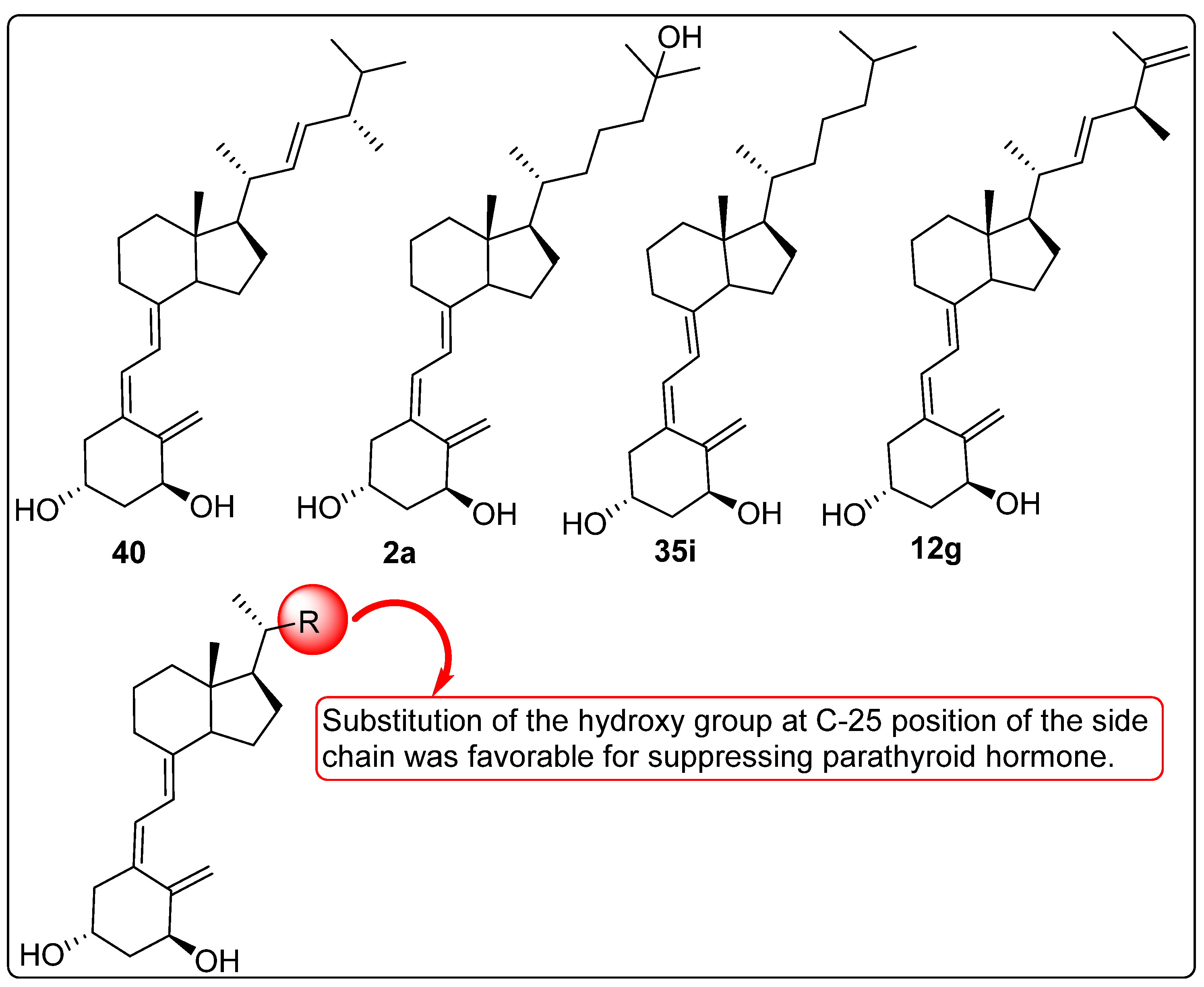
2.9. Vitamin D Analogues Altering the Secretion of Parathyroid Hormone (PTH)
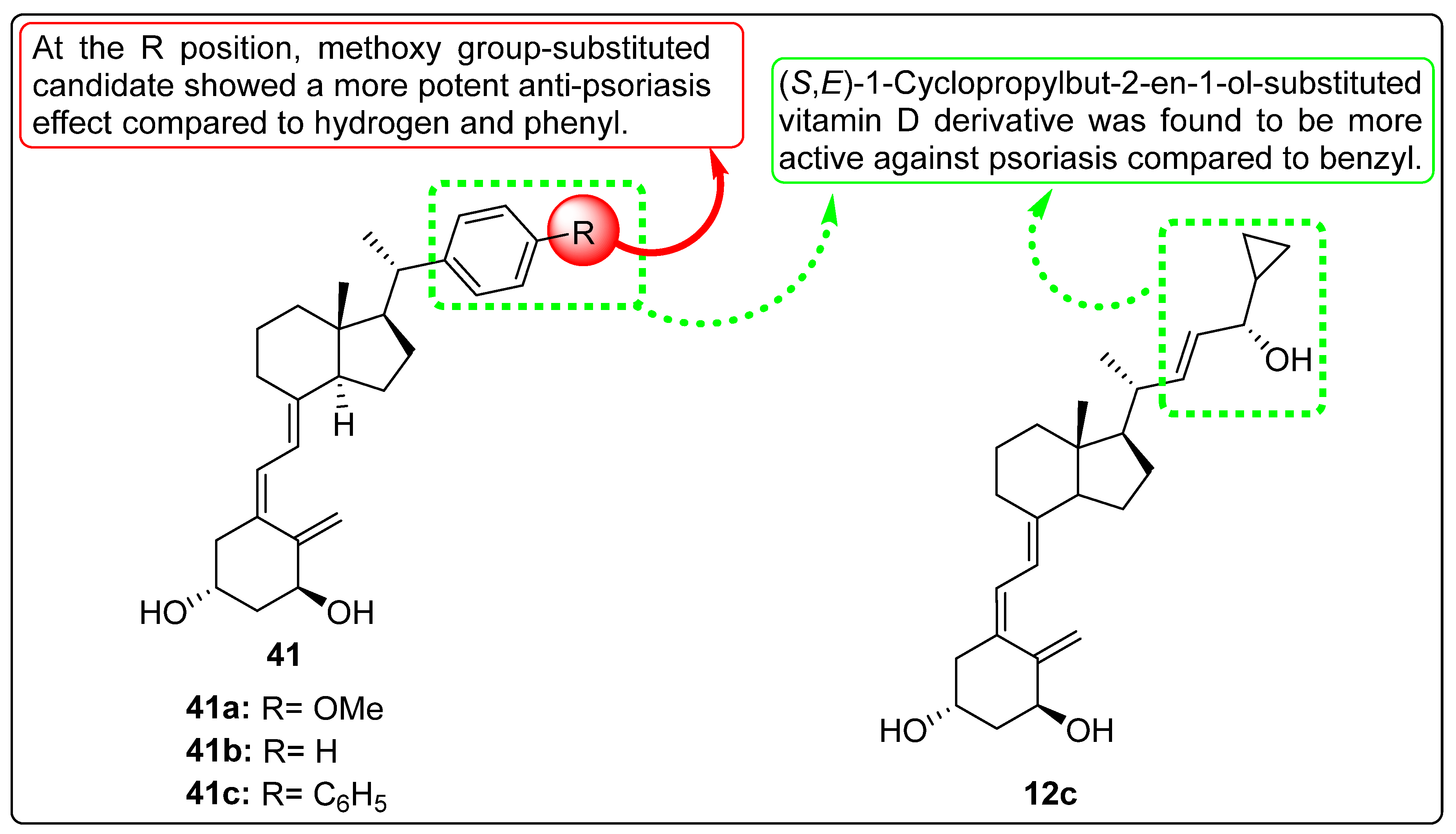
2.10. Vitamin D Analogues with Anti-Psoriasis Activity
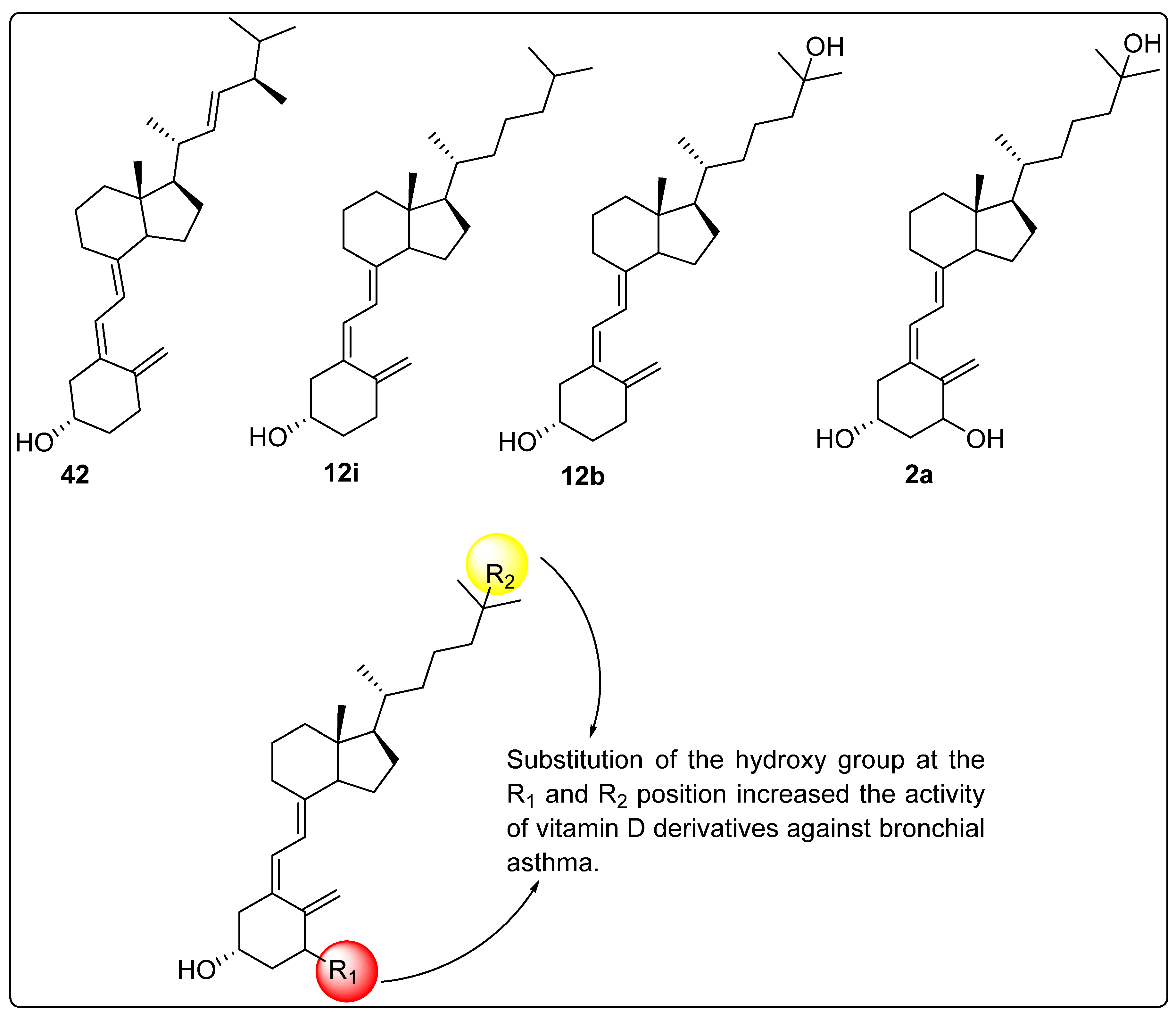
2.11. Activity of Vitamin D Analogues Against Bronchial Asthma
3. Summary and Future Perspectives
4. Conclusions
Funding
Conflicts of Interest
References
- Gezen-Ak, D.; Dursun, E. Vitamin D, a secosteroid hormone and its multifunctional receptor, vitamin D receptor, in Alzheimer’s type neurodegeneration. J. Alzheimer’s Dis. 2023, 95, 1273–1299. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Turner, A.G.; Morris, H.A. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin. Biochem. 2012, 45, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lou, Y.; Zhang, W.; Dong, Q.; Han, B. Vitamin D inhibition of lung adenocarcinoma cell proliferation in vitro. Tumor Biol. 2014, 35, 10953–10958. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, P.I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Śliwińska, A. The action of vitamin D in adipose tissue: Is there the link between vitamin D deficiency and adipose tissue-related metabolic disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef] [PubMed]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High prevalence of vitamin D deficiency among the South Asian adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef] [PubMed]
- Fiscaletti, M.; Stewart, P.; Munns, C.F. The importance of vitamin D in maternal and child health: A global perspective. Public Health Rev. 2017, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Rajappa, S.; Singh, M.; Uehara, R.; Schachterle, S.E.; Setia, S. Cancer incidence and mortality trends in Asia based on regions and human development index levels: An analyses from GLOBOCAN 2020. Curr. Med. Res. Opin. 2023, 39, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Jeyakumar, D.T.; Francis, T.V.; Misra, A. Impact of the vitamin D deficiency on COVID-19 infection and mortality in Asian countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Green, T.; Venn, B. Vitamin D in Asia. In Vitamin D: Physiology, Molecular Biology, and Clinical Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; pp. 563–587. [Google Scholar] [CrossRef]
- Dawodu, A.; Davidson, B.; Woo, J.G.; Peng, Y.M.; Ruiz-Palacios, G.M.; Guerrero, M.D.; Morrow, A.L. Sun exposure and vitamin D supplementation in relation to vitamin D status of breastfeeding mothers and infants in the global exploration of human milk study. Nutrients 2015, 7, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Ganesh, R. Vitamin D deficiency in exclusively breast-fed infants. Indian J. Med. Res. 2008, 127, 250–255. [Google Scholar] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, N.; Kaufman, J.; Rodda, C.P. Shedding light on vitamin D status and its complexities during pregnancy, infancy and childhood: An australian perspective. Int. J. Environ. Res. Public Health 2019, 16, 538. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.L.; Greer, F.R. Section on Breastfeeding and Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The impact of vitamin D on skin aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Chen, T.C.; Chimeh, F.; Lu, Z.; Mathieu, J.; Person, K.S.; Zhang, A.; Kohn, N.; Martinello, S.; Berkowitz, R.; Holick, M.F. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch. Biochem. Biophys. 2007, 460, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.S.; Volmer, D.A.; Grünhage, F.; Lammert, F. Vitamin D in chronic liver disease. Liver Int. 2013, 33, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, F.; Norlin, M.; Wikvall, K. Kidney microsomal 25-and 1α-hydroxylase in vitamin D metabolism: Catalytic properties, molecular cloning, cellular localization and expression during development. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2002, 1580, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Dereje, S.; Muradov, I.; Nazzal, S.; Nguyen, T. Cholecalciferol (D3) versus ergocalciferol (D2) in older adults. Consult. Pharm. 2017, 32, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Lhamo, Y.; Chugh, P.K.; Tripathi, C.D. Vitamin D supplements in the Indian market. Indian J. Pharm. Sci. 2016, 78, 41. [Google Scholar] [CrossRef] [PubMed]
- Okamura, W.H.; Midland, M.M.; Hammond, M.W.; Rahman, N.A.; Dormanen, M.C.; Nemere, I.; Norman, A.W. Chemistry and conformation of vitamin D molecules. J. Steroid Biochem. Mol. Biol. 1995, 53, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Potts, A.W. Electronic structure of vitamins D2 and D3. Biochim. Biophys. Acta (BBA)-Bioenergetics 1997, 1319, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Maswal, M.; Pandita, M.; Bashir, S. Terpenes, Terpenoids and Steroids: Properties, Biosynthesis and Functions. In Steroids and their Medicinal Potential; Bentham Science Publishers: Potomac, MD, USA, 2023; pp. 1–38. [Google Scholar] [CrossRef]
- Rios-Leyvraz, M.; Yao, Q. Calcium, zinc, and vitamin D in breast milk: A systematic review and meta-analysis. Int. Breastfeed. J. 2023, 18, 27. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition Novel Foods Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of vitamin D2 mushroom powder as a Novel food pursuant to Regulation (EU) 2015/2283 (NF 2019/1471). EFSA J. 2022, 20, 07326. [Google Scholar] [CrossRef]
- Magalhaes, P.J.; Carvalho, D.O.; Guido, L.F.; Barros, A.A. Detection and quantification of provitamin D 2 and vitamin D 2 in hop (Humulus lupulus L.) by liquid chromatography–diode array detection–electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2007, 55, 7995–8002. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of vitamin D3 (cholecalciferol) as a feed additive for pigs, piglets, bovines, ovines, calves, equines, chickens for fattening, turkeys, other poultry, fish and other animal species or categories, based on a dossier submitted by Fermenta Biotech Ltd. EFSA J. 2013, 11, 3289. [Google Scholar] [CrossRef]
- Almarri, F.; Haq, N.; Alanazi, F.K.; Mohsin, K.; Alsarra, I.A.; Aleanizy, F.S.; Shakeel, F. Solubility and thermodynamic function of vitamin D3 in different mono solvents. J. Mol. Liq. 2017, 229, 477–481. [Google Scholar] [CrossRef]
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, K.G.; Alexander, B.M.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Regioselective Hydroxylation in the Production of 25-Hydroxyvitamin D by Coprinopsis cinerea Peroxygenase. ChemCatChem 2015, 7, 283–290. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.L.; Sîrbe, C.; Benţa, G.; Mititelu, A.; Grama, A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 10705. [Google Scholar] [CrossRef] [PubMed]
- Aberger, S.; Schreiber, N.; Pilz, S.; Eller, K.; Rosenkranz, A.R.; Kirsch, A.H. Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity—A Comprehensive Review and Clinical Insight. Int. J. Mol. Sci. 2024, 25, 10003. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Pamphile, R.; Paris, E.; Kempf, C.; Schlichting, M.; Arnaud, S.; Garnero, P.; Garnero, P.; Meunier, P.J. Combined calcium and vitamin D3 supplementation in elderly women: Confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: The Decalyos II study. Osteoporos. Int. 2002, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Rochel, N. Vitamin D and Its Receptor from a Structural Perspective. Nutrients 2022, 14, 2847. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kesterson, R.A.; Yamamoto, H.; Taketani, Y.; Nishiwaki, E.; Tatsumi, S.; Inoue, Y.; Morita, K.; Takeda, E.; Pike, J.W. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol. Endocrinol. 1997, 11, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Chow, E.C.; Quach, H.P.; Groothuis, G.M.; Tirona, R.G.; Pang, K.S. Significance of the vitamin D receptor on crosstalk with nuclear receptors and regulation of enzymes and transporters. AAPS J. 2022, 24, 71. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hong, N.; Rhee, Y. A randomized controlled trial of the effect of raloxifene plus cholecalciferol versus cholecalciferol alone on bone mineral density in postmenopausal women with osteopenia. JBMR Plus 2024, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, R. Efficacy and safety of single or consecutive double high-dose oral cholecalciferol supplementation in adult patients with vitamin D deficiency. Steroids 2023, 199, 109308. [Google Scholar] [CrossRef] [PubMed]
- Schall, J.I.; Hediger, M.L.; Zemel, B.S.; Rutstein, R.M.; Stallings, V.A. Comprehensive Safety Monitoring of 12-Month Daily 7000-IU Vitamin D3 Supplementation in Human Immunodeficiency Virus–Infected Children and Young Adults. J. Parenter. Enter. Nutr. 2016, 40, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.; Faridi, M.M.; Aggarwal, A.; Madhu, S.V.; Malhotra, R.K. Oral Vitamin D Supplementation to Mothers During Lactation—Effect of 25 (OH) D Concentration on Exclusively Breastfed Infants at 6 Months of Age: A Randomized Double-Blind Placebo-Controlled Trial. Breastfeed. Med. 2020, 15, 237–245. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Murray, B.F. Vitamin D dose response is underestimated by Endocrine Society’s Clinical Practice Guideline. Endocr. Connect. 2013, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 20 January 2025).
- Schacht, E.; Richy, F.; Reginster, J.Y. The therapeutic effects of alfacalcidol on bone strength, muscle metabolism and prevention of falls and fractures. J. Musculoskelet. Neuronal Interact. 2005, 5, 273–284. [Google Scholar] [PubMed]
- Dennis, V.C.; Albertson, G.L. Doxercalciferol treatment of secondary hyperparathyroidism. Ann. Pharmacother. 2006, 40, 1955–1965. [Google Scholar] [CrossRef]
- Reichrath, J.; Holick, M.F. Chapter 109—Psoriasis and Other Skin Diseases. Vitamin D (Fourth Edition). Health Dis. Ther. 2018, 2, 1037–1051. [Google Scholar] [CrossRef]
- Egido, J.; Martínez-Castelao, A.; Bover, J.; Praga, M.; Torregrosa, J.V.; Fernández-Giráldez, E.; Solozábal, C. The pleiotropic effects of paricalcitol: Beyond bone-mineral metabolism. Nefrologia 2016, 36, 10–18, (In English and Spanish). [Google Scholar] [CrossRef]
- Kragballe, K. Treatment of psoriasis with calcipotriol and other vitamin D analogues. J. Am. Acad. Dermatol. 1992, 27 Pt 1, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Morii, H. Falecalcitriol as a new therapeutic agent for secondary hyperparathyroidism. Clin. Calcium 2005, 15, 29–33. [Google Scholar] [PubMed]
- Slominski, A.T.; Kim, T.K.; Hobrath JVet, a.l. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 2017, 7, 11434. [Google Scholar] [CrossRef] [PubMed]
- Kotwan, J.; Kühn, J.; Baur, A.C.; Stangl, G.I. Oral Intake of Lumisterol Affects the Metabolism of Vitamin D. Mol. Nutr. Food Res. 2021, 65, e2001165. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. [Google Scholar] [CrossRef]
- Black, L.J.; Lucas, R.M.; Sherriff, J.L.; Björn, L.O.; Bornman, J.F. In Pursuit of Vitamin D in Plants. Nutrients 2017, 9, 136. [Google Scholar] [CrossRef]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Correction: Vitamin D4 in Mushrooms. PLoS ONE 2021, 16, e0253992. [Google Scholar] [CrossRef]
- Saunders, W.B. Pharmacology and Therapeutics; Waldman, S.A., Terzic, A., Egan, L.J., Eds.; Elsevier Publishers: Philadelphia, PA, USA, 2009; pp. 1333–1378. ISBN 9781416032915. [Google Scholar] [CrossRef]
- Napoli, J.L.; Fivizzani, M.A.; Schnoes, H.K.; DeLuca, H.F. Synthesis of vitamin D5: Its biological activity relative to vitamins D3 and D2. Arch. Biochem. Biophys. 1979, 197, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Villette, C.; Delecolle, J. Vitamin D5 in Arabidopsis thaliana. Sci. Rep. 2018, 8, 16348. [Google Scholar] [CrossRef]
- Maj, E.; Filip-Psurska, B.; Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D derivatives potentiate the anticancer and anti-angiogenic activity of tyrosine kinase inhibitors in combination with cytostatic drugs in an A549 non-small cell lung cancer model. Int. J. Oncol. 2017, 52, 337–366. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and autoimmune rheumatic diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Marepally, S.R.; Goh, E.S.; Cheng, C.Y.; Janjetovic, Z.; Kim, T.K.; Miller, D.D.; Postlethwaite, A.E.; Slominski, A.T.; Tuckey, R.C.; et al. Investigation of 20 S-hydroxyvitamin D3 analogs and their 1α-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci. Rep. 2018, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-mediated hypercalcemia: Mechanisms, diagnosis, and treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnar, F.; Carlberg, C. Vitamin D and its synthetic analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishizawa, M.; Ikawa, T.; Sajiki, H.; Matsumoto, T.; Tokiwa, H.; Makishima, M.; Yamada, S. Syntheses of 25-Adamantyl-25-alkyl-2-methylidene-1α, 25-dihydroxyvitamin D3 Derivatives with Structure–Function Studies of Antagonistic and Agonistic Active Vitamin D Analogs. Biomolecules 2023, 13, 1082. [Google Scholar] [CrossRef] [PubMed]
- Belorusova, A.Y.; Rovito, D.; Chebaro, Y.; Doms, S.; Verlinden, L.; Verstuyf, A.; Metzger, D.; Rochel, N.; Laverny, G. Vitamin D Analogs Bearing C-20 Modifications Stabilize the Agonistic Conformation of Non-Responsive Vitamin D Receptor Variants. Int. J. Mol. Sci. 2022, 23, 8445. [Google Scholar] [CrossRef]
- Abe, J.; Nagai, Y.; Higashikuni, R.; Iida, K.; Hirokawa, T.; Nagai, H.; Kominato, K.; Tsuchida, T.; Hirata, M.; Inada, M.; et al. Synthesis of vitamin D 3 derivatives with nitrogen-linked substituents at A-ring C-2 and evaluation of their vitamin D receptor-mediated transcriptional activity. Org. Biomol. Chem. 2012, 10, 7826–7839. [Google Scholar] [CrossRef]
- Asano, L.; Waku, T.; Abe, R.; Kuwabara, N.; Ito, I.; Yanagisawa, J.; Nagasawa, K.; Shimizu, T. Regulation of the vitamin D receptor by vitamin D lactam derivatives. FEBS Lett. 2016, 590, 3270–3279. [Google Scholar] [CrossRef]
- Jamali, N.; Wang, S.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1,25(OH)2D3. PLoS ONE 2017, 12, e0190131. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Slominski, R.M.; Qayyum, S.; Kim, T.K.; Janjetovic, Z.; Raman, C.; Tuckey, R.C.; Song, Y.; Slominski, A.T. Molecular and structural basis of interactions of vitamin D3 hydroxyderivatives with aryl hydrocarbon receptor (AhR): An integrated experimental and computational study. Int. J. Biol. Macromol. 2022, 209, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Akagi, Y.; Usuda, K.; Yasui, K.; Shimizu, I.; Okamoto, M.; Uesugi, M.; Hosokawa, S.; Nagasawa, K. Synthesis of 24,24-difluoro-1,3-cis-25-dihydroxy-19-norvitamin D3 derivatives and evaluation of their vitamin D receptor-binding affinity. Biol. Pharm. Bull. 2016, 39, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Anami, Y.; Itoh, T.; Egawa, D.; Yoshimoto, N.; Yamamoto, K. A mixed population of antagonist and agonist binding conformers in a single crystal explains partial agonism against vitamin D receptor: Active vitamin D analogues with 22 R-alkyl group. J. Med. Chem. 2014, 57, 4351–4367. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Chida, T.; Takagi, K.; Horie, K.; Sawai, Y.; Nakamura, Y.; Harada, Y.; Takenouchi, K.; Kittaka, A. Synthesis of C-2 substituted vitamin D derivatives having ringed side chains and their biological evaluation, especially biological effect on bone by modification at the C-2 position. Org. Biomol. Chem. 2011, 9, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.M.; Binek, A.; Ahrends, T.; Nowacka, J.D.; Szydłowska, A.; Turczyk, Ł.; Wąsiewicz, T.; Wierzbicki, P.M.; Sądej, R.; Tuckey, R.C.; et al. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int. J. Oncol. 2015, 47, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Filip-Psurska, B.; Świętnicki, W.; Kutner, A.; Wietrzyk, J. Vitamin D analogs combined with 5-fluorouracil in human HT-29 colon cancer treatment. Oncol. Rep. 2014, 32, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef]
- Maj, E.; Trynda, J.; Maj, B.; Gębura, K.; Bogunia-Kubik, K.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Differential response of lung cancer cell lines to vitamin D derivatives depending on EGFR, KRAS, p53 mutation status and VDR polymorphism. J. Steroid Biochem. Mol. Biol. 2019, 193, 105431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Wigington, D.P.; Strugnell, S.A.; Knutson, J.C. Growth inhibition of cancer cells by an active metabolite of a novel vitamin D prodrug. Anticancer Res. 2005, 25, 4333–4339. [Google Scholar] [PubMed]
- DeBerardinis, A.M.; Madden, D.J.; Banerjee, U.; Sail, V.; Raccuia, D.S.; De Carlo, D.; Lemieux, S.M.; Meares, A.; Hadden, M.K. Structure–activity relationships for vitamin D3-based aromatic A-ring analogues as Hedgehog pathway inhibitors. J. Med. Chem. 2014, 57, 3724–3736. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, H.; Shen, H. Vitamin D enhances the sensitivity of breast cancer cells to the combination therapy of photodynamic therapy and paclitaxel. Tissue Cell 2022, 77, 101815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, Z. A novel glutathione-triggered theranostic prodrug for anticancer and imaging in living cells. RSC Adv. 2018, 8, 11419–11423. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Ślebioda, T.; Woźniak, M.; Tuckey, R.C.; Slominski, A.T.; Żmijewski, M.A. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids 2016, 110, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Martinesi, M.; Ambrosini, S.; Treves, C.; Zuegel, U.; Steinmeyer, A.; Vito, A.; Milla, M.; Bonanomi, A.G.; Stio, M. Role of vitamin D derivatives in intestinal tissue of patients with inflammatory bowel diseases. J. Crohn’s Colitis 2014, 8, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahab, F.K.; Al-Shawi, N.N. Effects of vitamin D3 on methotrexate-induced jejunum damage in rats. Iraqi J. Pharm. Sci. 2020, 29, 260–267. [Google Scholar] [CrossRef]
- Kudo, T.; Ishizawa, M.; Maekawa, K.; Nakabayashi, M.; Watarai, Y.; Uchida, H.; Tokiwa, H.; Ikura, T.; Ito, N.; Makishima, M.; et al. Combination of triple bond and adamantane ring on the vitamin D side chain produced partial agonists for vitamin D receptor. J. Med. Chem. 2014, 57, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Brożyna, A.A.; Kim, T.K.; Elsayed, M.M.; Janjetovic, Z.; Qayyum, S.; Slominski, R.M.; Oak, A.S.; Li, C.; Podgorska, E.; et al. CYP11A1-derived vitamin D hydroxyderivatives as candidates for therapy of basal and squamous cell carcinomas. Int. J. Oncol. 2022, 61, 96. [Google Scholar] [CrossRef] [PubMed]
- Piatek, K.; Kutner, A.; Cacsire Castillo-Tong, D.; Manhardt, T.; Kupper, N.; Nowak, U.; Chodyński, M.; Marcinkowska, E.; Kallay, E.; Schepelmann, M. Vitamin d analogs regulate the vitamin D system and cell viability in ovarian cancer cells. Int. J. Mol. Sci. 2021, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Wallbaum, P.; Rohde, S.; Ehlers, L.; Lange, F.; Hohn, A.; Bergner, C.; Schwarzenböck, S.M.; Krause, B.J.; Jaster, R. Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J. Gastroenterol. 2018, 24, 170. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, Y.; Sakamoto, R.; Nagata, A.; Sakane, S.; Kittaka, A.; Odagi, M.; Tera, M.; Nagasawa, K. Synthesis of C2-Alkoxy-Substituted 19-Nor Vitamin D3 Derivatives: Stereoselectivity and Biological Activity. Biomolecules 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, F.; Mendoza, A.; Hayata, Y.; Asano, L.; Kotake, K.; Mototani, S.; Kawamura, S.; Kurosaki, S.; Akagi, Y.; Takemoto, Y.; et al. Discovery of a vitamin D receptor-silent vitamin D derivative that impairs sterol regulatory element-binding protein in vivo. J. Med. Chem. 2021, 64, 5689–5709. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Kim, T.K.; Zabłocka, M.; Jóźwicki, W.; Yue, J.; Tuckey, R.C.; Jetten, A.M.; Slominski, A.T. Association among vitamin D, retinoic acid-related orphan receptors, and vitamin D hydroxyderivatives in ovarian cancer. Nutrients 2020, 12, 3541. [Google Scholar] [CrossRef] [PubMed]
- Rhieu, S.Y.; Annalora, A.J.; LaPorta, E.; Welsh, J.; Itoh, T.; Yamamoto, K.; Sakaki, T.; Chen, T.C.; Uskokovic, M.R.; Reddy, G.S. Potent antiproliferative effects of 25-hydroxy-16-ene-23-yne-vitamin D3 that resists the catalytic activity of both CYP27B1 and CYP24A1. J. Cell. Biochem. 2014, 115, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Gaschott, T.; Milovic, V.; Stein, J.; Steinhilber, D. Tributyrin, a stable and rapidly absorbed prodrug of butyric acid, enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells. J. Nutr. 2001, 131, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kano, A.; Masuno, H.; Songkram, C.; Kawachi, E.; Hirano, T.; Tanatani, A.; Kagechika, H. Design and synthesis of tetraol derivatives of 1, 12-dicarba-closo-dodecaborane as non-secosteroidal vitamin D analogs. Bioorg. Med. Chem. Lett. 2014, 24, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Miyauchi, Y.; Yoshida, S.; Morita, M.; Kobayashi, T.; Kanagawa, H.; Katsuyama, E.; Fujie, A.; Hao, W.; Tando, T.; et al. The vitamin D analogue ED71 but Not 1, 25 (OH) 2D3 targets HIF1α protein in osteoclasts. PLoS ONE 2014, 9, e111845. [Google Scholar] [CrossRef]
- Takeda, S.; Saito, M.; Sakai, S.; Yogo, K.; Marumo, K.; Endo, K. Eldecalcitol, an Active Vitamin D 3 Derivative, Prevents Trabecular Bone Loss and Bone Fragility in Type I Diabetic Model Rats. Calcif. Tissue Int. 2017, 101, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ochiai-Shino, H.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Promoting effect of 1,25(OH)2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015, 5, 140201. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Smith, S.Y.; Tamura, T.; Saito, H.; Takahashi, F.; Samadfam, R.; Haile, S.; Doyle, N.; Endo, K. Long-Term Treatment with Eldecalcitol (1 α, 25-Dihydroxy-2 β-(3-hydroxypropyloxy) Vitamin D 3) Suppresses Bone Turnover and Leads to Prevention of Bone Loss and Bone Fragility in Ovariectomized Rats. Calcif. Tissue Int. 2015, 96, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.D.; Farahmand, P.; Schacht, E. Alfacalcidol in men with osteoporosis: A prospective, observational, 2-year trial on 214 patients. Rheumatol. Int. 2013, 33, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H.; Ono, Y.; Ohta, M.; Itoh, S.; Ichikawa, F.; Harada, S.; Takeda, S.; Sekiguchi, N.; Ishigai, M.; Takahashi, T. A series of nonsecosteroidal vitamin D receptor agonists for osteoporosis therapy. Bioorg. Med. Chem. 2013, 21, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Nagata, A.; Ohshita, H.; Mizumoto, Y.; Iwaki, M.; Yasuda, K.; Sakaki, T.; Nagasawa, K. Chemical Synthesis of Side-Chain-Hydroxylated Vitamin D3 Derivatives and Their Metabolism by CYP27B1. ChemBioChem 2021, 22, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Byford, V.; West, S.; Masuda, S.; Ibrahim, G.; Kaufmann, M.; Knutson, J.C.; Strugnell, S.; Mehta, R. Hepatic activation and inactivation of clinically-relevant vitamin D analogs and prodrugs. Anticancer Res. 2006, 26, 2589–2595. [Google Scholar] [PubMed]
- Ogawa, S.; Ooki, S.; Shinoda, K.; Higashi, T. Analysis of urinary vitamin D 3 metabolites by liquid chromatography/tandem mass spectrometry with ESI-enhancing and stable isotope-coded derivatization. Anal. Bioanal. Chem. 2014, 406, 6647–6654. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Li, H.; Lin, M.; Xu, F.; Xia, X.; Dai, D.; Sun, R.; Ling, Y.; Qiu, L.; Wang, R.; et al. Effects of active vitamin D analogues on muscle strength and falls in elderly people: An updated meta-analysis. Front. Endocrinol. 2024, 15, 1327623. [Google Scholar] [CrossRef] [PubMed]
- Reiter, F.P.; Ye, L.; Bösch, F.; Wimmer, R.; Artmann, R.; Ziesch, A.; Kanitz, V.; Mayr, D.; Steib, C.J.; Trauner, M.; et al. Antifibrotic effects of hypocalcemic vitamin D analogs in murine and human hepatic stellate cells and in the CCl4 mouse model. Lab. Investig. 2019, 99, 1906–1917. [Google Scholar] [CrossRef]
- Brown, A.J.; Ritter, C.S.; Knutson, J.C.; Strugnell, S.A. The vitamin D prodrugs 1α (OH) D2, 1α (OH) D3 and BCI-210 suppress PTH secretion by bovine parathyroid cells. Nephrol. Dial. Transplant. 2006, 21, 644–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gu, J.; Rodriguez, K.X.; Kanda, Y.; Yang, S.; Ociepa, M.; Wilke, H.; Abrishami, A.V.; Jørgensen, L.; Skak-Nielsen, T.; Chen, J.S.; et al. Convergent total synthesis of (+)-calcipotriol: A scalable, modular approach to vitamin D analogs. Proc. Natl. Acad. Sci. USA 2022, 119, e2200814119. [Google Scholar] [CrossRef] [PubMed]
- Al-Thagfan, S.S.; Alolayan, S.O.; Ahmed, S.; Emara, M.M.; Awadallah, M.F. Impacts of deficiency in vitamin D derivatives on disease severity in adult bronchial asthma patients. Pulm. Pharmacol. Ther. 2021, 70, 102073. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of vitamin D by the liver. J. Clin. Investig. 1969, 48, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Helvig, C.; Cuerrier, D.; Collop, D.; Kharebov, A.; Ryder, K.; Epps, T.; Petkovich, M. Vitamin D analogues targeting CYP24 in chronic kidney disease. J. Steroid Biochem. Mol. Biol. 2010, 121, 13–19. [Google Scholar] [CrossRef] [PubMed]
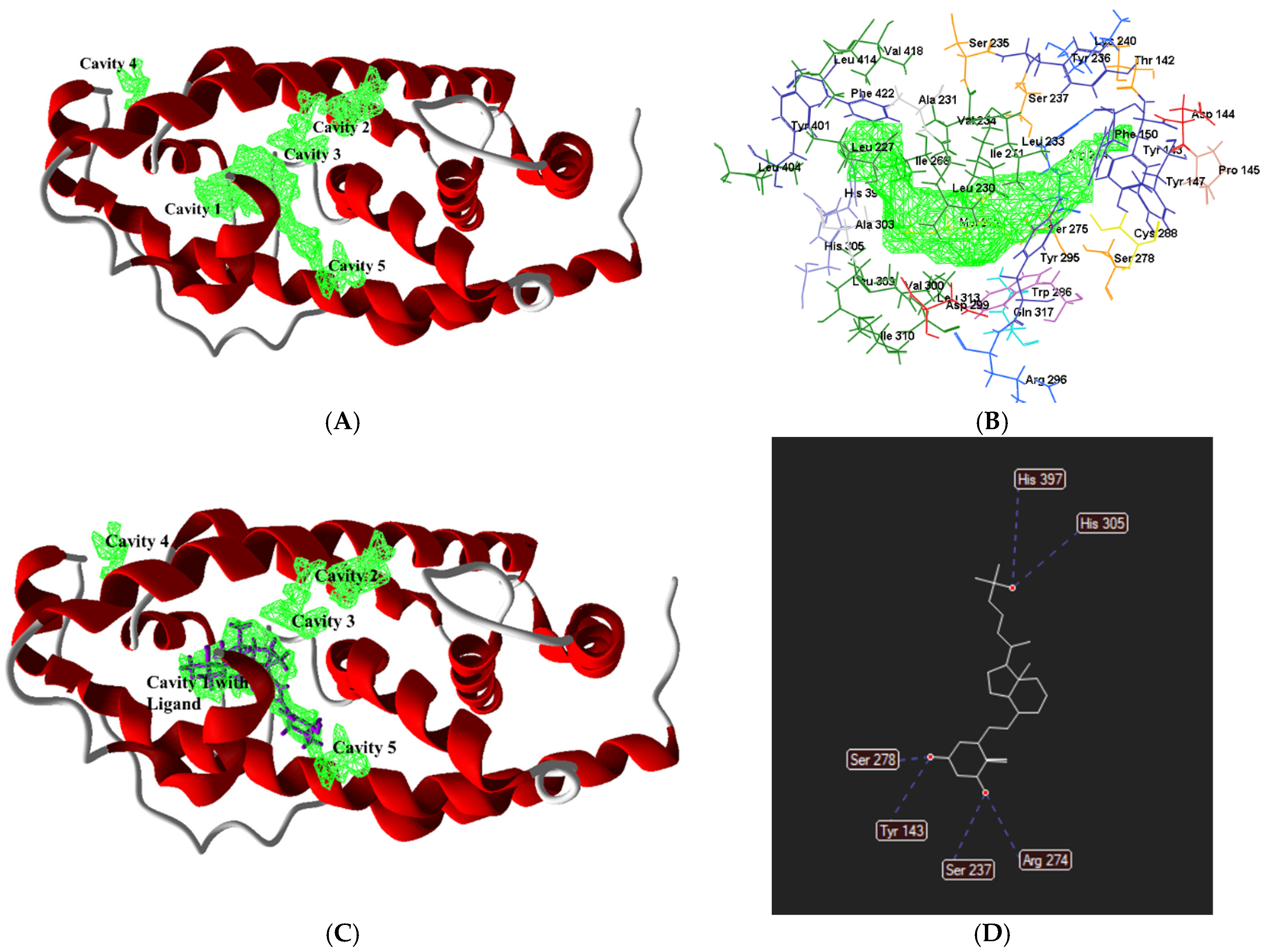



| S. No. | Cavity | Position | Volume (A3) | Surface (A2) |
|---|---|---|---|---|
| 1 | Cavity 1 | 10.692, 23.1393, 33.9638 | 202.752 | 437.76 |
| 2 | Cavity 2 | 3.6615, 26.5, 49.2174 | 25.6 | 110.08 |
| 3 | Cavity 3 | 1.33962, 25.9334, 45.0743 | 17.408 | 83.2 |
| 4 | Cavity 4 | 3.9975, 36.356, 303373 | 12.8 | 61.44 |
| 5 | Cavity 5 | 0.797499, 14.756, 43.8733 | 12.8 | 52.48 |
| Form of Vitamin D | Name | Structure | Uses |
|---|---|---|---|
| Vitamin D2 | Ergocalciferol |  | Treatment of vitamin D deficiency, management of hypoparathyroidism, used in fortified foods and supplements. |
| Vitamin D3 | Cholecalciferol |  | Treatment/prevention of vitamin D deficiency, rickets, and osteomalacia, used in fortified foods and supplements. |
| 25-Hydroxyvitamin D3 | Calcifediol | 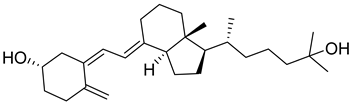 | Treatment of severe vitamin D deficiency, especially in patients with liver dysfunction. |
| 1,25 Dihydroxyvitamin D3 | Calcitriol | 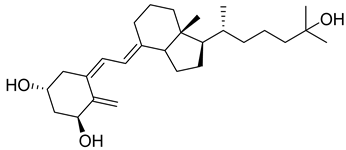 | Treatment of hypocalcemia, secondary hyperparathyroidism, osteoporosis, chronic kidney disease (CKD), and renal osteodystrophy. |
| 1-Hydroxyvitamin D3 | Alfacalcidol |  | Management of CKD-associated bone disease, reversal of myopathy, hypoparathyroidism, and other calcium-related disorders. |
| 1α-Hydroxyvitamin D2 | Doxercalciferol |  | Treatment of secondary hyperparathyroidism in CKD. |
| Synthetic Analogue | Paricalcitol | 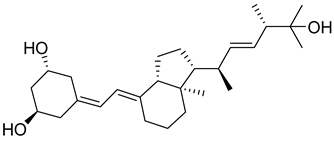 | Treatment of secondary hyperparathyroidism in CKD without causing hypercalcemia. |
| Synthetic Vitamin D3 analogue | Eldecalcitol |  | Treatment of osteoporosis; enhances calcium absorption in bones. |
| Synthetic Analogue | Calcipotriol (Calcipotriene) |  | Treatment of psoriasis; regulates keratinocyte differentiation and proliferation. |
| Synthetic Analogue | Maxacalcitol | 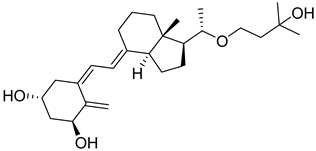 | Treatment of secondary hyperparathyroidism; management of psoriasis (in specific regions). |
| Fluorinated Synthetic Analogue | Falecalcitriol |  | Used for suppressing PTH in patients having chronic renal failure. |
| Metabolite of 7-dehydrocholesterol | Lumisterol |  | Used for anti-aging and photoprotection activity. |
| Vitamin D4 | 22-dihydroergocalciferol | 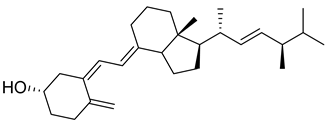 | Research studies for receptor binding and prevention of cancer and cardiovascular disease. |
| Vitamin D5 | Sitocalciferol |  | Explored for research in the area of oncology and plant-based nutrition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Nandini; Kharkwal, H.; Saha, M.; Sankaranarayanan, M.; Sharma, S.; Chander, S. Recent Advancements Towards the Use of Vitamin D Isoforms and the Development of Their Synthetic Analogues as New Therapeutics. Biomedicines 2025, 13, 1002. https://doi.org/10.3390/biomedicines13041002
Patel R, Nandini, Kharkwal H, Saha M, Sankaranarayanan M, Sharma S, Chander S. Recent Advancements Towards the Use of Vitamin D Isoforms and the Development of Their Synthetic Analogues as New Therapeutics. Biomedicines. 2025; 13(4):1002. https://doi.org/10.3390/biomedicines13041002
Chicago/Turabian StylePatel, Rajiv, Nandini, Harsha Kharkwal, Moumita Saha, Murugesan Sankaranarayanan, Saurabh Sharma, and Subhash Chander. 2025. "Recent Advancements Towards the Use of Vitamin D Isoforms and the Development of Their Synthetic Analogues as New Therapeutics" Biomedicines 13, no. 4: 1002. https://doi.org/10.3390/biomedicines13041002
APA StylePatel, R., Nandini, Kharkwal, H., Saha, M., Sankaranarayanan, M., Sharma, S., & Chander, S. (2025). Recent Advancements Towards the Use of Vitamin D Isoforms and the Development of Their Synthetic Analogues as New Therapeutics. Biomedicines, 13(4), 1002. https://doi.org/10.3390/biomedicines13041002








