Temporal Patterns of Holter-Detected Arrhythmias in Hypertrophic Cardiomyopathy Patients Treated with Mavacamten
Abstract
1. Introduction
2. Methods
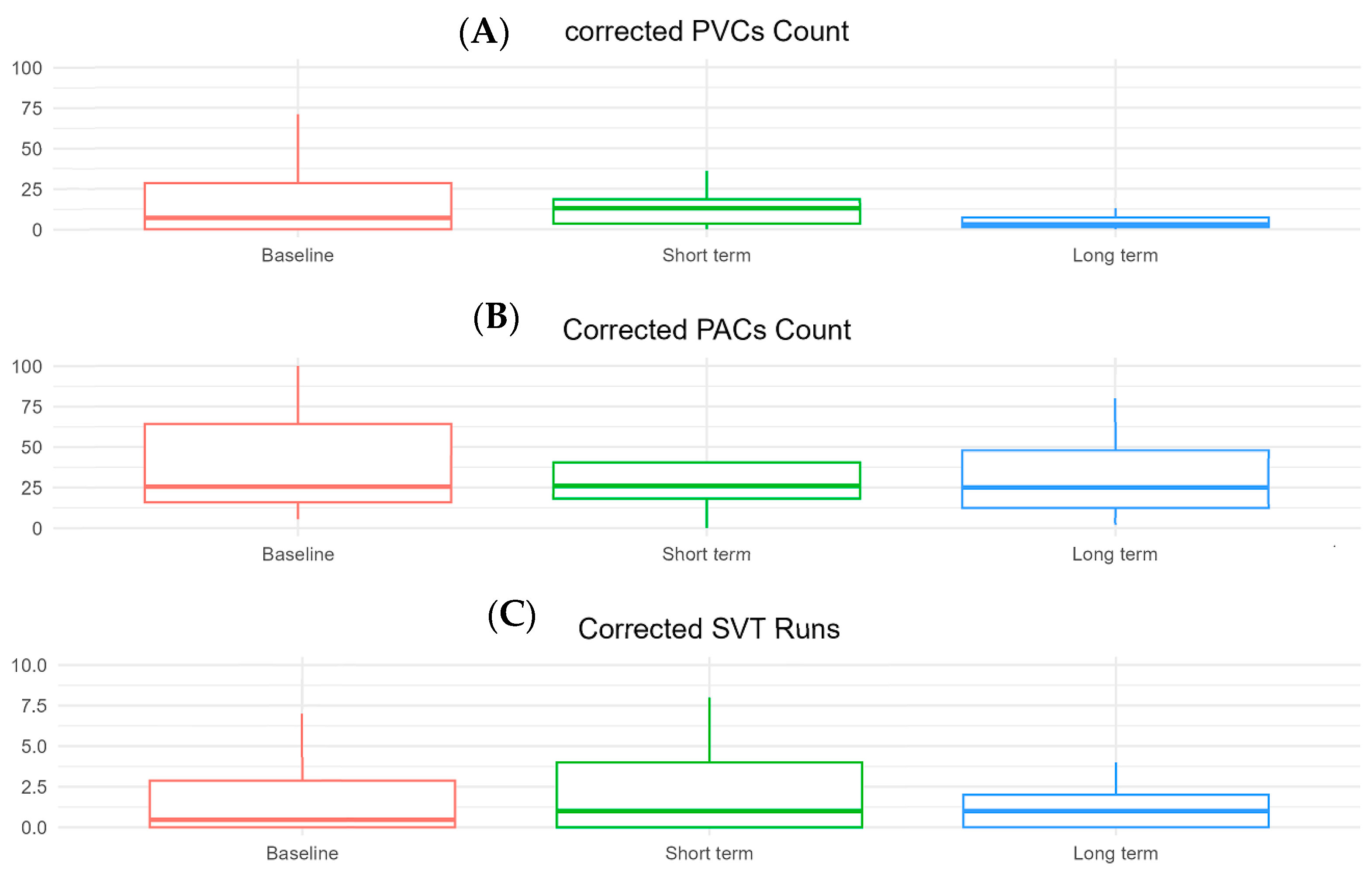
3. Results
4. Discussion
5. Conclusions
Clinical Significance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banthiya, S.; Check, L.; Atkins, J. Hypertrophic Cardiomyopathy as a Form of Heart Failure with Preserved Ejection Fraction: Diagnosis, Drugs, and Procedures. US Cardiol. Rev. 2024, 18, e17. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee, M.; Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Dearani, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Management of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 390–414. [Google Scholar] [CrossRef]
- Adabag, A.S.; Casey, S.A.; Kuskowski, M.A.; Zenovich, A.G.; Maron, B.J. Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 697–704. [Google Scholar] [CrossRef]
- Farinha, J.M.; Gupta, D.; Lip, G.Y.H. Frequent premature atrial contractions as a signalling marker of atrial cardiomyopathy, incident atrial fibrillation, and stroke. Cardiovasc. Res. 2023, 119, 429–439. [Google Scholar] [CrossRef]
- Palyam, V.; Azam, A.T.; Odeyinka, O.; Alhashimi, R.; Thoota, S.; Ashok, T.; Sange, I. Hypertrophic Cardiomyopathy and Atrial Fibrillation: A Review. Cureus 2022, 14, e21101. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Saberi, S.; Wong, T.C.; Pantazis, A.; Owens, A.; Leunig, A.; Alvarez, C.; Rader, F. Atrial Fibrillation in Hypertrophic Cardiomyopathy. JACC Adv. 2024, 3, 101210. [Google Scholar] [CrossRef]
- Du, M.; Wang, X.; Zhang, A.; Li, F.; Yi, M. Prognostic effect of atrial fibrillation on survival in patients with hypertrophic cardiomyopathy: A meta-analysis. J. Cardiothorac. Surg. 2023, 18, 196. [Google Scholar] [CrossRef]
- Perez-Silva, A.; Merino, J.L. Frequent ventricular extrasystoles: Significance, prognosis and treatment. E-J. Cardiol. Pract. 2011, 9, 22. [Google Scholar]
- Shen, H.; Dong, S.Y.; Ren, M.S.; Wang, R. Ventricular arrhythmia and sudden cardiac death in hypertrophic cardiomyopathy: From bench to bedside. Front. Cardiovasc. Med. 2022, 9, 949294. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ji, Z.; Zhou, W.; Pu, C.; Li, Y.; Zhou, C.; Hu, X.; Chen, C.; Sun, Y.; Huang, Q.; et al. Mean Scar Entropy by Late Gadolinium Enhancement Cardiac Magnetic Resonance Is Associated with Ventricular Arrhythmias Events in Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 758635. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Tomas, G.N.; James, A.W.; Sramko, M.; Pier, G.M.; Barison, A.; McKenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Park, C.H.; Kim, Y.; Kim, J.Y.; Min, P.K.; Yoon, Y.W.; Lee, K.A.; Lee, B.K.; Hong, B.K.; Kim, T.H.; et al. Burden of premature ventricular contractions beyond nonsustained ventricular tachycardia is related to the myocardial extracellular space expansion in patients with hypertrophic-cardiomyopathy. Clin. Cardiol. 2020, 43, 1317–1325. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Palandri, C.; Santini, L.; Argirò, A.; Margara, F.; Doste, R.; Bueno-Orovio, A.; Olivotto, I.; Coppini, R. Pharmacological Management of Hypertrophic Cardiomyopathy: From Bench to Bedside. Drugs 2022, 82, 889–912. [Google Scholar] [CrossRef]
- Nistri, S.; Olivotto, I.; Maron, M.S.; Ferrantini, C.; Coppini, R.; Grifoni, C.; Baldini, K.; Sgalambro, A.; Cecchi, F.; Maron, B.J. β Blockers for prevention of exercise-induced left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2012, 110, 715–719. [Google Scholar] [CrossRef]
- Gartzonikas, I.K.; Naka, K.K.; Anastasakis, A. Current and emerging perspectives on pathophysiology, diagnosis, and management of hypertrophic cardiomyopathy. Hell. J. Cardiol. 2023, 70, 65–74. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Grześk, G.; Osiak, J.; Wijata, A.; Mędlewska, M.; Gaborek, P.; Banach, J.; Wołowiec, A.; Głowacka, M. Beta-blockers in cardiac arrhythmias-Clinical pharmacologist’s point of view. Front. Pharmacol. 2022, 13, 1043714. [Google Scholar] [CrossRef]
- Seo, K.; Yamamoto, Y.; Kirillova, A.; Kawana, M.; Yadav, S.; Huang, Y.; Wang, Q.; Lane, K.V.; Pruitt, B.L.; Perez, M.V.; et al. Improved Cardiac Performance and Decreased Arrhythmia in Hypertrophic Cardiomyopathy with Non–β-Blocking R-Enantiomer Carvedilol. Circulation 2023, 148, 1691–1704. [Google Scholar] [CrossRef]
- Triska, J.; Tamargo, J.; Bozkurt, B.; Elkayam, U.; Taylor, A.; Birnbaum, Y. An Updated Review on the Role of Non-dihydropyridine Calcium Channel Blockers and Beta-blockers in Atrial Fibrillation and Acute Decompensated Heart Failure: Evidence and Gaps. Cardiovasc. Drugs Ther. 2023, 37, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.K.; Tian, L.; Belbachir, N.; Wnorowski, A.; Shrestha, R.; Ma, N.; Kitani, T.; Rhee, J.-W.; Wu, J.C. Identifying the Transcriptome Signatures of Calcium Channel Blockers in Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes. Circ. Res. 2019, 125, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Barac, I.; McKenna, W.J.; Elliott, P.M.; Dickie, S.; Chojnowska, L.; Casey, S.; Maron, B.J. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, N.J.; Coons, J.C. Disopyramide for Hypertrophic Cardiomyopathy: A Pragmatic Reappraisal of an Old Drug. Pharmacotherapy 2015, 35, 1164–1172. [Google Scholar] [CrossRef]
- Coppini, R.; Ferrantini, C.; Pioner, J.M.; Santini, L.; Wang, Z.J.; Palandri, C.; Scardigli, M.; Vitale, G.; Sacconi, L.; Stefàno, P.; et al. Electrophysiological and Contractile Effects of Disopyramide in Patients with Obstructive Hypertrophic Cardiomyopathy: A Translational Study. JACC Basic. Transl. Sci. 2019, 4, 795–813. [Google Scholar] [CrossRef]
- Kawas, R.F.; Anderson, R.L.; Ingle, S.R.B.; Song, Y.; Sran, A.S.; Rodriguez, H.M. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J. Biol. Chem. 2017, 292, 16571–16577. [Google Scholar] [CrossRef]
- Desai, M.Y.; Owens, A.; Wolski, K.; Geske, J.B.; Saberi, S.; Wang, A.; Sherrid, M.; Cremer, P.C.; Lakdawala, N.K.; Tower-Rader, A.; et al. Mavacamten in Patients with Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 56 Results from the VALOR-HCM Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 968–977. [Google Scholar] [CrossRef]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
- Spertus, J.A.; Fine, J.T.; Elliott, P.; Ho, C.Y.; Olivotto, I.; Saberi, S.; Li, W.; Dolan, C.; Reaney, M.; Sehnert, A.J.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): Health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2467–2475. [Google Scholar] [CrossRef]
- Cremer, P.C.; Geske, J.B.; Owens, A.; Jaber, W.A.; Harb, S.C.; Saberi, S.; Wang, A.; Sherrid, M.; Naidu, S.S.; Schaff, H.; et al. Myosin Inhibition and Left Ventricular Diastolic Function in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy: Insights from the VALOR-HCM Study. Circ. Cardiovasc. Imaging 2022, 15, e014986. [Google Scholar] [CrossRef]
- Hegde, S.M.; Lester, S.J.; Solomon, S.D.; Michels, M.; Elliott, P.M.; Nagueh, S.F.; Choudhury, L.; Zemanek, D.; Zwas, D.R.; Jacoby, D.; et al. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients with Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2518–2532. [Google Scholar] [CrossRef] [PubMed]
- Desai Milind, Y.; Okushi, Y.; Wolski, K.; Jeffrey, B.G.; Owens, A.; Saberi, S.; Wang, A.; Paul, C.C.; Sherrid, M.; Neal, K.L.; et al. Mavacamten-Associated Temporal Changes in Left Atrial Function in Obstructive HCM. JACC Cardiovasc. Imaging 2025, 18, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Del Franco, A.; Palinkas, E.D.; Bellagamba, C.C.A.; Biagioni, G.; Zampieri, M.; Marchi, A.; Olivotto, I. Long-Term Effects of Mavacamten on Electromechanical Dispersion and Deformation in Obstructive Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2024, 17, e011188. [Google Scholar] [CrossRef] [PubMed]
- Castrichini, M.; Alsidawi, S.; Geske, J.B.; Newman, D.B.; Arruda-Olson, A.M.; Bos, J.M.; Ommen, S.R.; Siontis, K.C.; Ackerman, M.J.; Giudicessi, J.R. Incidence of newly recognized atrial fibrillation in patients with obstructive hypertrophic cardiomyopathy treated with Mavacamten. Heart Rhythm. 2024, 21, 2065–2067. [Google Scholar] [CrossRef]
- Roehl, K.; Suppah, M.; Geske, J.; Newman, D.; Giudicessi, J.; Ackerman, M.; Ommen, S.; Alsidawi, S. Effect of Mavacamten on Hypertrophic Cardiomyopathy Patients with Left Ventricular Outflow Tract Obstruction Provoked Only Postexercise or with Amyl Nitrite. J. Am. Soc. Echocardiogr. 2024, 37, 566–568. [Google Scholar] [CrossRef]
- Suppah, M.; Roehl, K.; Lew, K.; Arsanjani, R.; Lester, S.; Ommen, S.; Geske, J.; Siontis, K.C.; Schaff, H.; Alsidawi, S. Assessment of Positive Cardiac Remodeling in Hypertrophic Obstructive Cardiomyopathy Using an Artificial Intelligence–Based Electrocardiographic Platform in Patients Treated with Mavacamten. Mayo Clin. Proc. Digit. Health 2024, 2, 255–257. [Google Scholar] [CrossRef]
- Carrick, R.T.; Maron, M.S.; Adler, A.; Wessler, B.; Hoss, S.; Chan, R.H.; Sridharan, A.; Huang, D.; Cooper, C.; Drummond, J.; et al. Development and Validation of a Clinical Predictive Model for Identifying Hypertrophic Cardiomyopathy Patients at Risk for Atrial Fibrillation: The HCM-AF Score. Circ. Arrhythm. Electrophysiol. 2021, 14, e009796. [Google Scholar] [CrossRef]
- Margara, F.; Psaras, Y.; Wang, Z.J.; Schmid, M.; Doste, R.; Garfinkel, A.C.; Repetti, G.G.; Seidman, J.G.; Seidman, C.E.; Rodriguez, B.; et al. Mechanism based therapies enable personalised treatment of hypertrophic cardiomyopathy. Sci. Rep. 2022, 12, 22501. [Google Scholar] [CrossRef]
- Saberi, S.; Cardim, N.; Yamani, M.; Schulz-Menger, J.; Li, W.; Florea, V.; Sehnert, A.J.; Kwong, R.Y.; Jerosch-Herold, M.; Masri, A.; et al. Mavacamten Favorably Impacts Cardiac Structure in Obstructive Hypertrophic Cardiomyopathy. Circulation 2021, 143, 606–608. [Google Scholar] [CrossRef]
- Suppah, M.; Abdalla, H.; Roehl, K.; Farina, J.; Arsanjani, R.; Geske, J.; Ommen, S.; Alsidawi, S. Sustained Benefits of Mavacamten in Patients with Obstructive Hypertrophic Cardiomyopathy: Long-Term Assessment Using Artificial Intelligence-Electrocardiogram and Echocardiographic Data. J. Am. Soc. Echocardiogr. 2025, 38, 47–49. [Google Scholar] [CrossRef]
- Grillo, M.P.; Erve, J.C.L.; Dick, R.; Driscoll, J.P.; Haste, N.; Markova, S.; Brun, P.; Carlson, T.J.; Evanchik, M. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica 2019, 49, 718–733. [Google Scholar] [CrossRef]
- Rader, F.; Oręziak, A.; Choudhury, L.; Saberi, S.; Fermin, D.; Wheeler, M.T.; Abraham, T.P.; Garcia-Pavia, P.; Zwas, D.R.; Masri, A.; et al. Mavacamten Treatment for Symptomatic Obstructive Hypertrophic Cardiomyopathy: Interim Results from the MAVA-LTE Study, EXPLORER-LTE Cohort. JACC Heart Fail. 2024, 12, 164–177. [Google Scholar] [CrossRef]
| Characteristic | n = 27 |
|---|---|
| Demographics | |
| Age, years (median [IQR]) | 66 [58–72] |
| Age group, n (%) | |
| <50 | 2 (7%) |
| 50–70 | 13 (48%) |
| >70 | 10 (37%) |
| Sex | |
| Male | 12 (35%) |
| Female | 15 (65%) |
| Clinical characteristics | |
| Hypertension, n (%) | 19 (70%) |
| Diabetes mellitus, n (%) | 3 (11%) |
| CKD, n (%) | 5 (18.5) |
| Asthma/COPD, n (%) | 6 (22%) |
| Afib, n (%) | 8 (30%) |
| Afib ablation, n (%) | 2 (7%) |
| ICD Implantation, n (%) | 13 (48%) |
| Medications | |
| Beta-blockers, n (%) | 23 (85%) |
| Calcium channel blockers, n (%) | 3 (11%) |
| Antiarrhythmics, n (%) | 1 (3%) |
| Echocardiographic measures | |
| Ejection fraction, % (median [IQR) | 71 [66.5–73.5] |
| LVOT MIG at rest, mmHg (median [IQR]) | 52 [17.5–101] |
| LVOT MIG with Valsalva, mmHg (median [IQR]) | 62 [33.5–73.25] |
| LV mass index, g/m2 (median [IQR]) | 126.5 [103.74–135.5] |
| Maximum LV wall thickness, mm (median [IQR]) | 19 [17–21] |
| LV septal thickness, mm (median [IQR]) | 15 [13–17] |
| Left atrial volume index, mL/m2 (median [IQR]) | 46 [35.5–54] |
| Baseline n = 10 | Short-Term n = 10 | p-Value 1 | |
|---|---|---|---|
| PVC, Median [Q1–Q3] | 24 (2, 286) | 19 (7, 494) | 0.63 |
| PAC, Median [Q1–Q3] | 70 (16, 231) | 145 (28, 622) | 0.69 |
| Number of SVT runs, Median [Q1–Q3] | 0.25 (0.00, 3.13) | 0.76 (0.00, 4.00) | 0.67 |
| SVT max-duration, Median [Q1–Q3] | 2 (0, 10) | 6 (0, 19) | 0.09 |
| SVT max-rate, Median [Q1–Q3] | 48 (0, 130) | 115 (0, 152) | 0.44 |
| Baseline n = 24 | Long-Term n = 23 | p-Value 1 | |
|---|---|---|---|
| PVC, Median [Q1–Q3] | 13 (1, 39) | 5 (1, 40) | 0.85 |
| PAC, Median [Q1–Q3] | 75 (24, 166) | 53 (18, 204) | 0.37 |
| Number of SVT runs, Median [Q1–Q3] | 0.47 (0.00, 3.00) | 1.00 (0.00, 2.00) | 0.27 |
| SVT max-duration, Median [Q1–Q3] | 3 (0, 10) | 3 (0, 9) | 0.46 |
| SVT max-rate, Median [Q1–Q3] | 102 (0, 141) | 109 (0, 142) | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.; Roehl, K.; Suppah, M.; Abo Abdullah, H.; Arsanjani, R.; Siontis, K.C.; Geske, J.B.; Ommen, S.R.; Giudicessi, J.R.; Alsidawi, S. Temporal Patterns of Holter-Detected Arrhythmias in Hypertrophic Cardiomyopathy Patients Treated with Mavacamten. Biomedicines 2025, 13, 1005. https://doi.org/10.3390/biomedicines13041005
Badr A, Roehl K, Suppah M, Abo Abdullah H, Arsanjani R, Siontis KC, Geske JB, Ommen SR, Giudicessi JR, Alsidawi S. Temporal Patterns of Holter-Detected Arrhythmias in Hypertrophic Cardiomyopathy Patients Treated with Mavacamten. Biomedicines. 2025; 13(4):1005. https://doi.org/10.3390/biomedicines13041005
Chicago/Turabian StyleBadr, Amro, Kaitlin Roehl, Mustafa Suppah, Humam Abo Abdullah, Reza Arsanjani, Konstantinos C. Siontis, Jeffrey B. Geske, Steve R. Ommen, John R. Giudicessi, and Said Alsidawi. 2025. "Temporal Patterns of Holter-Detected Arrhythmias in Hypertrophic Cardiomyopathy Patients Treated with Mavacamten" Biomedicines 13, no. 4: 1005. https://doi.org/10.3390/biomedicines13041005
APA StyleBadr, A., Roehl, K., Suppah, M., Abo Abdullah, H., Arsanjani, R., Siontis, K. C., Geske, J. B., Ommen, S. R., Giudicessi, J. R., & Alsidawi, S. (2025). Temporal Patterns of Holter-Detected Arrhythmias in Hypertrophic Cardiomyopathy Patients Treated with Mavacamten. Biomedicines, 13(4), 1005. https://doi.org/10.3390/biomedicines13041005






