The Cellular and Molecular Characteristics of Postnatal Human Thymus Stromal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thymus Tissue
2.2. Tissue Digestion and Thymic Stromal Cell Isolation
2.3. Thymic Stromal Cell Enrichment
2.4. Spheroid Culture
2.5. Growth Factor Treatment
2.6. Limiting Dilution Assay
2.7. 2′-Deoxyguanosine Treatment
2.8. Multiplex PCR
2.9. Flow Cytometry
2.10. Fluorescent and Confocal Microscopy
3. Results
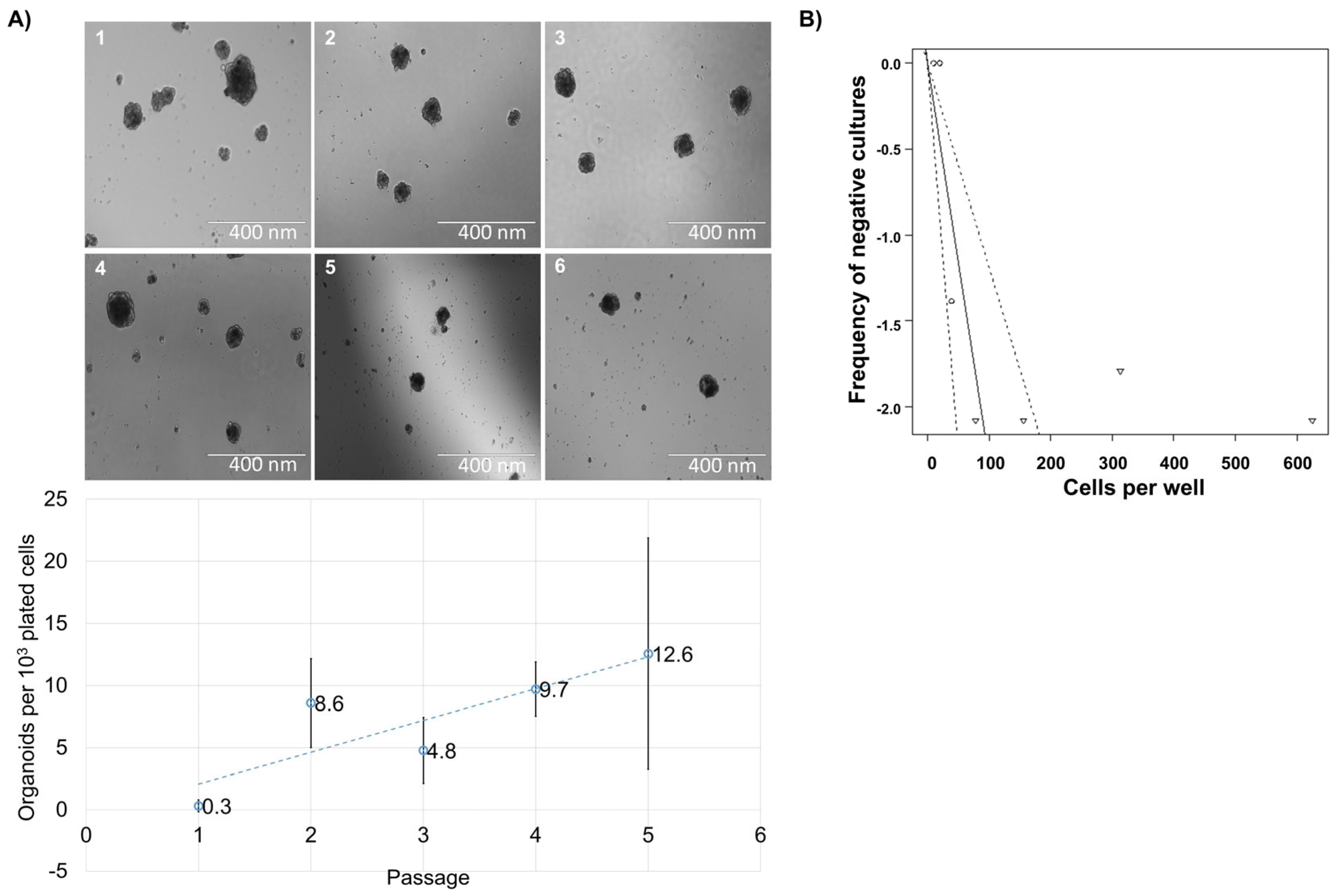
3.1. Spheroid Formation Potential of Cells from Human Thymus
3.2. Reformation Ability of Cells from Human Thymus
3.3. Expressional Analysis of Thymus Spheroid Cells
3.4. 2-Deoxyguanosine Treatment of the Stromal Cell Population
3.5. Cyr61 and Interleukin-22 Impact on Spheroid Formation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Sequence |
|---|---|
| GUSB | F1: ACTTCTCTGACAACCGACGC |
| F2: GTACGAACGGGAGGTGATCC | |
| R: CATTCACCCACACGATGGCA | |
| KRT5 | F1:AGGGCGAGGAATGCAGACTC |
| F2: TGGACCAGTCAACATCTCTGT | |
| R:ACTGCTACCTCCGGCAAGA | |
| KRT8 | F1:GCTCCAGGCTGAGATTGAGG |
| F2:TAAGGATGCCAACGCCAAGT | |
| R: CTCAGACCACCTGCATAGCC | |
| AIRE | F1:ACTTCTGGAGGGTGCTGTTC |
| F2: AGACTCCCCACCAAGAGGAA | |
| R: CCTTCAGTTGAGAGCCTGGG | |
| EpCAM | F1: AGAGCAAAACCTGAAGGGGC |
| F2: TCCATGTGCTGGTGTGTGAA | |
| R: TCTGAAGTGCAGTCCGCAAA | |
| PDGFRB1 | F1: CCCTTCCTCCATCCCTCTGT |
| F2: GGAGAGGGCAGTAAGGAGGA | |
| R: GAAGCCGCATGGTGTCCTTG | |
| HOXA3 | F1: GGCAAGGACACAGCATCTTG |
| F2: GACACCGGAAAAGGCGATTC | |
| R: CCCGCGCAGACCTAGAAAGA | |
| PAX1 | F1: GCTGCCCTACAACCACATCT |
| F2: TGGGCATCCGGACGTTTATG | |
| R: GGAGGCCGACTGAGTGTATTTA | |
| PAX9 | F1: CTTTTAGGGCGTGTCCCCAG |
| F2: CTTTCATCGGGGCACAGACT | |
| R: AAGGCTGGCTCCATTGCT |
References
- Boyd, R.L.; Tucek, C.L.; Godfrey, D.I.; Izon, D.J.; Wilson, T.J.; Davidson, N.J.; Bean, A.G.D.; Ladyman, H.M.; Ritter, M.A.; Hugo, P. The Thymic Microenvironment. Immunol. Today 1993, 14, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Steinmann, G.G.; Klaus, B.; Muller-Hermelink, H.K. The Involution of the Ageing Human Thymic Epithelium Is Independent of Puberty. A Morphometric Study. Scand. J. Immunol. 1985, 22, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Albergante, L.; Blackburn, C.C.; Newman, T.J. Thymic Involution and Rising Disease Incidence with Age. Proc. Natl. Acad. Sci. USA 2018, 115, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, E.W.; Nagelkerken, L. Age-Related Changes in Immune Reactivity: The Influence of Intrinsic Defects and of a Changed Composition of the CD4+ T Cell Compartment. Exp. Clin. Immunogenet. 1992, 9, 195–202. [Google Scholar]
- Chaudhry, M.S.; Velardi, E.; Dudakov, J.A.; van den Brink, M.R.M. Thymus: The Next (Re)Generation. Immunol. Rev. 2016, 271, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; Ucar, O.; Klug, P.; Matt, S.; Brunk, F.; Hofmann, T.G.; Kyewski, B. Adult Thymus Contains FoxN1- Epithelial Stem Cells That Are Bipotent for Medullary and Cortical Thymic Epithelial Lineages. Immunity 2014, 41, 257–269. [Google Scholar] [CrossRef]
- Campinoti, S.; Gjinovci, A.; Ragazzini, R.; Zanieri, L.; Ariza-McNaughton, L.; Catucci, M.; Boeing, S.; Park, J.E.; Hutchinson, J.C.; Muñoz-Ruiz, M.; et al. Reconstitution of a Functional Human Thymus by Postnatal Stromal Progenitor Cells and Natural Whole-Organ Scaffolds. Nat. Commun. 2020, 11, 6372. [Google Scholar] [CrossRef]
- Sheridan, J.M.; Keown, A.; Policheni, A.; Roesley, S.N.A.A.; Rivlin, N.; Kadouri, N.; Ritchie, M.E.; Jain, R.; Abramson, J.; Heng, T.S.P.P.; et al. Thymospheres Are Formed by Mesenchymal Cells with the Potential to Generate Adipocytes, but Not Epithelial Cells. Cell Rep. 2017, 21, 934–942. [Google Scholar] [CrossRef]
- Stoeckle, C.; Rota, I.A.; Tolosa, E.; Haller, C.; Melms, A.; Adamopoulou, E. Isolation of Myeloid Dendritic Cells and Epithelial Cells from Human Thymus. J. Vis. Exp. 2013, 79, e50951. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis HHS Public Access. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smyth, G.K. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 2009, 347, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, F.; Zúñiga-Pflücker, J.C.; Takahama, Y. In Vitro Systems for the Study of T Cell Development: Fetal Thymus Organ Culture and OP9-DL1 Cell Coculture. Curr. Protoc. Immunol. 2006, 71, 3–18. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Monteiro, M.; Rocha, B.; Veiga-fernandes, H. Quantification of Multiple Gene Expression in Individual Cells. Genome Res. 2004, 14, 1938–1947. [Google Scholar]
- Horvat, L.; Skelin, J.; Jelić Puškarić, B.; Feliciello, I.; Heckel, D.; Madunić, J.; Kardum-Skelin, I.; Matulić, M.; Radić-Krišto, D.; Antica, M. Notch pathway connections in primary leukaemia samples of limited size. Transl. Med. Commun. 2018, 3, 8. [Google Scholar] [CrossRef]
- Shezen, E.; Okon, E.; Ben-Hur, H.; Abramsky, O. Cytokeratin Expression in Human Thymus: Immunohistochemical Mapping. Cell Tissue Res. 1995, 279, 221–231. [Google Scholar] [CrossRef]
- Lee, E.N.; Park, J.K.; Lee, J.-R.; Oh, S.-O.; Baek, S.-Y.; Kim, B.-S.; Yoon, S. Characterization of the Expression of Cytokeratins 5, 8, and 14 in Mouse Thymic Epithelial Cells during Thymus Regeneration Following Acute Thymic Involution. Anat. Cell Biol. 2011, 44, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Peterson, P.; Scott, H.S.; Kudoh, J.; Minoshima, S.; Heino, M.; Krohn, K.J.; Lalioti, M.D.; Mullis, P.E.; Antonarakis, S.E.; et al. Positional Cloning of the APECED Gene. Nat. Genet. 1997, 17, 393–398. [Google Scholar] [CrossRef]
- Perniola, R. Twenty Years of AIRE. Front. Immunol. 2018, 9, 98. [Google Scholar] [CrossRef]
- Veevers-Lowe, J.; Ball, S.G.; Shuttleworth, A.; Kielty, C.M. Mesenchymal Stem Cell Migration Is Regulated by Fibronectin through A5β1-Integrin-Mediated Activation of PDGFR-β and Potentiation of Growth Factor Signals. J. Cell Sci. 2011, 124, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Farley, A.M.; Morris, L.X.; Vroegindeweij, E.; Depreter, M.L.G.; Vaidya, H.; Stenhouse, F.H.; Tomlinson, S.R.; Anderson, R.A.; Cupedo, T.; Cornelissen, J.J.; et al. Dynamics of Thymus Organogenesis and Colonization in Early Human Development. Development 2013, 140, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Unanue, E.R.; Turk, V.; Neefjes, J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu. Rev. Immunol. 2016, 34, 265–297. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef]
- Gracz, A.D.; Fuller, M.K.; Wang, F.; Li, L.; Stelzner, M.; Dunn, J.C.Y.Y.; Martin, M.G.; Magness, S.T. Brief Report: CD24 and CD44 Mark Human Intestinal Epithelial Cell Populations with Characteristics of Active and Facultative Stem Cells. Stem Cells 2013, 31, 2024–2030. [Google Scholar] [CrossRef]
- Jenkinson, E.J.; Franchi, L.L.; Kingston, R.; Owen, J.J.T. Effect of Deoxyguanosine on Lymphopoiesis in the Developing Thymus Rudimentin Vitro: Application in the Production of Chimeric Thymus Rudiments. Eur. J. Immunol. 1982, 12, 583–587. [Google Scholar] [CrossRef]
- Emre, Y.; Irla, M.; Dunand-Sauthier, I.; Ballet, R.; Meguenani, M.; Jemelin, S.; Vesin, C.; Reith, W.; Imhof, B.A. Thymic Epithelial Cell Expansion through Matricellular Protein CYR61 Boosts Progenitor Homing and T-Cell Output. Nat. Commun. 2013, 4, 2842. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; Jenq, R.R.; Young, L.F.; Ghosh, A.; Singer, N.V.; West, M.L.; Smith, O.M.; Holland, A.M.; Tsai, J.J.; et al. Interleukin-22 Drives Endogenous Thymic Regeneration in Mice. Science 2012, 336, 91–95. [Google Scholar] [CrossRef]
- Feng, D.; Kong, X.; Weng, H.; Park, O.; Wang, H.; Dooley, S.; Gershwin, M.E.; Gao, B. Interleukin-22 Promotes Proliferation of Liver Stem/Progenitor Cells in Mice and Patients with Chronic Hepatitis B Virus Infection. Gastroenterology 2012, 143, 188–198.e7. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 Promotes Intestinal-Stem-Cell-Mediated Epithelial Regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Plum, J.; De Smedt, M.; Defresne, M.P.; Leclercq, G.; Vandekerckhove, B. Human CD34+ fetal liver stem cells differentiate to T cells in a mouse thymic microenvironment. Blood 1994, 84, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.M.; Namikawa, R.; Kaneshima, H.; Shultz, L.D.; Lieberman, M.; Weissman, I.L. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science 1988, 241, 1632–1639. [Google Scholar] [CrossRef]
- Vandekerckhove, B.A.; Namikawa, R.; Bacchetta, R.; Roncarolo, M.G. Human hematopoietic cells and thymic epithelial cells induce tolerance via different mechanisms in the SCID-hu mouse thymus. J. Exp. Med. 1992, 175, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Montel-Hagen, A.; Tsai, S.; Seet, C.S.; Crooks, G.M. Generation of Artificial Thymic Organoids from Human and Murine Hematopoietic Stem and Progenitor Cells. Curr. Protoc. 2022, 2, e403. [Google Scholar] [CrossRef] [PubMed]
- Bleul, C.C.; Corbeaux, T.; Reuter, A.; Fisch, P.; Mönting, J.S.; Boehm, T. Formation of a Functional Thymus Initiated by a Postnatal Epithelial Progenitor Cell. Nature 2006, 441, 992–996. [Google Scholar] [CrossRef]
- Wong, K.; Lister, N.L.; Barsanti, M.; Lim, J.M.C.; Hammett, M.V.; Khong, D.M.; Siatskas, C.; Gray, D.H.D.; Boyd, R.L.; Chidgey, A.P. Multilineage Potential and Self-Renewal Define an Epithelial Progenitor Cell Population in the Adult Thymus. Cell Rep. 2014, 8, 1198–1209. [Google Scholar] [CrossRef]
- Ulyanchenko, S.; O’Neill, K.E.; Medley, T.; Farley, A.M.; Vaidya, H.J.; Cook, A.M.; Blair, N.F.; Blackburn, C.C. Identification of a Bipotent Epithelial Progenitor Population in the Adult Thymus. Cell Rep. 2016, 14, 2819–2832. [Google Scholar] [CrossRef]
- Lim, S.; J F van Son, G.; Wisma Eka Yanti, N.L.; Andersson-Rolf, A.; Willemsen, S.; Korving, J.; Lee, H.G.; Begthel, H.; Clevers, H. Derivation of functional thymic epithelial organoid lines from adult murine thymus. Cell Rep. 2024, 43, 114019. [Google Scholar] [CrossRef]
- Ocampo-Godinez, J.M.; Gonzalez-Quiroz, J.L.; Cote-Palafox, H.; George, E.; Vergara-Lope Nuñez, J.A.; Villagomez-Olea, G.; Vazquez-Vazquez, F.C.; Lopez-Villegas, E.O.; Leon-Avila, G.; Dominguez-Lopez, M.L.; et al. Primary explants of the postnatal thymus allow the expansion of clonogenic thymic epithelial cells that constitute thymospheres. Stem Cell Res. Ther. 2023, 14, 312. [Google Scholar] [CrossRef]
- Hübscher, T.; Lorenzo-Martín, L.F.; Barthlott, T.; Tillard, L.; Langer, J.J.; Rouse, P.; Blackburn, C.C.; Holländer, G.; Lutolf, M.P. Thymic epithelial organoids mediate T-cell development. Development 2024, 151, dev202853. [Google Scholar] [CrossRef]
- Bredenkamp, N.; Ulyanchenko, S.; O’Neill, K.E.; Manley, N.R.; Vaidya, H.J.; Blackburn, C.C. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat. Cell Biol. 2014, 16, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, D.N.; Isono, K.; Hikosaka-Kuniishi, M.; Yamazaki, H. Neural Crest-Derived Mesenchymal Cells Support Thymic Reconstitution After Lethal Irradiation. Eur. J. Immunol. 2025, 55, e202451305. [Google Scholar] [CrossRef]
- Chojnowski, J.L.; Masuda, K.; Trau, H.A.; Thomas, K.; Capecchi, M.; Manley, N.R. Multiple Roles for HOXA3 in Regulating Thymus and Parathyroid Differentiation and Morphogenesis in Mouse. Development 2014, 141, 3697–3708. [Google Scholar] [CrossRef]
- Figueiredo, M.; Zilhão, R.; Neves, H. Thymus Inception: Molecular Network in the Early Stages of Thymus Organogenesis. Int. J. Mol. Sci. 2020, 21, 5765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Swiggard, W.J.; Heufler, C.; Peng, M.; Mirza, A.; Steinman, R.M.; Nussenzweig, M.C. The Receptor DEC-205 Expressed by Dendritic Cells and Thymic Epithelial Cells Is Involved in Antigen Processing. Nature 1995, 375, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell. Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skelin Ilic, J.; Bódi, I.; Milkovic, L.; Prodan, Z.; Belina, D.; Heckel, D.; Cicin-Sain, L.; Grčević, D.; Delfino, D.V.; Radic Kristo, D.; et al. The Cellular and Molecular Characteristics of Postnatal Human Thymus Stromal Stem Cells. Biomedicines 2025, 13, 1004. https://doi.org/10.3390/biomedicines13041004
Skelin Ilic J, Bódi I, Milkovic L, Prodan Z, Belina D, Heckel D, Cicin-Sain L, Grčević D, Delfino DV, Radic Kristo D, et al. The Cellular and Molecular Characteristics of Postnatal Human Thymus Stromal Stem Cells. Biomedicines. 2025; 13(4):1004. https://doi.org/10.3390/biomedicines13041004
Chicago/Turabian StyleSkelin Ilic, Josipa, Ildikó Bódi, Lidija Milkovic, Zsolt Prodan, Dražen Belina, Darko Heckel, Lipa Cicin-Sain, Danka Grčević, Domenico Vittorio Delfino, Delfa Radic Kristo, and et al. 2025. "The Cellular and Molecular Characteristics of Postnatal Human Thymus Stromal Stem Cells" Biomedicines 13, no. 4: 1004. https://doi.org/10.3390/biomedicines13041004
APA StyleSkelin Ilic, J., Bódi, I., Milkovic, L., Prodan, Z., Belina, D., Heckel, D., Cicin-Sain, L., Grčević, D., Delfino, D. V., Radic Kristo, D., Matulić, M., & Antica, M. (2025). The Cellular and Molecular Characteristics of Postnatal Human Thymus Stromal Stem Cells. Biomedicines, 13(4), 1004. https://doi.org/10.3390/biomedicines13041004








