Development and Validation of a Predictive Nomogram for Venous Thromboembolism Risk in Multiple Myeloma Patients: A Single-Center Cohort Study in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Statistical Analysis and Construction of the Nomogram
3. Results
3.1. Clinical Characteristics of Patients
3.2. Independent Predictive Factors in the Training Cohort
3.3. Predictive Nomogram for VTE
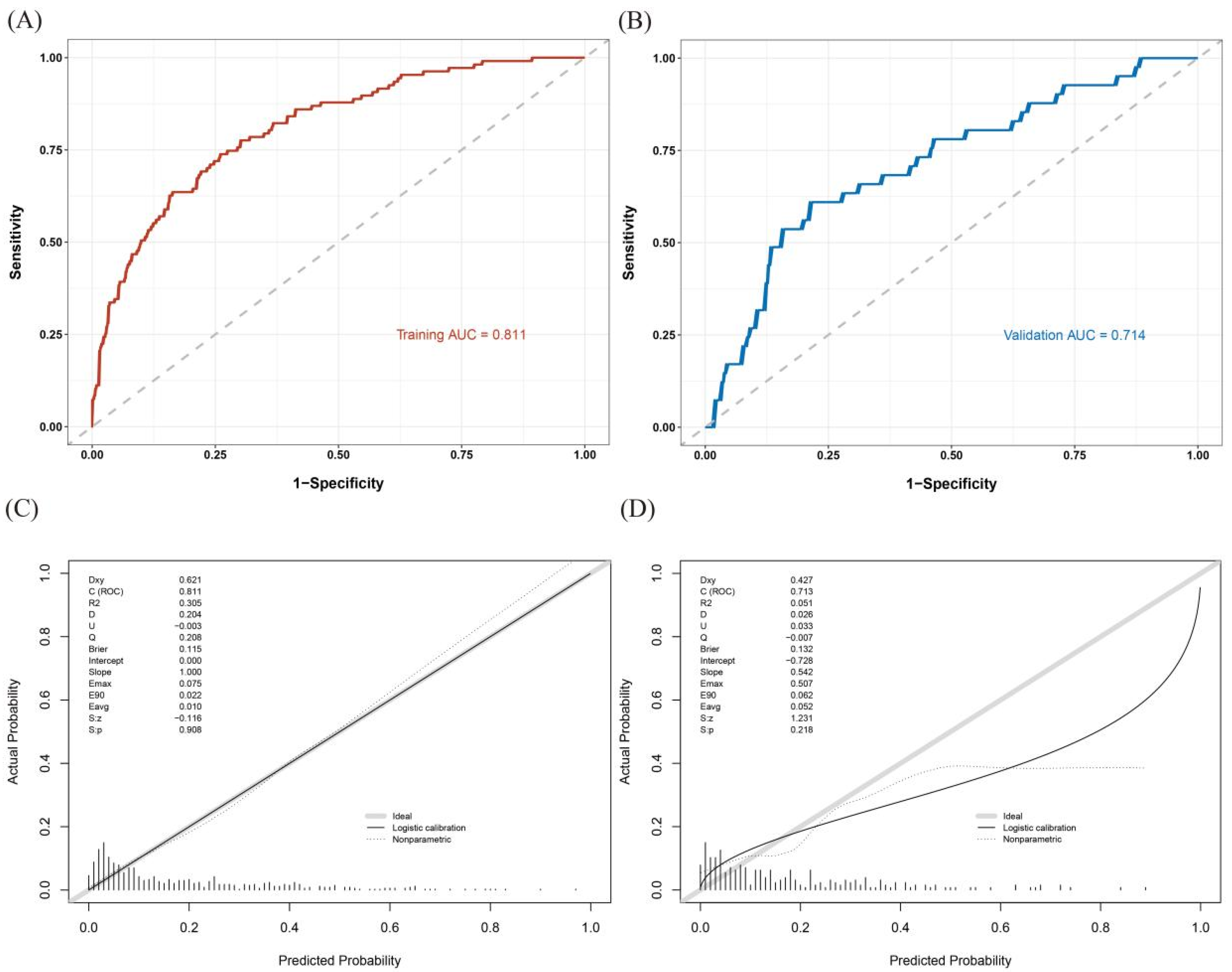
3.4. Validation and Calibration of the Nomogram
3.5. Evaluating the Effectiveness of the Nomogram in Clinical Decision-Making
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenger, N.; Sebastian, T.; Engelberger, R.P.; Kucher, N.; Spirk, D. Pulmonary embolism and deep vein thrombosis: Similar but different. Thromb. Res. 2021, 206, 88–98. [Google Scholar] [CrossRef]
- Blitzer, R.R.; Eisenstein, S. Venous Thromboembolism and Pulmonary Embolism: Strategies for Prevention and Management. Surg. Clin. N. Am. 2021, 101, 925–938. [Google Scholar] [CrossRef]
- Farmakis, I.T.; Barco, S.; Mavromanoli, A.C.; Agnelli, G.; Cohen, A.T.; Giannakoulas, G.; Mahan, C.E.; Konstantinides, S.V.; Valerio, L. Cost-of-Illness Analysis of Long-Term Health Care Resource Use and Disease Burden in Patients with Pulmonary Embolism: Insights from the PREFER in VTE Registry. J. Am. Heart Assoc. 2022, 11, e027514. [Google Scholar] [CrossRef]
- Charalampous, C.; Shah, D.; Kumar, S.; Chakraborty, R. Thromboprophylaxis in multiple myeloma: A case-based review with practical guidelines. Ann. Hematol. 2024, 103, 3881–3888. [Google Scholar] [CrossRef]
- Baker, H.A.; Brown, A.R.; Mahnken, J.D.; Shireman, T.I.; Webb, C.E.; Lipe, B.C. Application of risk factors for venous thromboembolism in patients with multiple myeloma starting chemotherapy, a real-world evaluation. Cancer Med. 2019, 8, 455–462. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Cheng, Q.; Zhao, F.; Li, J.; Yan, H.; Xu, A.; Sun, C.; Hu, Y. Hematologists’ awareness of venous thromboembolism in multiple myeloma: A national survey in China. Ann. Med. 2023, 55, 2263019. [Google Scholar] [CrossRef]
- Baljevic, M.; Sborov, D.W.; Lim, M.Y.; Hillengass, J.; Martin, T.; Castillo, J.J.; Streiff, M.B.; Kumar, S.K.; Callander, N.S. Optimizing Thromboembolism Prophylaxis for the Contemporary Age of Multiple Myeloma. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 91–95. [Google Scholar] [CrossRef]
- Dima, D.; Li, A.; Granat, L.M.; Dhillon, P.; Chamseddine, F.; Yalamanchali, A.; Mirzai, S.; Wei, W.; Samaras, C.J.; Valent, J.; et al. External validation of the SAVED score for venous thromboembolism risk stratification in patients with multiple myeloma receiving immunomodulatory drugs. Br. J. Haematol. 2023, 201, 280–284. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Luo, S.; Wang, T.F.; Fiala, M.; Schoen, M.; Wildes, T.M.; Mikhael, J.; Kuderer, N.M.; Calverley, D.C.; Keller, J.; et al. Predicting venous thromboembolism in multiple myeloma: Development and validation of the IMPEDE VTE score. Am. J. Hematol. 2019, 94, 1176–1184. [Google Scholar] [CrossRef]
- Chakraborty, R.; Rybicki, L.; Wei, W.; Valent, J.; Faiman, B.M.; Samaras, C.J.; Anwer, F.; Khorana, A.A. Abnormal metaphase cytogenetics predicts venous thromboembolism in myeloma: Derivation and validation of the PRISM score. Blood 2022, 140, 2443–2450. [Google Scholar] [CrossRef]
- Han, H.J.; Kim, M.; Lee, J.; Suh, H.S. The Risk of Venous Thromboembolism and Ischemic Stroke Stratified by VTE Risk Following Multiple Myeloma: A Korean Population-Based Cohort Study. J. Clin. Med. 2024, 13, 2829. [Google Scholar] [CrossRef] [PubMed]
- da Costa, I.H.F.; de Pádua, C.A.M.; de Miranda Drummond, P.L.; Silveira, L.P.; Malta, J.S.; Dos Santos, R.M.M.; Reis, A.M.M. Comparison of three risk assessment models for thromboembolism in multiple myeloma patients receiving immunomodulators: A Brazilian historical cohort. J. Thromb. Thrombolysis 2023, 56, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Covut, F.; Sanfilippo, K.M. Mitigating the risk of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory-based therapy. Hematology. Am. Soc. Hematology. Educ. Program 2022, 2022, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Fang, L.J.; Xiao, M.Y.; Lu, M.Q.; Chu, B.; Shi, L.; Gao, S.; Xiang, Q.Q.; Wang, Y.T.; Liu, X.; et al. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in Chinese patients with multiple myeloma: A single-center retrospective cohort study. Thromb. Res. 2024, 236, 130–135. [Google Scholar] [CrossRef]
- Falanga, A.; Giaccherini, C. VTE prophylaxis in multiple myeloma. Blood 2022, 140, 2413–2414. [Google Scholar] [CrossRef]
- Jarchowsky, O.; Avnery, O.; Ellis, M.H. Thrombosis in multiple myeloma: Mechanisms, risk assessment and management. Leuk. Lymphoma 2023, 64, 1905–1913. [Google Scholar] [CrossRef]
- Li, H.; Tian, Y.; Niu, H.; He, L.; Cao, G.; Zhang, C.; Kaiweisierkezi, K.; Luo, Q. Derivation, validation and assessment of a novel nomogram-based risk assessment model for venous thromboembolism in hospitalized patients with lung cancer: A retrospective case control study. Front. Oncol. 2022, 12, 988287. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mackman, N.; Falanga, A.; Pabinger, I.; Noble, S.; Ageno, W.; Moik, F.; Lee, A.Y.Y. Cancer-associated venous thromboembolism. Nat. Reviews. Dis. Primers 2022, 8, 11. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Fang, B.; Leng, Y.; Sun, F.; Wang, Y.; Wang, Q.; Jin, J.; Yang, M.; Xu, B.; et al. Development and validation of a new risk assessment model for immunomodulatory drug-associated venous thrombosis among Chinese patients with multiple myeloma. Thromb. J. 2023, 21, 105. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Tan, W.J.; Chen, L.; Yang, S.J.; Zhang, B.Y.; Sun, M.L.; Lin, Y.B.; Wang, X.H. Development and Validation of a Prediction Model for Venous Thrombus Embolism (VTE) in Patients With Colorectal Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231186790. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiang, W.M.; Wang, Y.; Wang, X.Y. Development and validation of a risk assessment nomogram for venous thromboembolism associated with hospitalized postoperative Chinese breast cancer patients. J. Adv. Nurs. 2021, 77, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Dong, H.; Li, X.; Liu, Y.; Hu, B.; Li, H.; Miao, J.; Chen, Q. Development and validation of a nomogram to assess postoperative venous thromboembolism risk in patients with stage IA non-small cell lung cancer. Cancer Med. 2023, 12, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, X.; Xu, Q.; Yang, Z.; Li, J.; Yang, T.; Wang, G.; Lei, H. Development and validation of a nomogram model for predicting the risk of venous thromboembolism in lymphoma patients undergoing chemotherapy: A prospective cohort study conducted in China. Ann. Med. 2023, 55, 2275665. [Google Scholar] [CrossRef]
- Massironi, S.; Gervaso, L.; Fanizzi, F.; Preatoni, P.; Dell’Anna, G.; Fazio, N.; Danese, S. Venous Thromboembolism in Patients with Neuroendocrine Neoplasms: A Systematic Review of Incidence, Types, and Clinical Outcomes. Cancers 2025, 17, 212. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamashita, Y.; Morimoto, T.; Tada, T.; Sakamoto, H.; Takase, T.; Hiramori, S.; Kim, K.; Oi, M.; Akao, M.; et al. Age and long-term outcomes of patients with venous thromboembolism: From the COMMAND VTE Registry. Int. J. Cardiol. 2023, 383, 89–95. [Google Scholar] [CrossRef]
- Schoen, M.W.; Carson, K.R.; Luo, S.; Gage, B.F.; Li, A.; Afzal, A.; Sanfilippo, K.M. Venous thromboembolism in multiple myeloma is associated with increased mortality. Res. Pract. Thromb. Haemost. 2020, 4, 1203–1210. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug-Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 840–847. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Fiala, M.A.; Feinberg, D.; Tathireddy, H.; Girard, T.; Vij, R.; Di Paola, J.; Gage, B.F. D-dimer predicts venous thromboembolism in multiple myeloma: A nested case-control study. Res. Pract. Thromb. Haemost. 2023, 7, 102235. [Google Scholar] [CrossRef]
- Costa, B.A.; Costa, T.A.; Saravia, S.D.; Felix, N.; Tan, C.R.; Korde, N.; Richter, J. Thromboembolic risk of carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for newly diagnosed multiple myeloma: A comparative systematic review and meta-analysis. Am. J. Hematol. 2024, 99, 1056–1065. [Google Scholar] [CrossRef]
- Sanfilippo, K.M. Assessing the risk of venous thromboembolism in multiple myeloma. Thromb. Res. 2020, 191 (Suppl. S1), S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhan, J.; Li, X.; Hua, J.; Lei, H.; Chen, X. A nomogram to predict the risk of venous thromboembolism in patients with colon cancer in China. Cancer Med. 2024, 13, e7231. [Google Scholar] [CrossRef]



| Variables | Overall (n = 845) | Training Cohort (n = 592) | Validation Cohort (n = 253) | p-Value |
|---|---|---|---|---|
| Age (year) | 63.53 ± 11.01 | 63.50 ± 11.02 | 63.61 ± 11.01 | 0.890 |
| KPS (points) | 75.74 ± 11.58 | 75.60 ± 11.78 | 76.06 ± 11.10 | 0.597 |
| BMI | ||||
| ≤28 | 791 (93.61) | 555 (93.75) | 236 (93.28) | 0.919 |
| >28 | 54 (6.39) | 37 (6.25) | 17 (6.72) | |
| Sex | ||||
| Male | 492 (58.22) | 343 (57.94) | 149 (58.89) | 0.856 |
| Female | 353 (41.78) | 249 (42.06) | 104 (41.11) | |
| Hypertension | ||||
| NO | 647 (76.57) | 458 (77.36) | 189 (74.70) | 0.455 |
| YES | 198 (23.43) | 134 (22.64) | 64 (25.30) | |
| Diabetes | ||||
| NO | 739 (87.46) | 523 (88.34) | 216 (85.38) | 0.280 |
| YES | 106 (12.54) | 69 (11.66) | 37 (14.62) | |
| CVP | ||||
| NO | 541 (64.02) | 383 (64.70) | 158 (62.45) | 0.586 |
| YES | 304 (35.98) | 209 (35.30) | 95 (37.55) | |
| Fracture | ||||
| NO | 678 (80.24) | 483 (81.59) | 195 (77.08) | 0.157 |
| YES | 167 (19.76) | 109 (18.41) | 58 (22.92) | |
| Paralysis | ||||
| NO | 736 (87.10) | 514 (86.82) | 222 (87.75) | 0.799 |
| YES | 109 (12.90) | 78 (13.18) | 31 (12.25) | |
| ISS | ||||
| I–II | 264 (31.24) | 179 (30.24) | 85 (33.60) | 0.377 |
| III | 581 (68.76) | 413 (69.76) | 168 (66.40) | |
| Surgery | ||||
| NO | 625 (73.96) | 434 (73.31) | 191 (75.49) | 0.564 |
| YES | 220 (26.04) | 158 (26.69) | 62 (24.51) | |
| Chemotherapy | ||||
| NO | 289 (34.20) | 196 (33.11) | 93 (36.76) | 0.344 |
| YES | 556 (65.80) | 396 (66.89) | 160 (63.24) | |
| Targeted | ||||
| NO | 386 (45.68) | 274 (46.28) | 112 (44.27) | 0.643 |
| YES | 459 (54.32) | 318 (53.72) | 141 (55.73) | |
| Anticoagulation | ||||
| NO | 523 (61.89) | 370 (62.50) | 153 (60.47) | 0.633 |
| YES | 322 (38.11) | 222 (37.50) | 100 (39.53) | |
| Erythropoietin | ||||
| NO | 734 (86.86) | 517 (87.33) | 217 (85.77) | 0.614 |
| YES | 111 (13.14) | 75 (12.67) | 36 (14.23) | |
| Dexamethasone | ||||
| NO | 214 (25.33) | 150 (25.34) | 64 (25.30) | 1.000 |
| YES | 631 (74.67) | 442 (74.66) | 189 (74.70) | |
| VTE | ||||
| NO | 697 (82.49) | 485 (81.93) | 212 (83.79) | 0.578 |
| YES | 148 (17.51) | 107 (18.07) | 41 (16.21) | |
| WBC (109/L) * | 5.43 [4.16, 6.98] | 5.46 [4.16, 6.99] | 5.24 [4.16, 6.78] | 0.621 |
| Hb (g/L) | 100.12 ± 27.91 | 99.61 ± 27.29 | 101.30 ± 29.34 | 0.419 |
| PLT (109/L) * | 176.00 [131.00, 239.00] | 176.00 [128.00, 240.00] | 176.00 [136.00, 233.00] | 0.845 |
| LYM (109/L) * | 1.32 [1.02, 1.74] | 1.33 [1.01, 1.73] | 1.31 [1.03, 1.76] | 0.940 |
| β2.Microglobulin (mg/L)* | 4.50 [3.00, 7.60] | 4.48 [3.00, 7.53] | 4.70 [3.00, 7.60] | 0.637 |
| Ca (mmol/L) | 2.34 ± 0.38 | 2.32 ± 0.38 | 2.36 ± 0.40 | 0.231 |
| APTT (s) | 28.20 ± 5.71 | 28.31 ± 5.88 | 27.94 ± 5.31 | 0.390 |
| PT (s) | 12.07 ± 1.89 | 12.10 ± 1.84 | 12.00 ± 2.03 | 0.484 |
| D.dimer (mg/L) * | 0.85 [0.37, 1.92] | 0.84 [0.38, 1.91] | 0.91 [0.33, 1.99] | 0.967 |
| Characteristics | No VTE (n = 485) | VTE (n = 107) | OR (Univariable) | OR (Multivariable) |
|---|---|---|---|---|
| Age (year) | 62.65 ± 10.83 | 67.36 ± 11.09 | 1.04 (1.02–1.07, p < 0.001) | 1.03 (1.01–1.06, p = 0.015) |
| KPS (points) | 76.42 ± 10.89 | 71.87 ± 14.67 | 0.97 (0.96–0.99, p < 0.001) | 0.97 (0.95–0.99, p = 0.002) |
| BMI | ||||
| ≤28 | 452 (93.20) | 103 (96.26) | ||
| >28 | 33 (6.80) | 4 (3.74) | 0.53 (0.18–1.53, p = 0.243) | |
| Sex | ||||
| Male | 275 (56.70) | 68 (63.55) | ||
| Female | 210 (43.30) | 39 (36.45) | 0.75 (0.49–1.16, p = 0.195) | |
| Hypertension | ||||
| NO | 376 (77.53) | 82 (76.64) | ||
| YES | 109 (22.47) | 25 (23.36) | 1.05 (0.64–1.73, p = 0.842) | |
| Diabetes | ||||
| NO | 429 (88.45) | 94 (87.85) | ||
| YES | 56 (11.55) | 13 (12.15) | 1.06 (0.56–2.02, p = 0.860) | |
| CVP | ||||
| NO | 330 (68.04) | 53 (49.53) | ||
| YES | 155 (31.96) | 54 (50.47) | 2.17 (1.42–3.32, p < 0.001) | |
| Fracture | ||||
| NO | 398 (82.06) | 85 (79.44) | ||
| YES | 87 (17.94) | 22 (20.56) | 1.18 (0.70–2.00, p = 0.527) | |
| Paralysis | ||||
| NO | 424 (87.42) | 90 (84.11) | ||
| YES | 61 (12.58) | 17 (15.89) | 1.31 (0.73–2.35, p = 0.361) | |
| ISS | ||||
| I–II | 150 (30.93) | 29 (27.10) | ||
| III | 335 (69.07) | 78 (72.90) | 1.20 (0.75–1.92, p = 0.436) | |
| Surgery | ||||
| NO | 366 (75.46) | 68 (63.55) | ||
| YES | 119 (24.54) | 39 (36.45) | 1.76 (1.13–2.75, p = 0.012) | 1.75 (1.04–2.94, p = 0.035) |
| Chemotherapy | ||||
| NO | 182 (37.53) | 14 (13.08) | ||
| YES | 303 (62.47) | 93 (86.92) | 3.99 (2.21–7.21, p < 0.001) | 2.16 (1.04–4.50, p = 0.040) |
| Targeted | ||||
| NO | 244 (50.31) | 30 (28.04) | ||
| YES | 241 (49.69) | 77 (71.96) | 2.60 (1.64–4.11, p < 0.001) | |
| Anticoagulation | ||||
| NO | 336 (69.28) | 34 (31.78) | ||
| YES | 149 (30.72) | 73 (68.22) | 4.84 (3.09–7.60, p < 0.001) | 4.14 (2.39–7.17, p < 0.001) |
| Erythropoietin | ||||
| NO | 435 (89.69) | 82 (76.64) | ||
| YES | 50 (10.31) | 25 (23.36) | 2.65 (1.55–4.53, p < 0.001) | 2.56 (1.36–4.83, p = 0.004) |
| Dexamethasone | ||||
| NO | 140 (28.87) | 10 (9.35) | ||
| YES | 345 (71.13) | 97 (90.65) | 3.94 (1.99–7.77, p < 0.001) | |
| WBC (109/L) * | 5.55 [4.20, 6.99] | 5.15 [3.96, 6.99] | 0.99 (0.93–1.05, p = 0.782) | |
| Hb (g/L) | 99.32 ± 27.83 | 100.91 ± 24.78 | 1.00 (0.99–1.01, p = 0.586) | 1.01 (1.01–1.02, p = 0.028) |
| PLT (109/L) * | 180.00 [127.00, 240.00] | 160.00 [131.50, 222.00] | 0.99 (0.99–1.00, p = 0.173) | 0.99 (0.99–0.99, p = 0.019) |
| LYM (109/L) * | 1.36 [1.06, 1.75] | 1.09 [0.88, 1.56] | 0.69 (0.49–0.98, p = 0.039) | |
| β2.Microglobulin (mg/L) * | 4.50 [3.03, 7.80] | 4.00 [2.80, 6.60] | 1.01 (0.98–1.03, p = 0.619) | |
| Ca (mmol/L) | 2.35 ± 0.39 | 2.23 ± 0.33 | 0.35 (0.18–0.71, p = 0.003) | 0.31 (0.14–0.68, p = 0.004) |
| APTT (s) | 28.53 ± 6.18 | 27.30 ± 4.10 | 0.96 (0.91–1.00, p = 0.049) | 0.94 (0.89–0.99, p = 0.031) |
| PT (s) | 12.11 ± 1.90 | 12.02 ± 1.55 | 0.97 (0.86–1.10, p = 0.638) | |
| D.dimer (mg/L) * | 0.78 [0.35, 1.78] | 1.25 [0.63, 3.12] | 1.08 (1.02–1.14, p = 0.013) | 1.11 (1.03–1.20, p = 0.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, X.; Li, X.; Xu, Q.; Yuan, Y.; Hu, Z.; Zhao, Y.; Liu, Y.; Zhang, Y.; Lei, H. Development and Validation of a Predictive Nomogram for Venous Thromboembolism Risk in Multiple Myeloma Patients: A Single-Center Cohort Study in China. Biomedicines 2025, 13, 770. https://doi.org/10.3390/biomedicines13040770
Zhang H, Zhang X, Li X, Xu Q, Yuan Y, Hu Z, Zhao Y, Liu Y, Zhang Y, Lei H. Development and Validation of a Predictive Nomogram for Venous Thromboembolism Risk in Multiple Myeloma Patients: A Single-Center Cohort Study in China. Biomedicines. 2025; 13(4):770. https://doi.org/10.3390/biomedicines13040770
Chicago/Turabian StyleZhang, Haolin, Xi Zhang, Xiaosheng Li, Qianjie Xu, Yuliang Yuan, Zuhai Hu, Yulan Zhao, Yao Liu, Yunyun Zhang, and Haike Lei. 2025. "Development and Validation of a Predictive Nomogram for Venous Thromboembolism Risk in Multiple Myeloma Patients: A Single-Center Cohort Study in China" Biomedicines 13, no. 4: 770. https://doi.org/10.3390/biomedicines13040770
APA StyleZhang, H., Zhang, X., Li, X., Xu, Q., Yuan, Y., Hu, Z., Zhao, Y., Liu, Y., Zhang, Y., & Lei, H. (2025). Development and Validation of a Predictive Nomogram for Venous Thromboembolism Risk in Multiple Myeloma Patients: A Single-Center Cohort Study in China. Biomedicines, 13(4), 770. https://doi.org/10.3390/biomedicines13040770






