Nucleus Accumbens Proteome Disbalance in an Adolescent Mouse Model of Schizophrenia and Nicotine Misuse Comorbidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Protein Extraction
2.4. Protein Digestion and nanoULPC-MSE Analysis
2.5. Data Processing
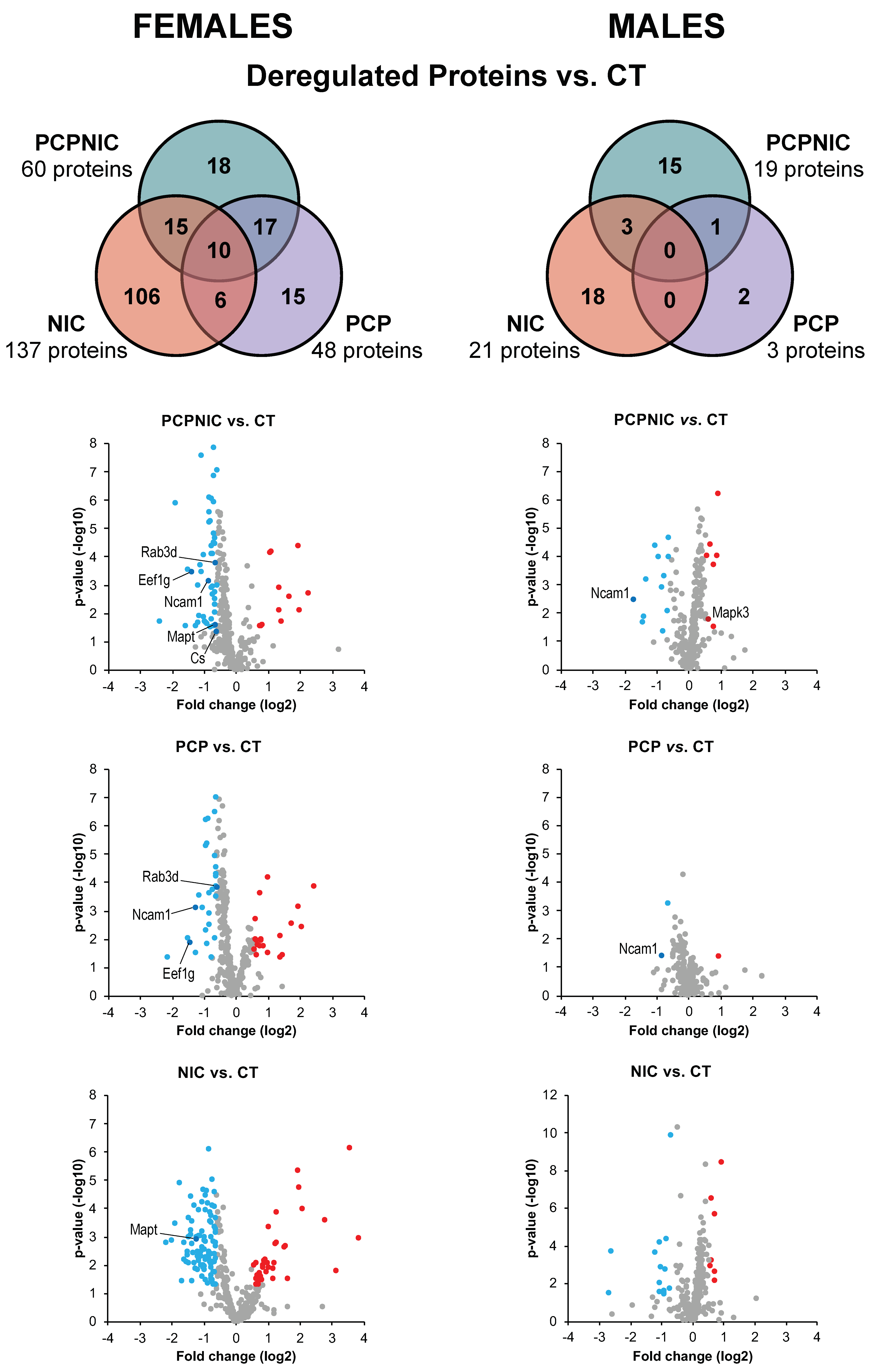
3. Results
3.1. Proteomic Profile of the NAcc of Mouse Models of SCHZ, Nicotine Misuse, and Their Co-Morbidity When Compared to Controls
3.2. Exclusively Deregulated Proteins
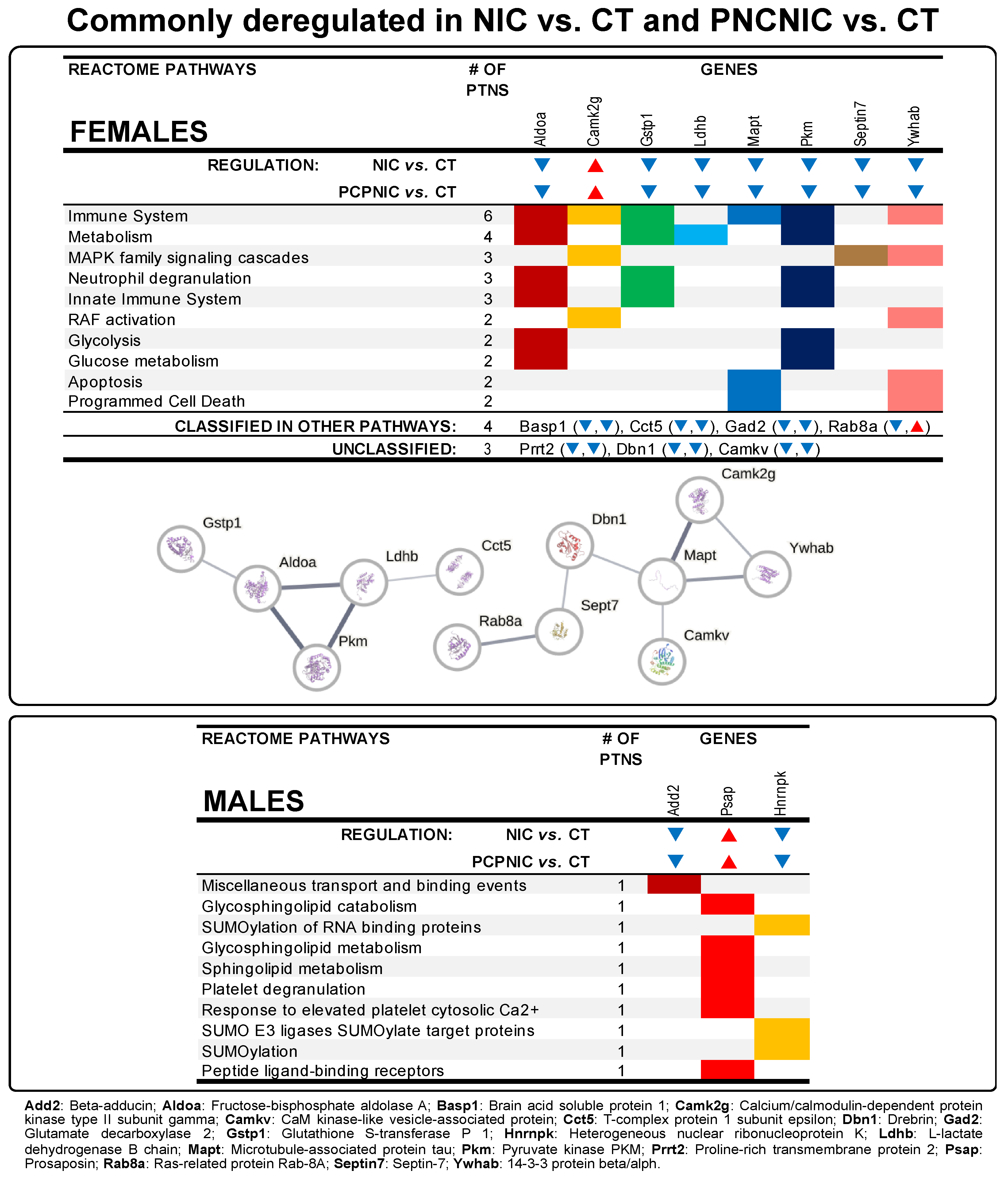
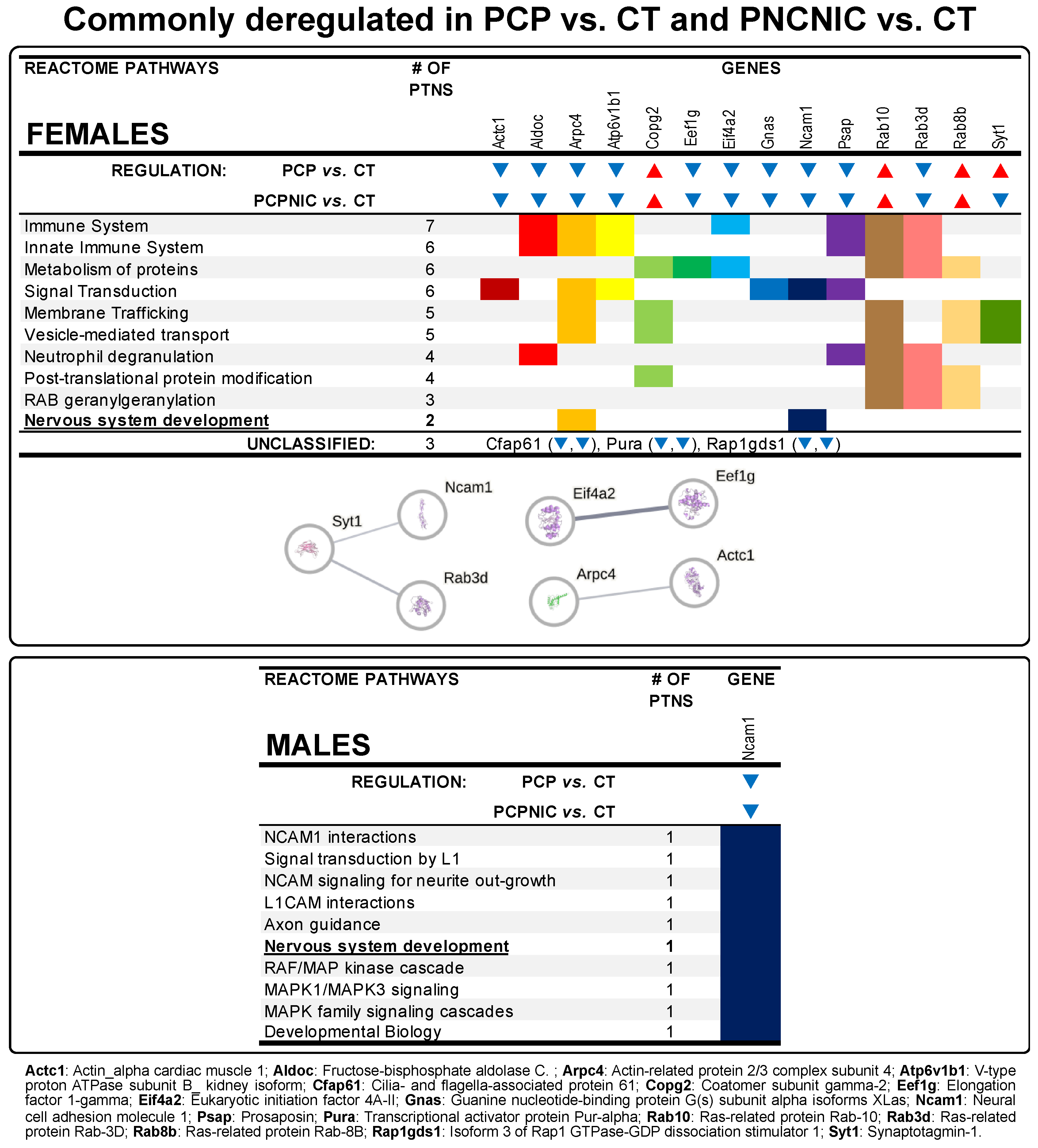
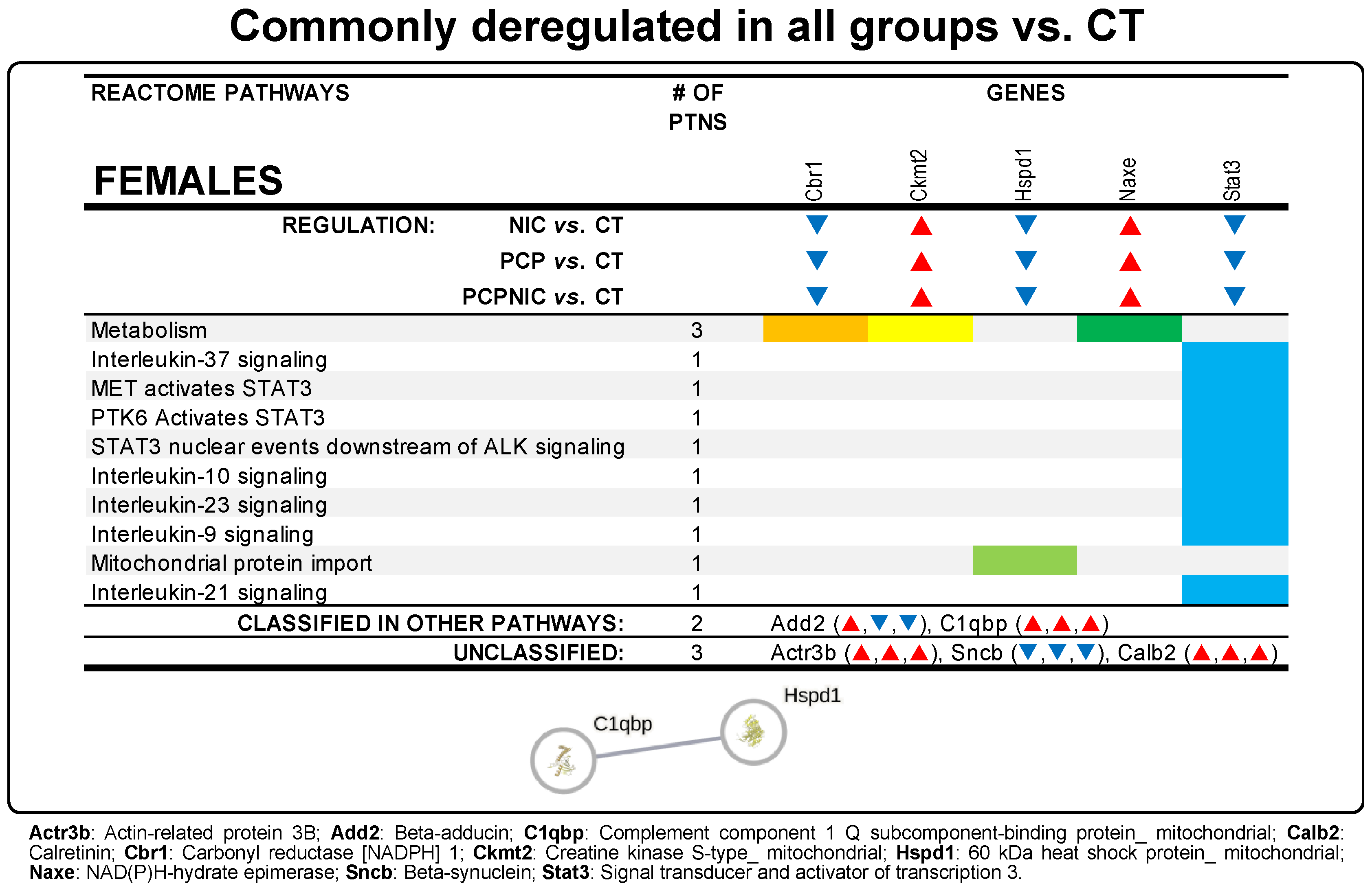
3.3. Commonly Deregulated Proteins
4. Discussion
4.1. Exclusively Deregulated Proteins in PCPNIC Mice
4.2. Commonly Deregulated Proteins in NIC and PCPNIC Mice
4.3. Commonly Deregulated Proteins in PCP and PCPNIC Mice
4.4. Commonly Deregulated Proteins in PCP, NIC, and PCPNIC Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACO2 | Aconitate hydratase_ mitochondrial |
| Acta1 | Actin alpha 1 |
| Actc1 | Actin_ alpha cardiac muscle |
| Add2 | Beta-adducin |
| Aldoa | Fructose-bisphosphate aldolase A |
| Arf | ADP Ribosylation Factor 4 |
| Arpc4 | Actin-related protein 2/3 complex subunit 4 |
| Atp1a2 | Sodium/potassium-transporting ATPase subunit alpha-2 |
| C1qbp | Complement C1q Binding Protein |
| Camk2a | Calcium/calmodulin-dependent protein kinase type II subunit alpha |
| Camk2g | Calcium/calmodulin-dependent protein kinase type II subunit gamma |
| Camkv | CaM kinase-like vesicle-associated protein |
| Cct5 | T-complex protein 1 subunit epsilon |
| CS | Citrate synthase |
| CT | Control group |
| Ctbp1 | C-terminal-binding protein 1 |
| DIA | Data-independent acquisition |
| Dbn1 | Drebrin |
| DTT | Dithiothreitol |
| Eef1g | Eukaryotic translation elongation factor 1 gamma |
| Eif4a2 | Eukaryotic initiation factor 4A-II |
| ENDS | Electronic nicotine delivery system |
| FDR | False discovery rate |
| GFP | [Glu1]-Fibrinopeptide B human |
| Gnb4 | Guanine nucleotide-binding protein subunit beta-4 |
| Gstp1 | Glutathione S-transferase P 1 |
| GWAS | Genome-wide association study |
| Hnrnpk | Heterogeneous nuclear ribonucleoprotein K |
| Hspd1 | 60 kDa heat shock protein_mitochondrial |
| IAA | Iodoacetamide |
| Idh3a | Isocitrate dehydrogenase [NAD] subunit alpha_ mitochondrial |
| Ldhb | L-lactate dehydrogenase B chain |
| Mapk1 | Mitogen-activated protein kinase 1 |
| Mapk3 | Mitogen-activated protein kinase 3 |
| Mapt | Microtubule-associated protein tau |
| MSE | Mass spectrometry Experimentation |
| NAcc | Nucleus Accumbens |
| Ncam1 | Neural cell adhesion molecule 1 |
| NIC | Nicotine-exposed group |
| PANTHER | Protein Analysis Through Evolutionary Relationships |
| PCP | Phencyclidine-exposed group |
| PCPNIC | Phencyclidine- and nicotine-exposed group |
| PN | Postnatal day |
| Prkaca | cAMP-dependent protein kinase catalytic subunit alpha |
| Psap | Prosaposin |
| Rab3d | Ras-related protein Rab-3D |
| Rab8a | Ras-related protein Rab-8A |
| SCHZ | Schizophrenia |
| Sept7 | Septin-7 |
| Syt1 | Synaptotagmin-1 |
| TCA | Tricarboxylic acid cycle, also known as the citric acid cycle and Krebs cycle |
| TEAB | Triethylammonium bicarbonate buffer |
| TWAS | transcriptome-wide association study |
| VTA | Ventral tegmental area |
| Ywhab | 14-3-3 protein beta/alpha |
| Ywhae | 14-3-3 protein epsilon (Ywhae) |
| Ywhag | 14-3-3 protein gamma |
References
- Matamales, M.; Bertran-Gonzalez, J.; Salomon, L.; Degos, B.; Deniau, J.-M.; Valjent, E.; Hervé, D.; Girault, J.-A. Striatal Medium-Sized Spiny Neurons: Identification by Nuclear Staining and Study of Neuronal Subpopulations in BAC Transgenic Mice. PLoS ONE 2009, 4, e4770. [Google Scholar]
- Groenewegen, H.J.; Wright, C.I.; Beijer, A.V.J.; Voorn, P. Convergence and Segregation of Ventral Striatal Inputs and Outputs. Ann. N. Y. Acad. Sci. 1999, 877, 49–63. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Totterdell, S. The Anatomy of Co-Morbid Neuropsychiatric Disorders Based on Cortico-Limbic Synaptic Interactions. Neurotox. Res. 2006, 10, 65–85. [Google Scholar] [CrossRef]
- Floresco, S.B. The Nucleus Accumbens: An Interface between Cognition, Emotion, and Action. Annu. Rev. Psychol. 2015, 66, 25–32. [Google Scholar] [CrossRef]
- Ikemoto, S.; Bonci, A. Neurocircuitry of Drug Reward. Neuropharmacology 2014, 76, 329–341. [Google Scholar] [CrossRef]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-Hypothalamic Circuitry and Food Motivation: Integration of Energy, Action and Reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef]
- Levita, L.; Hare, T.A.; Voss, H.U.; Glover, G.; Ballon, D.J.; Casey, B.J. The Bivalent Side of the Nucleus Accumbens. Neuroimage 2009, 44, 1178–1187. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The Brain Reward Circuitry in Mood Disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef]
- Andersen, S.L.; Thompson, A.T.; Rutstein, M.; Hostetter, J.C.; Teicher, M.H. Dopamine Receptor Pruning in Prefrontal Cortex during the Periadolescent Period in Rats. Synapse 2000, 37, 167–169. [Google Scholar] [CrossRef]
- Wahlstrom, D.; Collins, P.; White, T.; Luciana, M. Developmental Changes in Dopamine Neurotransmission in Adolescence: Behavioral Implications and Issues in Assessment. Brain Cogn. 2010, 72, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Luciana, M.; Collins, P.F. Incentive Motivation, Cognitive Control, and the Adolescent Brain: Is It Time for a Paradigm Shift? Child Dev. Perspect. 2012, 6, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, H.; Ahmadi-Soleimani, S.M.; Pachenari, N.; Azadi, M.; Halliwell, R.F.; Rubino, T.; Azizi, H. Adolescent Drug Exposure: A Review of Evidence for the Development of Persistent Changes in Brain Function. Brain Res. Bull. 2020, 156, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Jones, R.M. Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef]
- Weinberger, D.R. Implications of Normal Brain Development for the Pathogenesis of Schizophrenia. Arch. Gen. Psychiatry 1987, 44, 660–669. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Flores, C. Mesocorticolimbic Dopamine Pathways Across Adolescence: Diversity in Development. Front. Neural Circuits 2021, 15, 735625. [Google Scholar] [CrossRef]
- Barendse, M.E.A.; Lara, G.A.; Guyer, A.E.; Swartz, J.R.; Taylor, S.L.; Shirtcliff, E.A.; Lamb, S.T.; Miller, C.; Ng, J.; Yu, G.; et al. Sex and Pubertal Influences on the Neurodevelopmental Underpinnings of Schizophrenia: A Case for Longitudinal Research on Adolescents. Schizophr. Res. 2023, 252, 231–241. [Google Scholar] [CrossRef]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Peritogiannis, V.; Gogou, A.; Samakouri, M. Very Long-Term Outcome of Psychotic Disorders. Int. J. Soc. Psychiatry 2020, 66, 633–641. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III—The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Robison, A.J.; Thakkar, K.N.; Diwadkar, V.A. Cognition and Reward Circuits in Schizophrenia: Synergistic, Not Separate. Biol. Psychiatry 2020, 87, 204–214. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, Y.; Hanxiaoran, L.; Greenshaw, A.J.; Li, T. Anhedonia in Depression and Schizophrenia: Brain Reward and Aversion Circuits. Neuropsychiatr. Dis. Treat. 2022, 18, 1385–1396. [Google Scholar] [CrossRef]
- WHO Tobacco. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 24 September 2024).
- Abreu-Villaça, Y.; Manhães, A.C.; Krahe, T.E.; Filgueiras, C.C.; Ribeiro-Carvalho, A. Tobacco and Alcohol Use during Adolescence: Interactive Mechanisms in Animal Models. Biochem. Pharmacol. 2017, 144, 1–17. [Google Scholar] [CrossRef]
- Colyer-Patel, K.; Kuhns, L.; Weidema, A.; Lesscher, H.; Cousijn, J. Age-Dependent Effects of Tobacco Smoke and Nicotine on Cognition and the Brain: A Systematic Review of the Human and Animal Literature Comparing Adolescents and Adults. Neurosci. Biobehav. Rev. 2023, 146, 105038. [Google Scholar] [CrossRef]
- Kumari, V.; Postma, P. Nicotine Use in Schizophrenia: The Self Medication Hypotheses. Neurosci. Biobehav. Rev. 2005, 29, 1021–1034. [Google Scholar] [CrossRef]
- Ohi, K.; Shimada, T.; Kuwata, A.; Kataoka, Y.; Okubo, H.; Kimura, K.; Yasuyama, T.; Uehara, T.; Kawasaki, Y. Smoking Rates and Number of Cigarettes Smoked per Day in Schizophrenia: A Large Cohort Meta-Analysis in a Japanese Population. Int. J. Neuropsychopharmacol. 2019, 22, 19–27. [Google Scholar] [CrossRef]
- Häfner, H. From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness. Psychiatry J. 2019, 2019, 9804836. [Google Scholar] [CrossRef]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “Just the Facts” 4. Clinical Features and Conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef]
- Miller, T.J.; McGlashan, T.H.; Rosen, J.L.; Cadenhead, K.; Ventura, J.; McFarlane, W.; Perkins, D.O.; Pearlson, G.D.; Woods, S.W. Prodromal Assessment With the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive Validity, Interrater Reliability, and Training to Reliability. Schizophr. Bull. 2003, 29, 703–715. [Google Scholar] [CrossRef]
- Lanza, S.T.; Vasilenko, S.A. New Methods Shed Light on Age of Onset as a Risk Factor for Nicotine Dependence. Addict. Behav. 2015, 50, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.A.; Ambrose, B.K.; Gentzke, A.S.; Apelberg, B.J.; Jamal, A.; King, B.A. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students—United States, 2011–2018. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Miech, R.; Johnston, L.; O’Malley, P.M.; Bachman, J.G.; Patrick, M.E. Adolescent Vaping and Nicotine Use in 2017–2018—U.S. National Estimates. N. Engl. J. Med. 2019, 380, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Skokou, M.; Ferentinou, E.; Gourzis, P. Nicotine Consumption during the Prodromal Phase of Schizophrenia—A Review of the Literature. Neuropsychiatr. Dis. Treat. 2019, 15, 2943–2958. [Google Scholar] [CrossRef]
- Lally, J.; Spaducci, G.; Gardner-Sood, P.; Atakan, Z.; Greenwood, K.; Di Forti, M.; Ismail, K.; Murphy, K.C.; Smith, S.; McNeill, A.; et al. Tobacco Smoking and Nicotine Dependence in First Episode and Established Psychosis. Asian J. Psychiatr. 2019, 43, 125–131. [Google Scholar] [CrossRef]
- Myles, N.; Newall, H.D.; Curtis, J.; Nielssen, O.; Shiers, D.; Large, M. Tobacco Use before, at, and after First-Episode Psychosis: A Systematic Meta-Analysis. J. Clin. Psychiatry 2012, 73, 468–475. [Google Scholar] [CrossRef]
- Parikh, V.; Kutlu, M.G.; Gould, T.J. NAChR Dysfunction as a Common Substrate for Schizophrenia and Comorbid Nicotine Addiction: Current Trends and Perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef]
- Featherstone, R.E.; Siegel, S.J. The Role of Nicotine in Schizophrenia. Int. Rev. Neurobiol. 2015, 124, 23–78. [Google Scholar]
- Aguilar, M.C.; Gurpegui, M.; Diaz, F.J.; De Leon, J. Nicotine Dependence and Symptoms in Schizophrenia. Br. J. Psychiatry 2005, 186, 215–221. [Google Scholar] [CrossRef]
- de Leon, J.; Diaz, F.J. A Meta-Analysis of Worldwide Studies Demonstrates an Association between Schizophrenia and Tobacco Smoking Behaviors. Schizophr. Res. 2005, 76, 135–157. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Manhães, A.C.; Semeão, K.A.; Maia, J.G.; Couto, L.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Abreu-Villaça, Y. Does Nicotine Exposure during Adolescence Modify the Course of Schizophrenia-like Symptoms? Behavioral Analysis in a Phencyclidine-Induced Mice Model. PLoS ONE 2021, 16, e0257986. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Bandeira-Martins, A.; Silva, J.O.; Couto, L.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Adolescent Nicotine Potentiates the Inhibitory Effect of Raclopride, a D2R Antagonist, on Phencyclidine-Sensitized Psychotic-like Behavior in Mice. Toxicol. Appl. Pharmacol. 2022, 456, 116282. [Google Scholar] [CrossRef] [PubMed]
- Dutra-Tavares, A.C.; Couto, L.A.; Souza, T.P.; Bandeira-martins, A.; Silva, J.O.; Filgueiras, C.C.; Ribeiro-carvalho, A.; Manhães, A.C.; Abreu-villaça, Y. Nicotine’s Effects on Schizophrenia-like Symptoms in a Mice Model: Time Matters. Brain Sci. 2024, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vega, A.; Dutra-Tavares, A.C.; Souza, T.P.; Semeão, K.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Nicotine Exposure in a Phencyclidine-Induced Mice Model of Schizophrenia: Sex-Selective Medial Prefrontal Cortex Protein Markers of the Combined Insults in Adolescent Mice. Int. J. Mol. Sci. 2023, 24, 14634. [Google Scholar] [CrossRef]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2018, 64, 5–17. [Google Scholar] [CrossRef]
- Cadinu, D.; Grayson, B.; Podda, G.; Harte, M.K.; Doostdar, N.; Neill, J.C. NMDA Receptor Antagonist Rodent Models for Cognition in Schizophrenia and Identification of Novel Drug Treatments, an Update. Neuropharmacology 2018, 142, 41–62. [Google Scholar] [CrossRef]
- Bubeníková-Valešová, V.; Horáček, J.; Vrajová, M.; Höschl, C. Models of Schizophrenia in Humans and Animals Based on Inhibition of NMDA Receptors. Neurosci. Biobehav. Rev. 2008, 32, 1014–1023. [Google Scholar] [CrossRef]
- Yee, B.K.; Chang, D.L.T.; Feldon, J. The Effects of Dizocilpine and Phencyclidine on Prepulse Inhibition of the Acoustic Startle Reflex and on Prepulse-Elicited Reactivity in C57BL6 Mice. Neuropsychopharmacology 2004, 29, 1865–1877. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal Models of Schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Hambsch, B.; Keyworth, H.; Lind, J.; Otte, D.M.; Racz, I.; Kitchen, I.; Bailey, A.; Zimmer, A. Chronic Nicotine Improves Short-Term Memory Selectively in a G72 Mouse Model of Schizophrenia. Br. J. Pharmacol. 2014, 171, 1758–1771. [Google Scholar] [CrossRef]
- Nunes-Freitas, A.L.; Ribeiro-Carvalho, A.; Lima, C.S.; Dutra-Tavares, A.C.; Manhães, A.C.; Lisboa, P.C.; Oliveira, E.; Gaspar de Moura, E.; Filgueiras, C.C.; Abreu-Villaça, Y. Nicotine Exposure during the Third Trimester Equivalent of Human Gestation: Time Course of Effects on the Central Cholinergic System of Rats. Toxicol. Sci. 2011, 123, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Davalieva, K.; Kostovska, I.M.; Dwork, A.J. Proteomics Research in Schizophrenia. Front. Cell. Neurosci. 2016, 10, 18. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Garcia, S.; Saia-Cereda, V.M.; Santana, A.G.; Brandao-Teles, C.; Zuccoli, G.S.; Junqueira, D.G.; Reis-de-Oliveira, G.; Baldasso, P.A.; Cassoli, J.S.; et al. Proteomics and Molecular Tools for Unveiling Missing Links in the Biochemical Understanding of Schizophrenia. Proteom.—Clin. Appl. 2016, 10, 1148–1158. [Google Scholar] [CrossRef]
- Matsuura, K.; Otani, M.; Takano, M.; Kadoyama, K.; Matsuyama, S. The Influence of Chronic Nicotine Treatment on Proteins Expressed in the Mouse Hippocampus and Cortex. Eur. J. Pharmacol. 2016, 780, 16–25. [Google Scholar] [CrossRef]
- Cullen, K.A.; Liu, S.T.; Bernat, J.K.; Slavit, W.I.; Tynan, M.A.; King, B.A.; Neff, L.J. Flavored Tobacco Product Use Among Middle and High School Students—United States, 2014–2018. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 839–844. [Google Scholar] [CrossRef]
- Singh, T.; Arrazola, R.A.; Corey, C.G.; Husten, C.G.; Neff, L.J.; Homa, D.M.; King, B.A. Tobacco Use Among Middle and High School Students—United States, 2011–2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 361–367. [Google Scholar] [CrossRef]
- Massadeh, A.M.; Gharaibeh, A.A.; Omari, K.W. A Single-Step Extraction Method for the Determination of Nicotine and Cotinine in Jordanian Smokers’ Blood and Urine Samples by RP-HPLC and GC-MS. J. Chromatogr. Sci. 2009, 47, 170–177. [Google Scholar] [CrossRef]
- Wood, T.; Ellen Wewers, M.; Groner, J.; Ahijevych, K. Smoke Constituent Exposure and Smoking Topography of Adolescent Daily Cigarette Smokers. Nicotine Tob. Res. 2004, 6, 853–862. [Google Scholar] [CrossRef]
- Beraki, S.; Diaz-Heijtz, R.; Tai, F.; Ögren, S.O. Effects of Repeated Treatment of Phencyclidine on Cognition and Gene Expression in C57BL/6 Mice. Int. J. Neuropsychopharmacol. 2009, 12, 243. [Google Scholar] [CrossRef]
- Engel, M.; Snikeris, P.; Jenner, A.; Karl, T.; Huang, X.-F.; Frank, E. Neuregulin 1 Prevents Phencyclidine-Induced Behavioral Impairments and Disruptions to GABAergic Signaling in Mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu114. [Google Scholar] [CrossRef]
- Spielewoy, C.; Markou, A. Strain-Specificity in Nicotine Attenuation of Phencyclidine-Induced Disruption of Prepulse Inhibition in Mice: Relevance to Smoking in Schizophrenia Patients. Behav. Genet. 2004, 34, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Selvendra, A.; Stewart, A.; Castle, D. Risk Factors in Early and Late Onset Schizophrenia. Compr. Psychiatry 2018, 80, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Nunes, F.; do E Queiroz-Gomes, F.; Manhães, A.C.; Filgueiras, C.C. Combined Exposure to Nicotine and Ethanol in Adolescent Mice Differentially Affects Anxiety Levels during Exposure, Short-Term, and Long-Term Withdrawal. Neuropsychopharmacology 2008, 33, 599–610. [Google Scholar] [CrossRef]
- Abreu-Villaça, Y.; Medeiros, A.H.; Lima, C.S.; Faria, F.P.; Filgueiras, C.C.; Manhães, A.C. Combined Exposure to Nicotine and Ethanol in Adolescent Mice Differentially Affects Memory and Learning during Exposure and Withdrawal. Behav. Brain Res. 2007, 181, 136–146. [Google Scholar] [CrossRef]
- Ribeiro-Carvalho, A.; Lima, C.S.; Nunes-Freitas, A.L.; Filgueiras, C.C.; Manhães, A.C.; Abreu-Villaça, Y. Exposure to Nicotine and Ethanol in Adolescent Mice: Effects on Depressive-Like Behavior during Exposure and Withdrawal. Behav. Brain Res. 2011, 221, 282–289. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab Proteins as Membrane Organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Rodríguez-Vega, A.; Losada-Barragán, M.; Berbert, L.R.; Mesquita-Rodrigues, C.; Bombaça, A.C.S.; Menna-Barreto, R.; Aquino, P.; Carvalho, P.C.; Padrón, G.; de Jesus, J.B.; et al. Quantitative Analysis of Proteins Secreted by Leishmania (Viannia) Braziliensis Strains Associated to Distinct Clinical Manifestations of American Tegumentary Leishmaniasis. J. Proteom. 2021, 232, 104077. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.Z.; Vissers, J.P.C.; Geromanos, S.J. Absolute Quantification of Proteins by LCMSE: A Virtue of Parallel MS Acquisition. Mol. Cell. Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER Version 11: Expanded Annotation Data from Gene Ontology and Reactome Pathways, and Data Analysis Tool Enhancements. Nucleic Acids Res. 2017, 45, 183–189. [Google Scholar] [CrossRef]
- Hill, R.A. Sex Differences in Animal Models of Schizophrenia Shed Light on the Underlying Pathophysiology. Neurosci. Biobehav. Rev. 2016, 67, 41–56. [Google Scholar] [CrossRef]
- Hayes, E.; Gavrilidis, E.; Kulkarni, J. The Role of Oestrogen and Other Hormones in the Pathophysiology and Treatment of Schizophrenia. Schizophr. Res. Treat. 2012, 2012, 540273. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, A.; Mancini-Marïe, A. Sex/Gender Differences in the Brain and Cognition in Schizophrenia. Neurosci. Biobehav. Rev. 2016, 67, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Wickens, M.M.; Bangasser, D.A.; Briand, L.A. Sex Differences in Psychiatric Disease: A Focus on the Glutamate System. Front. Mol. Neurosci. 2018, 11, 197. [Google Scholar] [CrossRef]
- McGregor, C.; Riordan, A.; Thornton, J. Estrogens and the Cognitive Symptoms of Schizophrenia: Possible Neuroprotective Mechanisms. Front. Neuroendocrinol. 2017, 47, 19–33. [Google Scholar] [CrossRef]
- Dutra-Tavares, A.C.; Souza, T.P.; Silva, J.O.; Semeão, K.A.; Mello, F.F.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Neonatal Phencyclidine as a Model of Sex-Biased Schizophrenia Symptomatology in Adolescent Mice. Psychopharmacology 2023, 240, 2111–2129. [Google Scholar] [CrossRef]
- Beltz, A.M.; Berenbaum, S.A.; Wilson, S.J. Sex Differences in Resting State Brain Function of Cigarette Smokers and Links to Nicotine Dependence. Exp. Clin. Psychopharmacol. 2015, 23, 247–254. [Google Scholar] [CrossRef]
- Flores, R.J.; Uribe, K.P.; Swalve, N.; O’Dell, L.E. Sex Differences in Nicotine Intravenous Self-Administration: A Meta-Analytic Review. Physiol. Behav. 2019, 203, 42–50. [Google Scholar] [CrossRef]
- Pogun, S.; Yararbas, G.; Nesil, T.; Kanit, L. Sex Differences in Nicotine Preference. J. Neurosci. Res. 2017, 95, 148–162. [Google Scholar] [CrossRef]
- Kanniah, G.; Kumar, R. A Selective Literature Review Exploring the Role of the Nicotinic System in Schizophrenia. Gen. Psychiatry 2023, 36, e100756. [Google Scholar] [CrossRef]
- Henkel, N.D.; Wu, X.; O’Donovan, S.M.; Devine, E.A.; Jiron, J.M.; Rowland, L.M.; Sarnyai, Z.; Ramsey, A.J.; Wen, Z.; Hahn, M.K.; et al. Schizophrenia: A Disorder of Broken Brain Bioenergetics. Mol. Psychiatry 2022, 27, 2393–2404. [Google Scholar] [CrossRef]
- Martins-De-Souza, D.; Harris, L.W.; Guest, P.C.; Bahn, S. The Role of Energy Metabolism Dysfunction and Oxidative Stress in Schizophrenia Revealed by Proteomics. Antioxid. Redox Signal. 2011, 15, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D. Mitochondrial Dysfunction in Schizophrenia: A Possible Linkage to Dopamine. J. Neurochem. 2002, 83, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, F. The Interplay of Dopamine Metabolism Abnormalities and Mitochondrial Defects in the Pathogenesis of Schizophrenia. Transl. Psychiatry 2022, 12, 464. [Google Scholar] [CrossRef]
- Brenner-Lavie, H.; Klein, E.; Zuk, R.; Gazawi, H.; Ljubuncic, P.; Ben-Shachar, D. Dopamine Modulates Mitochondrial Function in Viable SH-SY5Y Cells Possibly via Its Interaction with Complex I: Relevance to Dopamine Pathology in Schizophrenia. Biochim. Biophys. Acta—Bioenerg. 2008, 1777, 173–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, M.; Yu, Y.; Wang, J.; Jiao, Y.; Zheng, M.; Zhang, S. 14–3-3ε: A Protein with Complex Physiology Function but Promising Therapeutic Potential in Cancer. Cell Commun. Signal. 2024, 22, 72. [Google Scholar] [CrossRef]
- Cho, E.; Park, J.-Y. Emerging Roles of 14-3-3γ in the Brain Disorder. BMB Rep. 2020, 53, 500–511. [Google Scholar] [CrossRef]
- Bergman, L.M.; Blaydes, J.P. C-Terminal Binding Proteins: Emerging Roles in Cell Survival and Tumorigenesis. Apoptosis 2006, 11, 879–888. [Google Scholar] [CrossRef]
- Grooteclaes, M.; Deveraux, Q.; Hildebrand, J.; Zhang, Q.; Goodman, R.H.; Frisch, S.M. C-Terminal-Binding Protein Corepresses Epithelial and Proapoptotic Gene Expression Programs. Proc. Natl. Acad. Sci. USA 2003, 100, 4568–4573. [Google Scholar] [CrossRef]
- Fu, H.; Subramanian, R.R.; Masters, S.C. 14-3-3 Proteins: Structure, Function, and Regulation. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 617–647. [Google Scholar] [CrossRef]
- Saia-Cereda, V.M.; Cassoli, J.S.; Martins-de-Souza, D.; Nascimento, J.M. Psychiatric Disorders Biochemical Pathways Unraveled by Human Brain Proteomics. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 3–17. [Google Scholar] [CrossRef]
- Venkataramaiah, C. Modulations in the ATPases during Ketamine-Induced Schizophrenia and Regulatory Effect of “3-(3, 4-Dimethoxy Phenyl)-1-(4-Methoxyphenyl) Prop-2-En-1-One”: An in Vivo and in Silico Studies. J. Recept. Signal Transduct. 2020, 40, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Vawter, M.P.; Ginsberg, S.D. Target Identification for CNS Diseases by Transcriptional Profiling. Neuropsychopharmacology 2009, 34, 18–54. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Marttunen, T.; Blackwell, K.T.; Akkouh, I.; Shadrin, A.; Valstad, M.; Elvsåshagen, T.; Linne, M.-L.; Djurovic, S.; Einevoll, G.T.; Andreassen, O.A. Genetic Mechanisms for Impaired Synaptic Plasticity in Schizophrenia Revealed by Computational Modeling. Proc. Natl. Acad. Sci. USA 2024, 121, e2312511121. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Ericson, M.; Söderpalm, B. Chronic Phencyclidine Increases Synapsin-1 and Synaptic Adaptation Proteins in the Medial Prefrontal Cortex. Int. Sch. Res. Not. 2013, 2013, 620361. [Google Scholar] [CrossRef]
- Reszka, A.A.; Segert, R.; Diltz, C.D.; Krebs, E.G.; Fischer, E.H. Association of Mitogen-Activated Protein Kinase with the Microtubule Cytoskeleton. Proc. Natl. Acad. Sci. USA 1995, 92, 8881–8885. [Google Scholar] [CrossRef]
- Morishima-Kawashima, M.; Kosik, K.S. The Pool of MAP Kinase Associated with Microtubules Is Small but Constitutively Active. Mol. Biol. Cell 1996, 7, 893–905. [Google Scholar] [CrossRef]
- Rodrigues, F.F.; Harris, T.J.C. Key Roles of Arf Small G Proteins and Biosynthetic Trafficking for Animal Development. Small GTPases 2019, 10, 403–410. [Google Scholar] [CrossRef]
- El Rawas, R.; Amaral, I.M.; Hofer, A. The Anti-Social Brain in Schizophrenia: A Role of CaMKII? Front. Psychiatry 2022, 13, 868244. [Google Scholar] [CrossRef]
- Mohanan, A.G.; Gunasekaran, S.; Jacob, R.S.; Omkumar, R. V Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Mediating Function and Dysfunction at Glutamatergic Synapses. Front. Mol. Neurosci. 2022, 15, 855752. [Google Scholar]
- Blizinsky, K.D.; Diaz-Castro, B.; Forrest, M.P.; Schürmann, B.; Bach, A.P.; Martin-de-Saavedra, M.D.; Wang, L.; Csernansky, J.G.; Duan, J.; Penzes, P. Reversal of Dendritic Phenotypes in 16p11.2 Microduplication Mouse Model Neurons by Pharmacological Targeting of a Network Hub. Proc. Natl. Acad. Sci. USA 2016, 113, 8520–8525. [Google Scholar] [CrossRef]
- Olde Loohuis, N.F.M.; Ba, W.; Stoerchel, P.H.; Kos, A.; Jager, A.; Schratt, G.; Martens, G.J.M.; van Bokhoven, H.; Nadif Kasri, N.; Aschrafi, A. MicroRNA-137 Controls AMPA-Receptor-Mediated Transmission and MGluR-Dependent LTD. Cell Rep. 2015, 11, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Mancuso, N.; Won, H.; Kousi, M.; Finucane, H.K.; Reshef, Y.; Song, L.; Safi, A.; McCarroll, S.; Neale, B.M.; et al. Transcriptome-Wide Association Study of Schizophrenia and Chromatin Activity Yields Mechanistic Disease Insights. Nat. Genet. 2018, 50, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.E.; Chen, J.; Jagadapillai, R.; Jang, H.; Abomoelak, B.; Brock, G.; Greene, R.M.; Michele Pisano, M. Developmental Cigarette Smoke Exposure: Hippocampus Proteome and Metabolome Profiles in Low Birth Weight Pups. Toxicology 2014, 317, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tobore, T.O. On the Potential Harmful Effects of E-Cigarettes (EC) on the Developing Brain: The Relationship between Vaping-Induced Oxidative Stress and Adolescent/Young Adults Social Maladjustment. J. Adolesc. 2019, 76, 202–209. [Google Scholar] [CrossRef]
- Ursic, D.; Sedbrook, J.C.; Himmel, K.L.; Culbertson, M.R. The Essential Yeast Tcp1 Protein Affects Actin and Microtubules. Mol. Biol. Cell 1994, 5, 1065–1080. [Google Scholar] [CrossRef]
- Chu, T.T.; Liu, Y. An Integrated Genomic Analysis of Gene-Function Correlation on Schizophrenia Susceptibility Genes. J. Hum. Genet. 2010, 55, 285–292. [Google Scholar]
- Kane, J.K.; Konu, Ö.; Ma, J.Z.; Li, M.D. Nicotine Coregulates Multiple Pathways Involved in Protein Modification/Degradation in Rat Brain. Mol. Brain Res. 2004, 132, 181–191. [Google Scholar] [CrossRef]
- Lasser, M.; Tiber, J.; Lowery, L.A. The Role of the Microtubule Cytoskeleton in Neurodevelopmental Disorders. Front. Cell. Neurosci. 2018, 12, 165. [Google Scholar]
- Herzmann, S.; Krumkamp, R.; Rode, S.; Kintrup, C.; Rumpf, S. PAR-1 Promotes Microtubule Breakdown during Dendrite Pruning in Drosophila. EMBO J. 2017, 36, 1981–1991. [Google Scholar] [CrossRef]
- Brent, B.K.; Thermenos, H.W.; Keshavan, M.S.; Seidman, L.J. Gray Matter Alterations in Schizophrenia High-Risk Youth and Early-Onset Schizophrenia: A Review of Structural MRI Findings. Child Adolesc. Psychiatr. Clin. N. Am. 2013, 22, 689–714. [Google Scholar] [CrossRef]
- Li, C.; Pang, D.; Lin, J.; Yang, T.; Shang, H. Shared Genetic Links between Frontotemporal Dementia and Psychiatric Disorders. BMC Med. 2022, 20, 131. [Google Scholar] [CrossRef]
- Andreou, D.; Jørgensen, K.N.; Nerland, S.; Smelror, R.E.; Wedervang-Resell, K.; Johannessen, C.H.; Myhre, A.M.; Andreassen, O.A.; Blennow, K.; Zetterberg, H.; et al. Lower Plasma Total Tau in Adolescent Psychosis: Involvement of the Orbitofrontal Cortex. J. Psychiatr. Res. 2021, 144, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Grizzell, J.A.; Patel, S.; Barreto, G.E.; Echeverria, V. Cotinine Improves Visual Recognition Memory and Decreases Cortical Tau Phosphorylation in the Tg6799 Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 75–81. [Google Scholar] [CrossRef]
- Elsonbaty, S.M.; Ismail, A.F.M. Nicotine Encourages Oxidative Stress and Impairment of Rats’ Brain Mitigated by Spirulina Platensis Lipopolysaccharides and Low-Dose Ionizing Radiation. Arch. Biochem. Biophys. 2020, 689, 108382. [Google Scholar] [CrossRef]
- Liang, Z.; Zhan, Y.; Shen, Y.; Wong, C.C.L.; Yates, J.R.; Plattner, F.; Lai, K.-O.; Ip, N.Y. The Pseudokinase CaMKv Is Required for the Activity-Dependent Maintenance of Dendritic Spines. Nat. Commun. 2016, 7, 13282. [Google Scholar] [CrossRef]
- Cheung, J.S.; van Woerden, G.M.; Veenma, D.C.M. CAMK2; Four Genes, One Syndrome? Delineation of Genotype–Phenotype Correlations. Curr. Opin. Neurobiol. 2025, 90, 102935. [Google Scholar] [CrossRef]
- Litersky, J.M.; Johnson, G.V.W.; Jakes, R.; Goedert, M.; Lee, M.; Seubert, P. Tau Protein Is Phosphorylated by Cyclic AMP-Dependent Protein Kinase and Calcium/Calmodulin-Dependent Protein Kinase II within Its Microtubule-Binding Domains at Ser-262 and Ser-356. Biochem. J. 1996, 316, 655–660. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Z.; Yu, C.; Dong, H.; Wang, S.; Wang, G.; Wang, D. Tau Aggravates Stress-Induced Anxiety by Inhibiting Adult Ventral Hippocampal Neurogenesis in Mice. Cereb. Cortex 2023, 33, 3853–3865. [Google Scholar] [CrossRef]
- Novak, G.; Seeman, M.V. Dopamine, Psychosis, and Symptom Fluctuation: A Narrative Review. Healthcare 2022, 9, 1713. [Google Scholar] [CrossRef]
- Jia, W.; Kawahata, I.; Cheng, A.; Fukunaga, K. The Role of CamkII and ERK Signaling in Addiction. Int. J. Mol. Sci. 2021, 22, 3189. [Google Scholar] [CrossRef]
- Grintsevich, E.E. Effects of Neuronal Drebrin on Actin Dynamics. Biochem. Soc. Trans. 2021, 49, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.; Yadav, S. Phosphoregulation of the Septin Cytoskeleton in Neuronal Development and Disease. Cytoskeleton 2022, 80, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Tang, B.L. Rab GTPases and Their Roles in Brain Neurons and Glia. Brain Res. Rev. 2008, 58, 236–246. [Google Scholar] [CrossRef]
- Shikanai, M.; Yuzaki, M.; Kawauchi, T. Rab Family Small GTPases-Mediated Regulation of Intracellular Logistics in Neural Development. Histol. Histopathol. 2018, 33, 765–771. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, K.T. Secretory Autophagy—A New Paradigm Regulating Synaptic Plasticity. Autophagy 2024, 20, 2354–2356. [Google Scholar] [CrossRef]
- Forsyth, J.K.; Lewis, D.A. Mapping the Consequences of Impaired Synaptic Plasticity in Schizophrenia through Development: An Integrative Model for Diverse Clinical Features. Trends Cogn. Sci. 2017, 21, 760–778. [Google Scholar] [CrossRef]
- Pandey, S.; Miller, C.A. IUPHAR-Review: Targeting the Cytoskeleton as a Therapeutic Approach to Substance Use Disorders. Pharmacol. Res. 2024, 202, 107143. [Google Scholar] [CrossRef]
- Stevens, R.J.; Littleton, J.T. Synaptic Growth: Dancing with Adducin. Curr. Biol. 2011, 21, R402–R405. [Google Scholar] [CrossRef]
- Bednarek, E.; Caroni, P. β-Adducin Is Required for Stable Assembly of New Synapses and Improved Memory upon Environmental Enrichment. Neuron 2011, 69, 1132–1146. [Google Scholar] [CrossRef]
- Bosia, M.; Pigoni, A.; Zagato, L.; Merlino, L.; Casamassima, N.; Lorenzi, C.; Pirovano, A.; Smeraldi, E.; Manunta, P.; Cavallaro, R. ADDing a Piece to the Puzzle of Cognition in Schizophrenia. Eur. J. Med. Genet. 2016, 59, 26–31. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, H.; He, J.; Liu, L.; Xue, W.; Fox, A.; Tickner, J.; Xu, J. The Emerging Roles of HnRNPK. J. Cell. Physiol. 2020, 235, 1995–2008. [Google Scholar] [CrossRef]
- Martins-de-Souza, D.; Gattaz, W.F.; Schmitt, A.; Novello, J.C.; Marangoni, S.; Turck, C.W.; Dias-Neto, E. Proteome Analysis of Schizophrenia Patients Wernicke’s Area Reveals an Energy Metabolism Dysregulation. BMC Psychiatry 2009, 9, 17. [Google Scholar] [CrossRef]
- Breiden, B.; Sandhoff, K. Lysosomal Glycosphingolipid Storage Diseases. Annu. Rev. Biochem. 2019, 88, 461–485. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.C.; Giddens, M.M.; Coleman, B.M.; Hall, R.A. The Protective Role of Prosaposin and Its Receptors in the Nervous System. Brain Res. 2014, 1585, 1–12. [Google Scholar] [CrossRef]
- He, Y.; Kaya, I.; Shariatgorji, R.; Lundkvist, J.; Wahlberg, L.U.; Nilsson, A.; Mamula, D.; Kehr, J.; Zareba-Paslawska, J.; Biverstål, H.; et al. Prosaposin Maintains Lipid Homeostasis in Dopamine Neurons and Counteracts Experimental Parkinsonism in Rodents. Nat. Commun. 2023, 14, 5804. [Google Scholar] [CrossRef]
- Jungerius, B.J.; Hoogendoorn, M.L.C.; Bakker, S.C.; van’t Slot, R.; Bardoel, A.F.; Ophoff, R.A.; Wijmenga, C.; Kahn, R.S.; Sinke, R.J. An Association Screen of Myelin-Related Genes Implicates the Chromosome 22q11 PIK4CA Gene in Schizophrenia. Mol. Psychiatry 2008, 13, 1060–1068. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhao, F.; Tian, H.; Chen, J.; Li, Q.; Yang, L.; Ping, J.; Li, R.; Wang, L.; Xu, Y.; et al. Acid Sphingomyelinase/Ceramide System in Schizophrenia: Implications for Therapeutic Intervention as a Potential Novel Target. Transl. Psychiatry 2022, 12, 260. [Google Scholar] [CrossRef]
- Keshri, N.; Nandeesha, H. Dysregulation of Synaptic Plasticity Markers in Schizophrenia. Indian J. Clin. Biochem. 2023, 38, 4–12. [Google Scholar] [CrossRef]
- Shiwaku, H.; Katayama, S.; Kondo, K.; Nakano, Y.; Tanaka, H.; Yoshioka, Y.; Fujita, K.; Tamaki, H.; Takebayashi, H.; Terasaki, O.; et al. Autoantibodies against NCAM1 from Patients with Schizophrenia Cause Schizophrenia-Related Behavior and Changes in Synapses in Mice. Cell Rep. Med. 2022, 3, 100597. [Google Scholar] [CrossRef]
- Chattopadhyaya, B.; Baho, E.; Huang, Z.J.; Schachner, M.; Di Cristo, G. Neural Cell Adhesion Molecule-Mediated Fyn Activation Promotes GABAergic Synapse Maturation in Postnatal Mouse Cortex. J. Neurosci. 2013, 33, 5957–5968. [Google Scholar] [CrossRef]
- An, H.; Zhou, L.; Yu, Y.; Fan, H.; Fan, F.; Tan, S.; Wang, Z.; Shi, J.; Yang, F.; Zhang, X.; et al. Serum NCAM Levels and Cognitive Deficits in First Episode Schizophrenia Patients versus Health Controls. Schizophr. Res. 2018, 192, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Keefe, R.S.E.; Lange, L.A.; Lange, E.M.; Stroup, T.S.; Lieberman, J.; Maness, P.F. NCAM1 and Neurocognition in Schizophrenia. Biol. Psychiatry 2007, 61, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Brennand, K.; Savas, J.N.; Kim, Y.; Tran, N.; Simone, A.; Hashimoto-Torii, K.; Beaumont, K.G.; Kim, H.J.; Topol, A.; Ladran, I.; et al. Phenotypic Differences in HiPSC NPCs Derived from Patients with Schizophrenia. Mol. Psychiatry 2015, 20, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Courtney, N.A.; Bao, H.; Briguglio, J.S.; Chapman, E.R. Synaptotagmin 1 Clamps Synaptic Vesicle Fusion in Mammalian Neurons Independent of Complexin. Nat. Commun. 2019, 10, 4076. [Google Scholar] [CrossRef]
- Tucker, W.C.; Weber, T.; Chapman, E.R. Reconstitution of Ca2+-Regulated Membrane Fusion by Synaptotagmin and SNAREs. Science 2004, 304, 435–438. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Wu, X.; Xie, Z.; Wang, Y.; Wang, B.; Li, X.; Pei, Y.; Gu, Y.; Huang, K.; et al. Synaptotagmin-1 Is a Bidirectional Ca2+ Sensor for Neuronal Endocytosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2111051119. [Google Scholar] [CrossRef]
- Sokolov, B.P.; Tcherepanov, A.A.; Haroutunian, V.; Davis, K.L. Levels of MRNAs Encoding Synaptic Vesicle and Synaptic Plasma Membrane Proteins in the Temporal Cortex of Elderly Schizophrenic Patients. Biol. Psychiatry 2000, 48, 184–196. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Schlüter, O.M.; Schmitz, F.; Jahn, R.; Rosenmund, C.; Südhof, T.C. A Complete Genetic Analysis of Neuronal Rab3 Function. J. Neurosci. 2004, 24, 6629–6637. [Google Scholar] [CrossRef]
- Marchisella, F.; Coffey, E.T.; Hollos, P. Microtubule and Microtubule Associated Protein Anomalies in Psychiatric Disease. Cytoskeleton 2016, 73, 596–611. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Chorev, D.S.; Geiger, B.; Cossart, P. The Diverse Family of Arp2/3 Complexes. Trends Cell Biol. 2017, 27, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hotulainen, P.; Llano, O.; Smirnov, S.; Tanhuanpää, K.; Faix, J.; Rivera, C.; Lappalainen, P. Defining Mechanisms of Actin Polymerization and Depolymerization during Dendritic Spine Morphogenesis. J. Cell Biol. 2009, 185, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, S.; Hashimoto, T.; Berry, K.J.; Tsubomoto, M.; Yamaguchi, Y.; Enwright, J.F.; Chen, K.; Kawabata, R.; Kikuchi, M.; Kishimoto, T.; et al. Expression of Actin- and Oxidative Phosphorylation-Related Transcripts across the Cortical Visuospatial Working Memory Network in Unaffected Comparison and Schizophrenia Subjects. Neuropsychopharmacology 2022, 47, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Block, K.; Sittka, A.; Oelgeschläger, M.; Seiler, A.E.M.; Luch, A. MicroRNA Profiling as Tool for In Vitro Developmental Neurotoxicity Testing: The Case of Sodium Valproate. PLoS ONE 2014, 9, e98892. [Google Scholar]
- Wanibuchi, M.; Ohtaki, S.; Ookawa, S.; Kataoka-Sasaki, Y.; Sasaki, M.; Oka, S.; Kimura, Y.; Akiyama, Y.; Mikami, T.; Mikuni, N.; et al. Actin, Alpha, Cardiac Muscle 1 (ACTC1) Knockdown Inhibits the Migration of Glioblastoma Cells in Vitro. J. Neurol. Sci. 2018, 392, 117–121. [Google Scholar] [CrossRef]
- Chu, J.; Cargnello, M.; Topisirovic, I.; Pelletier, J. Translation Initiation Factors: Reprogramming Protein Synthesis in Cancer. Trends Cell Biol. 2016, 26, 918–933. [Google Scholar] [CrossRef]
- Sasikumar, A.N.; Perez, W.B.; Kinzy, T.G. The Many Roles of the Eukaryotic Elongation Factor 1 Complex. WIREs RNA 2012, 3, 543–555. [Google Scholar] [CrossRef]
- Weng, J.; Zhu, X.; Ouyang, Y.; Liu, Y.; Lu, H.; Yao, J.; Pan, B. Identification of Immune-Related Biomarkers of Schizophrenia in the Central Nervous System Using Bioinformatic Methods and Machine Learning Algorithms. Mol. Neurobiol. 2024, 62, 3226–3243. [Google Scholar] [CrossRef]
- Gangadin, S.S.; Enthoven, A.D.; van Beveren, N.J.M.; Laman, J.D.; Sommer, I.E.C. Immune Dysfunction in Schizophrenia Spectrum Disorders. Annu. Rev. Clin. Psychol. 2024, 20, 229–257. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zimbron, J.; Dalman, C.; Lewis, G.; Jones, P.B. Childhood Infection and Adult Schizophrenia: A Meta-Analysis of Population-Based Studies. Schizophr. Res. 2012, 139, 161–168. [Google Scholar] [CrossRef]
- Pearlman, D.M.; Najjar, S. Meta-Analysis of the Association between N-Methyl-d-Aspartate Receptor Antibodies and Schizophrenia, Schizoaffective Disorder, Bipolar Disorder, and Major Depressive Disorder. Schizophr. Res. 2014, 157, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Benros, M.E.; Pedersen, M.G.; Rasmussen, H.; Eaton, W.W.; Nordentoft, M.; Mortensen, P.B. A Nationwide Study on the Risk of Autoimmune Diseases in Individuals With a Personal or a Family History of Schizophrenia and Related Psychosis. Am. J. Psychiatry 2014, 171, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The Complement System: An Unexpected Role in Synaptic Pruning during Development and Disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Halling, M.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Alaedini, A.; Dupont, D.; Dickerson, F.B.; et al. Complement C1q Formation of Immune Complexes with Milk Caseins and Wheat Glutens in Schizophrenia. Neurobiol. Dis. 2012, 48, 447–453. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Vo, D.D.; Jops, C.; Kim, M.; Wen, C.; Hervoso, J.L.; Pasaniuc, B.; Gandal, M.J. Isoform-Level Transcriptome-Wide Association Uncovers Genetic Risk Mechanisms for Neuropsychiatric Disorders in the Human Brain. Nat. Genet. 2023, 55, 2117–2128. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-Translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 Critically Regulates Endogenous IL-1β Production in Activated Microglia by Stimulating NLRP3 Inflammasome Pathway. J. Neuroinflamm. 2018, 15, 177. [Google Scholar] [CrossRef]
- Marino, C.; Krishnan, B.; Cappello, F.; Taglialatela, G. Hsp60 Protects against Amyloid β Oligomer Synaptic Toxicity via Modification of Toxic Oligomer Conformation. ACS Chem. Neurosci. 2019, 10, 2858–2867. [Google Scholar] [CrossRef]
- Magen, D.; Georgopoulos, C.; Bross, P.; Ang, D.; Segev, Y.; Goldsher, D.; Nemirovski, A.; Shahar, E.; Ravid, S.; Luder, A.; et al. Mitochondrial Hsp60 Chaperonopathy Causes an Autosomal-Recessive Neurodegenerative Disorder Linked to Brain Hypomyelination and Leukodystrophy. Am. J. Hum. Genet. 2008, 83, 30–42. [Google Scholar] [CrossRef]
- Pouget, J.G. The Emerging Immunogenetic Architecture of Schizophrenia. Schizophr. Bull. 2018, 44, 993–1004. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.P.; Rodríguez-Vega, A.; Dutra-Tavares, A.C.; Semeão, K.A.; Filgueiras, C.C.; Ribeiro-Carvalho, A.; Manhães, A.C.; Abreu-Villaça, Y. Nucleus Accumbens Proteome Disbalance in an Adolescent Mouse Model of Schizophrenia and Nicotine Misuse Comorbidity. Biomedicines 2025, 13, 901. https://doi.org/10.3390/biomedicines13040901
Souza TP, Rodríguez-Vega A, Dutra-Tavares AC, Semeão KA, Filgueiras CC, Ribeiro-Carvalho A, Manhães AC, Abreu-Villaça Y. Nucleus Accumbens Proteome Disbalance in an Adolescent Mouse Model of Schizophrenia and Nicotine Misuse Comorbidity. Biomedicines. 2025; 13(4):901. https://doi.org/10.3390/biomedicines13040901
Chicago/Turabian StyleSouza, Thainá Pereira, Andrés Rodríguez-Vega, Ana Carolina Dutra-Tavares, Keila A. Semeão, Claudio Carneiro Filgueiras, Anderson Ribeiro-Carvalho, Alex Christian Manhães, and Yael Abreu-Villaça. 2025. "Nucleus Accumbens Proteome Disbalance in an Adolescent Mouse Model of Schizophrenia and Nicotine Misuse Comorbidity" Biomedicines 13, no. 4: 901. https://doi.org/10.3390/biomedicines13040901
APA StyleSouza, T. P., Rodríguez-Vega, A., Dutra-Tavares, A. C., Semeão, K. A., Filgueiras, C. C., Ribeiro-Carvalho, A., Manhães, A. C., & Abreu-Villaça, Y. (2025). Nucleus Accumbens Proteome Disbalance in an Adolescent Mouse Model of Schizophrenia and Nicotine Misuse Comorbidity. Biomedicines, 13(4), 901. https://doi.org/10.3390/biomedicines13040901




