Abstract
Heart failure (HF) has become an immense health concern affecting almost 1–2% of the population globally. It is a complex syndrome characterized by activation of the sympathetic nervous system and the Renin–Angiotensin–Aldosterone (RAAS) axis as well as endothelial dysfunction, oxidative stress, and inflammation. The recent literature points towards the interaction between the intestinal flora and the heart, also called the gut–heart axis. The human gastrointestinal tract is naturally inhabited by various microbes, which are distinct for each patient, regulating the functions of many organs. Alterations of the gut microbiome, a process called dysbiosis, may result in systemic diseases and have been associated with heart failure through inflammatory and autoimmune mechanisms. The disorder of intestinal permeability favors the translocation of microbes and many metabolites capable of inducing inflammation, thus further contributing to the deterioration of normal cardiac function. Besides diet modifications and exercise training, many studies have revealed possible gut microbiota targeted treatments for managing heart failure. The aim of this review is to demonstrate the impact of the inflammatory environment induced by the gut microbiome and its metabolites on heart failure and the elucidation of these novel therapeutic approaches.
1. Introduction
Heart failure (HF) has become an immense health concern affecting almost 1–2% of the population globally [1]. The lifetime risk of HF approaches up to 25%, while its community-wide mortality rate may be exacerbated by the ongoing aging of the population [2]. Although established treatments have ameliorated survival from 1 to 6 years, the prognosis of these patients remains poor, with severe morbidity and mortality, low quality of life, and additional socioeconomic health burden [3].
HF is defined as a complex clinical syndrome resulting from any structural or functional abnormality impairing cardiac filling and/or blood pumping [4]. As a result, activation of the sympathetic nervous system, the Renin–Angiotensin–Aldosterone (RAAS) axis, endothelial dysfunction, oxidative stress, and inflammation may contribute to the chronic deterioration of cardiac function [5]. Among these mechanisms, inflammation plays a pivotal role in the evolution of HF, which is mostly mediated by several pro-inflammatory cytokines, such as increased levels of tumor necrosis factor-α (TNF-a), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17 (IL-17), interleukin-18 (IL-18) and reduced concentrations of interleukin-5, (IL-5), interleukin-7 (IL-7), and interleukin-33 (IL-33) [6,7]. The role of the NLRP3 inflammasome is critical in this regard, as it is considered the orchestrator of inflammation in HF (Figure 1). It is widely known that chronic inflammatory disorders (e.g., arterial hypertension, diabetes mellitus, hyperlipidemia, ischemic cardiomyopathy, atrial fibrillation, chronic kidney disease, asthma/ chronic obstructive pulmonary disease, depression) as well as metabolic factors (e.g., obesity and metabolic syndrome) and lifestyle behaviors (e.g., smoking, physical inactivity, alcohol abuse) are regarded as significant contributors to the progression of HF [8].
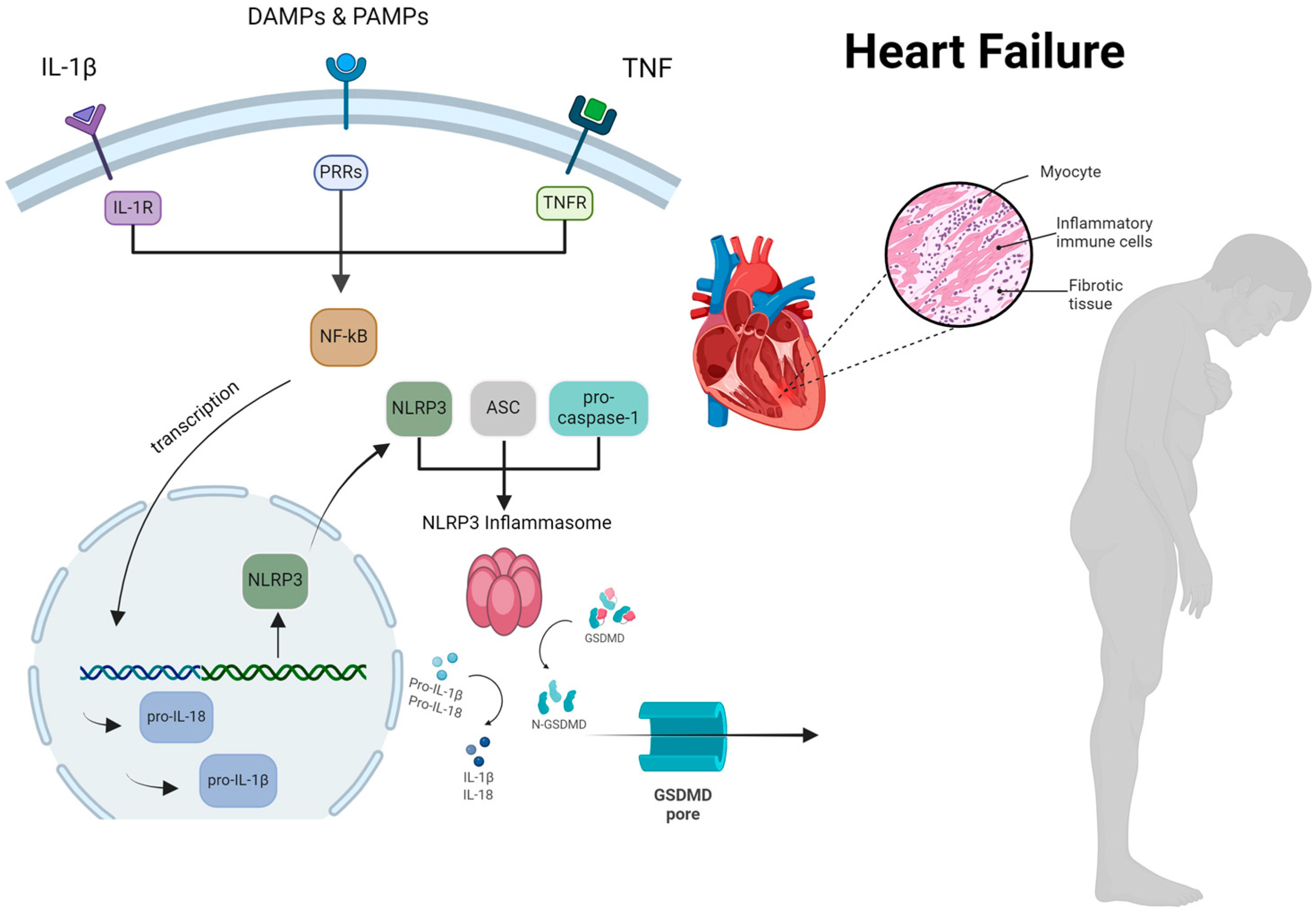
Figure 1.
The comprehensive process by which the NLRP3 inflammasome activation occurs, resulting in myocardial cell hypertrophy and inflammation in patients with heart failure. ASC, apoptosis-associated speck-like protein containing a CARD; DAMP, damage-associated molecular patterns; GSDMD, gasdermin D; IL, interleukin; NF-kB, nuclear factor k-light-chain enhancer of activated B cells; NLRP3, nucleotideoligomerization domain (NOD)-like receptor P3; PRRs, pattern recognition receptors; PAMP, pathogen-associated molecular patterns; TNF, tumor necrosis factor. Reproduced with permission from Vlachakis PK et al. [9], International Journal of Molecular Sciences; published by MDPI, 2024, and used under Creative Commons CC BY 4.0 license.
Because the etiology of inflammation in HF has not been totally understood, recent studies have focused their interest on the gut microbiome. The gastrointestinal tract is always inhabited by many microorganisms, whose genome, transcription, and translation products are responsible for numerous vital functions of our organism [10]. However, dysfunction of the intestinal microbiome, a process called dysbiosis, has been associated with increased cardiovascular risk [11]. Dysbiosis also favors the production of toxic metabolites through inflammatory pathways, which may induce or worsen preexisting coronary artery disease, hypertension, diabetes mellitus, hyperlipidemia, and HF [12,13,14]. However, these diet-related traditional cardiovascular risk factors may induce a chronic inflammatory environment, which may result not only in the alteration of the gut microbiome but also its epigenetic inheritance to descendants [15].
Growing evidence reveals an interrelationship between gut dysbiosis and HF, which is mediated by inflammation. The most prominent theory supporting the induction of HF refers to the “leaky gut” hypothesis [16]. Gut dysbiosis may be the result of (i) the reduction of intestinal bacterial diversity, (ii) the overproduction of harmful bacteria, and (iii) loss of the beneficial bacterial gut population [17]. The two most common mechanisms impairing the intestinal population in HF include reduced cardiac output and overactivity of the sympathetic nervous system, thus deteriorating the natural function of the intestinal wall barrier through vasoconstriction [18]. The pathophysiologic pathways differ depending on overall systolic cardiac function. Therefore, the disruption of the intestinal barrier permeability wall favors bacterial translocation and the diffusion of toxic bacterial compounds into systemic circulation through the TLR4/NF-κB activation pathway and the NLRP3 inflammasome, resulting in sub-acute or chronic inflammation [19]. Continuous exposure to systemic inflammation accelerates the evolution of HF. Consequently, gut dysbiosis and HF interact bidirectionally as inflammation may aggravate equally each of these two entities.
Τhis interaction between the gut microbiome and HF has progressively triggered scientific interest and can be characterized as an emerging field of study for the discovery of novel HF treatments [14]. The aim of this review is to demonstrate the impact of the inflammatory environment induced by the gut microbiome and its metabolites on HF and the elucidation of these novel molecular targets.
2. Gut Microbiota
Our gastrointestinal tract abounds with trillions of microbes, which mostly include bacteria and viruses [20]. The normal gut flora may contain more than 3 million genes, compared to the almost 23,000 genes of the human genome, demonstrating the significant impact of these species on human health [21]. The genetic information and the functions of these microbes are described as gut microbiomes.
According to recent studies, the community of the adult intestinal tract is mostly dominated by Bacteroidetes, Firmicutes, and, to a lesser extent, Actinobacteria, Proteobacteria, Verrucomicrobia, Cyanobacteria, and Eukarya [22]. In particular, the small intestine is mostly colonized by Gram-positive bacteria of the phylum Firmicutes, which comprise the classes of Streptococcus, Veillonella, and Clostridium [23]. However, the large intestine has greater microbial diversity, with domination of the phyla of Bacteroidetes (class of Bacteroides) and Firmicutes (class of Clostridium, Faecalibacterium, Lactobacillus and Ruminococcus) [24]. The composition of the bacterial community colonizing the gastrointestinal tract may be influenced mainly by eating habits and much less by other factors, such as the method of delivery, geographic origin, host genes, age, gender, body mass index (BMI), and treatment with antibiotics and/or probiotics [25]. It is noteworthy that Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria may be affected by numerous factors in our everyday life, including, in particular, overuse of chlorinated water, consumption of food additives, and exposure to pollutants, such as mycotoxins, heavy metals, pesticides, and antibiotics [26]. Dysregulation of the residing Verrucomicrobia population, especially Akkermensiamuciniphila, could reduce their efficacy in restraining inflammatory bowel disease (IBD) and obesity and may modify the intestinal environment, thus increasing the risk of carcinogenesis [27]. Additionally, the imbalance of the Eukarya, especially yeasts, a minor portion of the intestinal flora, is associated with certain autoimmune diseases, such as irritable bowel syndrome (IBS), as well as diabetes and colorectal cancer [28]. Exposure to the inflammatory effect of the Cyanobacterial toxin may result in neurodegenerative pathologies, including Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease [29].
The interaction between the intestinal microbiome and the gastrointestinal tract is not only symbiotic but also beneficial for human health. The human body (host) is responsible for the growth and protection of the intestinal bacteria, while these, in turn, facilitate digestion, the production of vitamins, the inhibition of pathogen overgrowth and colonization, and the regulation of the immune response. The significance of the intestinal microbiome in overall health and longevity has been proven recently, although Hippocrates highlighted the importance of a healthy digestive system [30]. This symbiotic interaction is moderated during different stages of life [31].
The main functions of the intestinal microbiome in human health include the following:
- 1.
- Nutrient metabolismThe intestinal microbiota produces a variety of important nutrients for human health. In particular, a significant amount of nutrients is received from carbohydrates, which are either absorbed directly or firstly digested and then absorbed as simple sugars in the upper parts of the gastrointestinal (GI) tract. Members of Bacteroides are mostly involved in carbohydrate metabolism [32]. A portion of these ingested carbohydrates escapes digestion in the upper gastrointestinal tract and is redirected for further fermentation by microbial populations, such as Bacteroides, Roseburia, Bifidobacterium, Fecalibacterium, and Enterobacteria [33]. The products are gases and short-chain fatty acids, such as butyric, acetic, and propionic acid, resulting from the interaction of the ligand–receptor of SCFAs with a G-protein-coupled receptor, Gpr41, representing important energy resources for the host [34].Proteins also remain an essential part of the human diet, which are mostly metabolized by the host’s digestive enzymes. The gut microbiome retains effective protein metabolism through microbial proteinases and peptidases as well as human proteinases [35]. Another important function that should be emphasized is the synthesis of vitamins K and B (such as B1 or “thiamine”, B2 or “riboflavin”, B3 or “niacin”, B5 or “pantothenic acid”, B6 or “pyridoxine”, B7 or “biotin”, B9 or “folic acid”, and B12 or “cobalamin”) [36]. Members of the genus Bacteroides also produce conjugated linoleic acid, an agent with antiatherogenic, anticancer, hypolipidemic, and immunomodulatory properties [37].Regarding lipids, the intestinal microbiome suppresses lipoprotein lipase activity in adipocytes. It is noteworthy that the Bacteroides thetaiotaomicron stimulates lipid hydrolysis in these tissues [38]. The most important fatty acids produced by the fermentation of plant fibers include the following. I. Acetic acid, which regulates appetite and lipid and cholesterol metabolism. II. Butyric acid, which produces energy, especially for colon cells, regulates glucose metabolism, and contributes to insulin resistance and cancer cell apoptosis. III. Propionic acid, which may affect the regulation of appetite and glucose metabolism [39]. Short-chain fatty acids generally reduce the risk of obesity and diabetes and empower the immune system or even favor brain development [40]. Interestingly, the study by Firrman et al. on animal models revealed that changes in the environmental pH resulted not only in an altered intestinal microbiome but also reduced pH, which was accompanied by significantly reduced levels of short-chain fatty acids (SCFA) [41].In 2009, Fukiya et al. demonstrated the conversion of primary bile acids into secondary bile acids and deoxycholic and lithocholic acids in the human colon by Bacteroidetes intestinalis, Bacteroidetes fragilis and Escherichia coli [42]. In conclusion, the intestinal microbiome plays a significant role in nutrient metabolism and balance within the host organism.
- 2.
- Regulation of the immune response and antimicrobial protectionThe gut microbiome of a healthy individual is in constant interaction with the native immune system. This homeostatic balance includes “immunological tolerance” against the host’s antigens and an effective immune response against harmful agents. The factors participating in the immunomodulatory process include the Gut Associated Lymphoid Tissue (GALT), T lymphocytes (CD4 and CD8), and IgA produced by plasma cells, as well as local macrophages and dendritic cells [43]. The immune system constantly supervises the intestinal flora by preventing their overgrowth through mechanisms like mucus production by goblet cells, the secretion of antimicrobial peptides by Paneth cells, and the production of IgA by B-cells [44,45]. Disturbance of this balance may increase the risk of infection, inflammation, cancer, and cardiovascular events.The mucus layer of the intestinal mucosa is described as the first barrier against pathogens. A layer of anaerobic bacteria covers the intestinal mucus layer, protecting epithelial colon cells from adhesion and overgrowth of aerobic Gram-negative bacteria. When intestinal dysbiosis occurs, this protective layer is destroyed, facilitating pathogen translocation into systemic circulation [46]. Epithelial cells also contribute to the immune response of the intestinal mucosa, not only directly but also through the secretion of cytokines and chemokines from the intestinal mucosa [47].The balance of the intestinal microbiome and the immune system reveals the presence of a least-inflammatory environment, which is capable of perceiving signals emitted by the inhabiting intestinal flora of the host. These signaling markers, which include liposaccharides, peptides, and peptidoglycan, are referred to as pathogen-associated molecular patterns or PAMPS and are recognized through specific pathways [48].When imbalance of the intestinal barrier occurs, pathogens will submit to the process of phagocytosis induced by tissue macrophages. Activation of the autophagy pathway affects Paneth cell function, increases IL-1β production, and promotes the activation of regulatory T-cells (T-reg) and IgA production by B lymphocytes [49]. MYD88 may mediate ΙgA secretion by B-lymphocytes of the intestinal epithelium through activation of nuclear factor–kappa–B (Nf-κB), which controls the immune and inflammatory response of the host organism [50].The gut microbiome may also affect neutrophil migration and their differentiation into macrophages, the differentiation of the T cell population into Th1, Th2, and Th17, which promote inflammation, as well as the function of regulatory T cells (T-regs), which restrict the inflammatory process [51,52].Innate lymphoid cells (ILCs) located on the intestinal epithelium may produce cytokines and exert an immunomodulatory effect on inflammation. ILCs can be divided into three groups: group 1/T-bet +, group 2/GATA-3 +, and group 3/RORγt + [53]. IECs release an enzyme called “intestinal alkaline phosphatase”, which dephosphorylates the endotoxin LPS and may reduce neutrophil migration through the gastrointestinal tract [54].In conclusion, a healthy intestinal microbiome contributes to the preservation of the intestinal barrier’s function, thus inhibiting the entrance of harmful microbes or metabolites into systemic circulation. Through this continuous interaction, the immune system acquires an effective defense system against numerous pathogens, contributing to the maintenance of the host’s homeostasis.
- 3.
- Maintenance of the gut barrier’s integrityNumerous studies have depicted the pivotal role of the intestinal microbiome in the integrity of the intestinal barrier and the protection of the gastrointestinal tract. Firstly, the Bacteroides thetaiotaomicron bacterium triggers the expression of a 2A protein, which is useful for the protection of desmosomes on the intestinal villi [55]. Mechanisms that also contribute to the maintenance of the gut microbiome barrier’s function include toll-like receptor 2 (TRL2) signaling, mediated by the peptidoglycan of the microbial cell wall and the endocannabinoid system [56,57]. According to Matar et al., a high-fat diet may increase the permeability of the intestinal barrier, whereas a diet including zinc, fiber, and vitamin D may reduce its permeability [58]. Quite interestingly, Chittimalli et al. have proven the protective effect of angiotensin-(1–7) on the balance of the intestinal barrier and the inhibitory effect on inflammation in the colon of old animal models through recovery of the intestinal stem cell layer and modulation of the residing gut microbiome [59]. A recent study by Lv et al. denoted the importance of maintaining levels of uric acid within normal range, as hyperuricemia favors the overgrowth of microbes, including, in particular, Bacteroides, Ruminiclostridium, Akkermansiaceae, Burkholderiaceae, Bilophila and Parasutterella, thus disturbing the intestinal barrier [60].
- 4.
- Metabolism of drugs and xenobioticsAmong other functions of the intestinal microbiome, the metabolism of drugs and xenobiotics should also be mentioned. Xenobiotics are defined as substances unnaturally produced by the host and include drugs, environmental toxins, and heavy metals. According to Collins and Patterson, the intestinal microbes may reduce xenobiotic absorption, thus altering their pharmacokinetics and favoring detoxification in the host organism [61]. Drug metabolism is mediated by enzymatic catalysis, among which the most frequent include reduction and hydrolysis, resulting in either drug activation or inhibition [62]. The best-studied case of enzymatic drug modification is digoxin, administered in cases of heart failure and atrial fibrillation. In certain circumstances, the bacterium Eggerthella lenta, belonging to the Actinobacteria phylum, reduces the bioavailability of digoxin through its conversion to the inactive product, dihydrodigoxin [63]. In 2009, the study by Clayton et al. highlighted the positive effect of the microbial metabolite p-cresol on the metabolism of acetaminophen due to the inhibition of the hepatic sulfotransferases [64]. Additionally, β-glucuronidase, an enzyme derived from the disintegration of the anticancer drug irinotecan, may affect its side effects, such as diarrhea, anorexia, weight loss, and inflammation [65]. Therefore, drugs and xenobiotic metabolism may reveal the possibility of novel therapeutic strategies to treat various diseases in the future.Better understanding of these metabolic modifications may hold the key for the discovery of novel HF therapeutic strategies.
3. Gut Dysbiosis, Inflammation, and Heart Failure
Alterations in the population or function of the gut microbiome, a process called dysbiosis, may result in pathologies ranging from local gastrointestinal disorders to diseases of the nervous, respiratory, and cardiovascular systems [66]. Dysbiosis may be the consequence of the imbalance between protective and possibly harmful bacteria or a reduction of microbial diversity [17].
It is widely known that the intestinal epithelium is the first line of defense and responsible for a “screening” process of the incoming molecules through specialized cell junctions, known as “tight junctions”. These junctions absorb important nutrients and remove harmful agents (such as toxins). However, in certain circumstances, their function is deteriorated, allowing toxic agents or metabolites to enter the bloodstream directly. This is referred to as “leaky gut” and may induce numerous pathologies, such as seasonal allergic reactions or asthma, digestive disorders or irritable bowel syndrome (IBS), hormonal imbalances (premenstrual syndrome (PMS) or polycystic ovary syndrome (PCOS)), depression, anxiety, autoimmune diseases (rheumatoid arthritis and celiac disease), chronic fatigue syndrome and fibromyalgia, dermatological diseases, such as acne and eczema, and food allergies and intolerances [67].
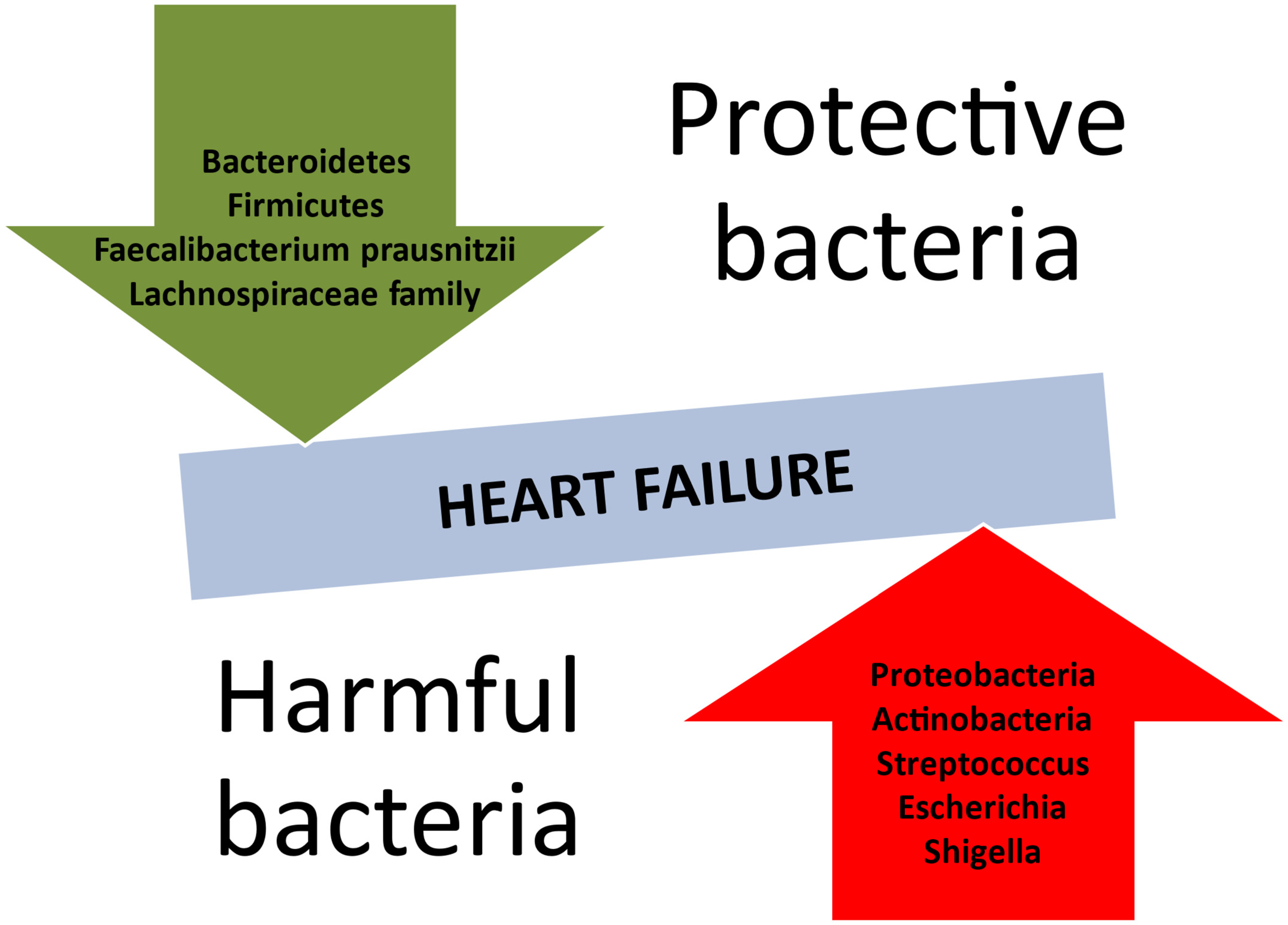
HF predisposes the body to modifications in the composition of the intestinal microbiome (Table 1). The recent literature reveals a reduction in microbial diversity or an absence of crucial gut microbes (Figure 2), such as those producing butyrate, e.g., Faecalibacterium prasusnitzii, the Lachnospiraceae family, and Eubacterium hallii [68,69]. Inflammation-mediated HF is mostly associated with a predominance of Gram-negative bacteria. According to a meta-analysis by Huang et al., Proteobacteria and Actinobacteria held the lion’s share of the gut microbiota in patients with HF [70]. It is also noteworthy that Streptococcus spp., Escherichia-Shigella, Veillonella sp., Klebsiella, and Enterococcus were also part of the intestinal community in the HF population [71].
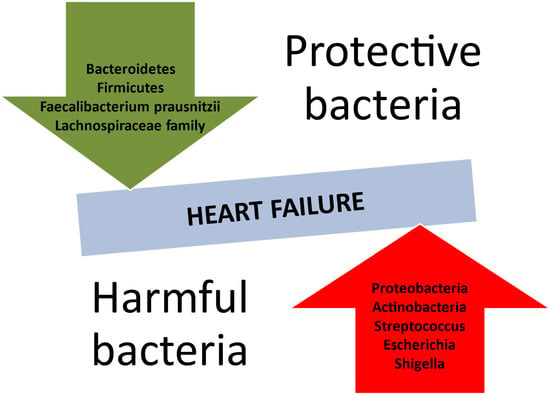
Figure 2.
Protective and harmful bacterial species concerning the development of heart failure.
Major differences have been described among the intestinal microbiota, as indicated by the stage of HF measured according to the left ventricular ejection fraction (LVEF). Patients with reduced LVEF had more abundance of Ruminococcus gnavus and decreased amounts of S. Faecalibacterium [72]. Nevertheless, the GUMPTION Study clearly states that patients with preserved LVEF presented higher levels of Enterococcus and Lactobacillus and reduced colonies of anti-inflammatory microorganisms (Butyricicoccus, Sutterella, Lachnospira, Ruminiclostridium) [73]. Therefore, these patients may be more susceptible to pseudomembranous colitis, most often due to overgrowth of the bacterium Clostridium difficile [74].


Table 1.
Gut microbiota in heart failure—bacterial diversity and its impact.
Table 1.
Gut microbiota in heart failure—bacterial diversity and its impact.
| Bacterial Group | Impact on Heart Failure | Mechanism | Key References |
|---|---|---|---|
| Bacteroidetes | Part of normal gut flora; changes linked to dysbiosis | Supports gut barrier integrity; altered composition linked to inflammation and endotoxemia | Huang et al. (2024) [70], Desai et al. (2023) [71] |
| Firmicutes | Reduced in heart failure patients; essential for SCFA production | Produces anti-inflammatory SCFAs (butyrate); depletion leads to inflammation | Cui et al. (2018) [72], Tousoulis et al. (2022) [69] |
| Proteobacteria | Dominant in HF patients; associated with inflammation | Enriched in endotoxin-producing bacteria; triggers NF-κB pathway | Huang et al. (2024) [70] |
| Actinobacteria | Increased in HF patients; correlated with worsened outcomes | Produces metabolites that exacerbate inflammation and oxidative stress | Huang et al. (2024) [70] |
| Faecalibacterium prausnitzii | Anti-inflammatory properties; reduced in HF patients | Produces butyrate; depletion leads to immune dysfunction and inflammation | Cui et al. (2018) [72], Paraskevaidis et al. (2023) [75] |
| Lachnospiraceae family | Decreased levels linked to poor cardiac outcomes | Produces butyrate; supports gut barrier and reduces systemic inflammation | Huang et al. (2024) [70] |
| Streptococcus spp. | Presence associated with increased inflammation | Overgrowth linked to gut permeability and cytokine production | Desai et al. (2023) [71] |
| Escherichia-Shigella | Linked to gut permeability and inflammation in HF patients | Produces endotoxins; triggers TLR4/NF-κB pathway and systemic inflammation | Méndez-Bailón et al. (2020) [74] |
It is widely known that inflammation plays a significant role in the pathogenesis of atherosclerotic cardiovascular disease and the evolution of HF. Cytokines are pro-inflammatory proteins produced by the damaged myocardium and include tumor necrosis factor-α, interleukin-1, interleukin-6, and interleukin-18. Their overproduction is enhanced by the excessive stimulation of the sympathetic nervous system. The injured myocardium and reduced cardiac output activate monocytes to release these cytokines. The following cardiomyocyte apoptosis may cause diffuse loss of myocardial tissue and lead to HF [76,77,78]. These factors also disturb cardiac hemodynamic balances, stimulate left ventricular hypertrophy, and induce the secretion of more cytokines. Additionally, tumor necrosis factor α (TNF-α) inhibits cardiac contractility by reducing the production of sarcoplasmic reticulum proteins, thus contributing to the deterioration of cardiac function [79]. Monocyte chemoattractant protein 1 (MCP-1) is a low molecular protein inducing the recruitment and migration of white blood cells, resulting in aggravation of the inflammatory environment [80]. Systemic inflammation favors the extensive collagen deposition of collagen. As a result, collagen becomes more rigid, contributing to the deterioration of both diastolic and systolic cardiac function [81,82].
The interaction between the gut microbiota, inflammation, and HF may be interpreted through the “leaky gut” hypothesis [83]. According to the recent literature, the most common factors favoring the alteration of the gut bacterial population in HF include reduced cardiac output and overdrive of the sympathetic nervous system, deteriorating the natural function of the intestinal wall barrier through vasoconstriction [18]. Cardiac systolic dysfunction, as measured by the left ventricular ejection fraction, may affect differently the inflammatory pathways associating HF with the gut microbiome. HF with reduced ejection fraction (HFrEF) induces hypoperfusion, resulting in ischemia and swelling of the intestinal mucosa. The following progressive visceral congestion disturbs the integrity of the intestinal barrier through which microbes and/or their metabolites may interact and infiltrate the mucosal elements initiating or exacerbating inflammatory processes and immune response. However, HF with preserved ejection fraction (HFpEF), mostly found in patients with metabolic syndrome, may be the consequence of insulin resistance and an increase in adipose tissue caused by the impaired gut microbiome, which provide the elements for inflammation and oxidative stress [84]. Therefore, these persisting mechanisms may favor sub-acute or chronic inflammation, the precursor of HF [75]. When dysbiosis occurs, an imbalance in the composition and function of the intestinal microflora is observed, resulting in modification of the immune response, susceptibility to inflammatory diseases, allergies, and metabolic diseases. Therefore, gut dysbiosis and HF result in an endless loop where each part of the scale aggravates the other through systemic inflammation.
A growing number of studies have revealed the constant interaction among inflammation, HF, and the gut microbiome (Figure 3). According to Zhang et al., infants with HF related to congenital heart disease presented apart from alterations of the intestinal microbial population predominance of Enteroccocus, which may induce the secretion of certain inflammatory mediators, such as interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), thus favoring the evolution of HF [85]. Additionally, the crucial role of inflammation in gut–heart interplay is depicted by fecal calprotectin (FC), an inflammatory biomarker of the intestine produced by monocytes and neutrophils affecting the status of HF, probably through stimulation of the NF-kB e p38 MAPK pathway [86]. Yuzefpolskaya et al. support that changes in the intestinal flora and endotoxinemia were more profound in patients with a left ventricular assist device (LVAD) or heart transplantation (HT), whereas the group with a worsening HF class had more elevated inflammatory and oxidative stress biomarkers [87]. One study on animal models with HF with preserved ejection fraction (HFpEF) suggested that high-protein and high-fat diets resulted in decreased gut diversity, imbalance of the intestinal barrier, and potential translocation of TMAO and endotoxins in systemic circulation capable of generating an inflammatory environment. It is noteworthy that modification of the gut microbiome was occurring simultaneously with the progression of HF [88]. Furthermore, Halade et al. have proven that sleep loss, when combined with excessive dietary fat consumption in post-myocardial infarction (MI) rats, triggered not only changes in the gastro-intestinal microbiome but also an increase in inflammatory prostaglandins and cytokines, such as IL1-β and IL-6, aggravating ongoing HF [89]. Consequently, inflammation remains one of the most important pathophysiologic mechanisms between the gut microbiome and HF.
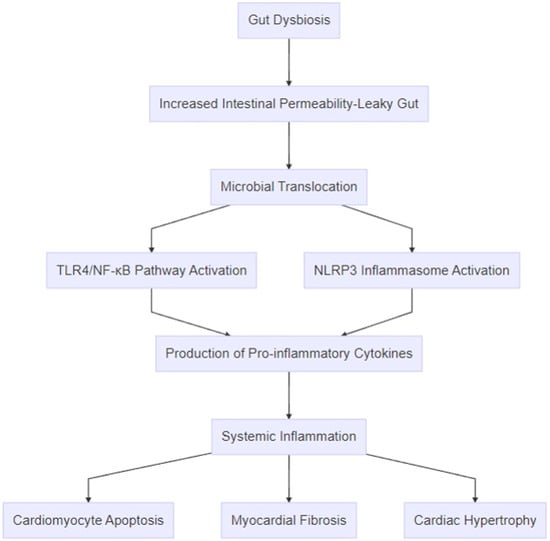
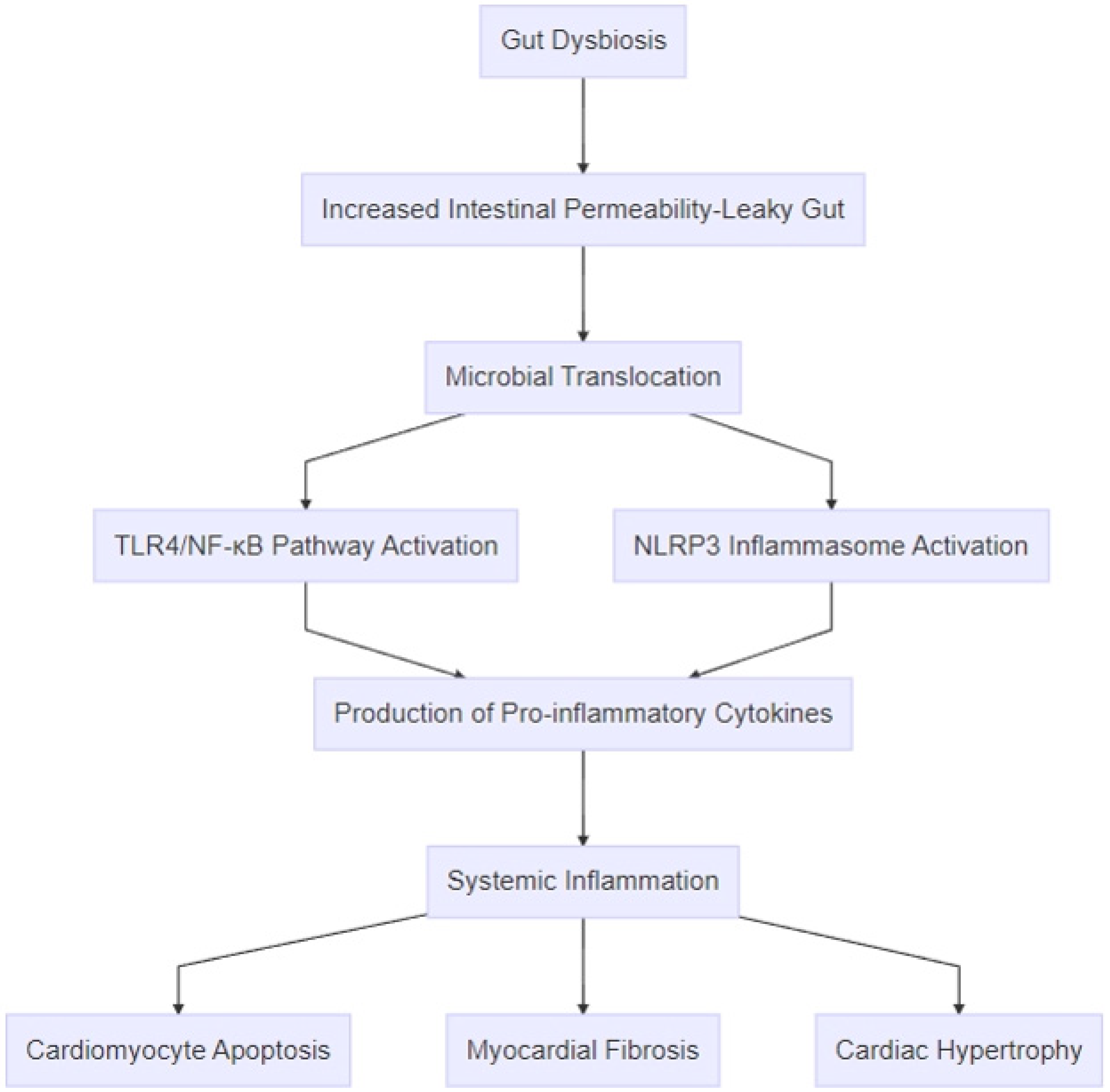
Figure 3.
Pathophysiologic mechanisms linking gut dysbiosis to heart failure. The process begins with gut dysbiosis, which leads to increased intestinal permeability (“leaky gut”) and microbial translocation of bacterial components, such as lipopolysaccharides, and metabolites, like trimethylamine-N-oxide. These microbial products activate inflammatory pathways, including the TLR4/NF-κB pathway and the NLRP3 inflammasome, resulting in the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-18. The amplification of systemic inflammation promotes cardiac remodeling through mechanisms like cardiomyocyte apoptosis, myocardial fibrosis, and cardiac hypertrophy.
4. The Role of Inflammatory Mediators of the Gut–Heart Axis
The gut–heart axis has become, over the years, a significant notion referring to the mutual interaction between the gut microbiota and cardiovascular diseases through numerous pathways [90]. Among these, inflammation plays a central role in the evolution of HF [9,91]. Therefore, the discovery of novel inflammatory biomarkers is required for a better understanding of this bidirectional association. So far, recent studies have revealed markers, such as endotoxins, trimethylamine n-oxide (TMAO), short-chain fatty acids, zonulin, and amino acids, that may be promising (Table 2).

Table 2.
Mediators contributing to the inflammatory process in the gut–heart axis.
4.1. Lipopolysaccharide
Lipopolysaccharide (LPS), also known as endotoxin, is a significant membrane component of the Gram-negative bacteria, such as Escherichia coli and Salmonella, inhabiting the gut and may result in pathologies in case of imbalance of the intestinal barrier [102]. LPS has become an important endothelial pro-inflammatory activator through the nuclear factor kappa-B (NF-κB) pathway in the pathogenesis of HF and may modify the prognosis of these patients [103,104]. Interestingly, higher LPS activity has been found more often in patients with decompensated/congestive HF. In fact, Nguyen et al. suggest that almost half of the population hospitalized for acute HF had excessive endotoxin levels and acidosis of the intestinal mucosal cells [92]. LPS has also been identified as one of the most potent triggers of innate immune activation, while other evidence suggests the development of allergy, autoimmune response, and chronic inflammation [105]. Asgharzadeh et al. found that LPS induced in animal models several pro-inflammatory mediators, mainly interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and myocardial accumulation of inflammatory cells [93]. Yuzefpolskaya et al. confirmed the presence of oxidative stress and chronic inflammation, especially in advanced HF stages, especially class IV, according to the New York Heart Association (NYHA) functional classification for HF [106]. Under septic conditions, LPS may bind to the CD14/TLR4/MD2 complex, promoting the secretion of pro-inflammatory cytokines and leading to myocardial apoptosis [107]. In addition, Toll-like receptor-4 (TLR-4) activated by LPS is capable of activating the innate immune response through pro-inflammatory cytokines, inflicting myocardial apoptosis and fibrosis and further worsening normal cardiac function [108]. Moreover, Lipid A (or endotoxin molecule) is a lipid element of endotoxin that can initiate inflammatory reactions in the cardiac tissue after binding to TLR-4 [109]. LPS is also a mediator of platelet activation and can influence the coagulation status, aggravating the already existing inflammatory environment [110]. It is worth mentioning that appropriate treatment in newly diagnosed HF patients significantly ameliorated their inflammatory status after 12 months by reducing several pro-inflammatory mediators, which included TNF-a, IL-6, IL-1, C-reactive protein (CRP), and Vascular cell adhesion protein 1 (VCAM-1) [97].
4.2. Trimethylamine N-Oxide
Trimethylamine N-oxide (TMAO) is an active metabolite produced by the intestinal microbiota as the result of dietary choline, betaine, and L-Carnitin metabolism [111]. The bacterial populations of Peptostreptococcaceae and Clostridiaceae may raise the plasma levels of TMAO [112]. A growing number of studies have proven that increased levels of TMAO are strongly associated with the progression of HF stages and may affect the prognosis of cardiovascular disease. Abnormal concentrations of TMAO are correlated to advanced diastolic dysfunction of the left ventricle and may predict prolonged adverse outcomes in chronic HF [113]. TMAO may contribute to the deterioration of cardiac function through the following pathways: myocardial hypertrophy and fibrosis, endothelial and mitochondrial dysfunction, oxidative stress, and inflammation [114]. It should be noted that TMAO is responsible for the reduced expression of anti-inflammatory cytokines (e.g., interleukin 10 (IL-10)) and the overproduction of pro-inflammatory cytokines, such as TNF-α, interleukin 1β and 6 (IL-1b, IL-6), and transforming growth factor-β (TGF-β) [95,115]. A research by Seldin et al. revealed that TMAO may promote inflammation of the endothelium through the NF-κB pathway [94]. Furthermore, the activation of the NLRP3 inflammasome by TMAO enhances vascular and cellular inflammation, favoring pathologic cardiac remodeling and risk of ventricular arrhythmias [116,117]. Another interesting pathway proposed by Savi et al. suggests that mitochondrial impairment by TMAO is capable of altering normal myocardial function [118].
4.3. Short-Chain Fatty Acids
Short-chain fatty acids (SCFAs) are mostly produced by the gut bacteria and include acetate, propionate, and butyrate derived from the digestion of dietary fibers [119]. The Bifidobacterium species are mostly responsible for the production of acetate, which may affect the levels of butyrate and propionate secreted by the Firmicutes and Bacterioidetes populations [120]. Because they represent an energy source for the colonic mucosa, they may influence cell growth, gut motility, and proliferation in order to keep the intestinal barrier intact [96]. Apart from the absorption of sodium and water, short-chain fatty acids may alter the function of the immune and nervous systems, applying a potential anti-inflammatory effect [121].
Under normal circumstances, the myocardium covers its metabolic needs mostly through oxidation of long chain fatty acids (LCFAs) and, to a lesser degree, glucose, ketones, and lactate [122]. The failing heart muscle presents an impaired metabolic profile with reduced oxidation of LCFA and a decreased ability to produce SCFAs [123]. Patients with chronic HF have been found with decreased levels of SCFAs regardless of the LEVF [124]. The main reason for this metabolic rotation relies on the depletion of the gut bacteria producing SCFA, therefore resulting in deterioration of the SCFA’s cardio-protective anti-inflammatory potential [125].
Although the exact underlying mechanism is not yet elucidated, the role of SCFAs in cardiac function is pivotal. SCFAs may protect from HF through the following pathways: prevention of mitochondrial dysfunction, maintenance of normal blood pressure levels, modulation of the inflammatory environment through inhibition of histone deacetylases (HDACs), and moderation of inflammatory genes via G-Protein-Coupled Receptors (GPRs) [126]. The inhibitory effect of HDACs and NF-κB activity may restrain the rate of cardiac hypertrophy [127]. SCFAs exert an agonistic effect on GPRs by reducing the risk of increased left-ventricular end-diastolic pressures, perivascular fibrosis, arterial hypertension, and stiffness, as depicted in GPR41-, GPR43-, and GPR 109A-knockout mice fed normal chow [128,129].
Bartolomaeus et al. have revealed that the impact of the cardiac inflammatory response on hypertension levels may be mediated by regulatory T-cells (Treg) [130]. SCFAs may also lower the production of certain pro-inflammatory cytokines, such as IL-6 and IL-8, and inhibit the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [131].
It is remarkable that SCFAs, especially butyrate, can keep intact the intestinal barrier. Butyrate is capable of ameliorating the intestinal barrier by stimulating tight junction protein Claudin-1 transcription and the macrophage/WNT/ERK signaling pathway [132,133]. Consequently, SCFAs are beneficial for cardiovascular health, as they may stabilize myocardial metabolism, regulate inflammation, protect from left ventricular hypertrophy, and support the intestinal barrier.
4.4. Zonulin
Zonulin, also known as pre-haptoglobin 2, is a protein equivalent of the zonula ocludens toxin of Vibrio cholera [134]. It is responsible for the balance of the intestinal barrier, especially in the overweight population, and it is mostly produced by the liver and the intestine [135]. The continuous consumption of wheat favors zonulin secretion [136]. Auto-immune diseases, celiac disease, and diabetes mellitus are characterized by pathologic zonulin plasma levels [137]. It is regulated by the C-X-C chemokine receptor type 3 (CXCR3) and may control a protein complex referred to as tight junctions in order to reversibly moderate intestinal permeability [138]. As a result, translocation of bacteria and/or their products may follow in blood circulation. Endotoxemia is the consequence of LPS translocation from the intestine to systemic circulation, contributing to chronic inflammation through the activation of Toll-like receptors in HF patients [139,140].
Recent studies demonstrate that zonulin may be a novel mediator between the impaired intestinal mucosa and HF. According to Oliva et al., patients with COVID-19 had increased gut permeability markers of zonulin and serum LPS and were predisposed to thrombotic events [99]. Ahmad et al. revealed that patients with chronic HF of ischemic and non-ischemic etiology had a more profound inflammatory profile accompanied by increased levels of plasma C-reactive protein (CRP) and zonulin, which could affect the prognosis of cardiac systolic function and their functional capacity [98]. It is worth noting that statin treatment significantly induced the secretion of CRP and zonulin in patients with chronic HF and further reduced the physical performance of this population [141]. Perticone et al. suggest that although the HF population had elevated inflammatory biomarkers, the levels of zonulin were reduced and associated with the severity of HF [142]. Notably, chronic treatment with probiotics reduced zonulin concentrations, restricted sarcopenia, and improved the performance status of this population [143]. These findings imply the importance of zonulin as a contributor to the inflammatory process and a potential therapeutic target in the complex relationship between the gut and HF.
4.5. Amino Acids
Amino acids are another category of biomarkers mostly found in high-protein meals, and they retain a crucial role in the gut–heart axis. Bacterial enzymes mainly produced by Bacteroides, Propionibacterium, Clostridium, Fusobacterium, Streptococcus, and Lactobacillus are capable of dissolving protein substrates to amino acids [144]. Chronic HF patients often have reduced amounts of amino acids, which are associated with the severity of the clinical syndrome and left ventricular systolic function [145]. The heart’s dependence on amino acids is the consequence of an enhanced myocardial anabolic environment and the lack of cardiomyocyte energy [146].
The amino acid tryptophan, which is degraded to its major metabolites, kynurenine and indole-3-propionate, is a contributor to cardiovascular disease [147]. In particular, HF induces the secretion of elevated plasma levels of kynurenine independent from the left ventricular ejection fraction [148]. The kynurenine pathway is activated by inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and Interferon gamma (IFN-γ) [100]. The overproduction of kynurenine pathway metabolites in patients with HF was associated with higher mortality and restricted functional capacity [149]. Similarly, increased levels of the ratio of the inflammatory markers kynurenine–tryptophan and kynurenic acid were strongly correlated with elevated risk of HF [150]. Recently, Wang et al. proposed that elevated serum concentrations of the kynurenine to tryptophan ratio (KTR), an inflammatory biomarker, was associated with poor prognosis in HF, especially with values of KTR more than 0.044 [101]. Interestingly, indoxyl sulfate (IS), another metabolite of tryptophane, moderated the expression of the NLRP3 inflammasome via the AHR/NF-κB pathway, raised the concentrations of numerous inflammatory cytokines, such as IL-1, IL-18, and TNF-a, and rendered the myocardium susceptible to fibrosis, hypertrophy, and left ventricular systolic dysfunction [151].
5. Possible Gut Microbiota Targeted Treatments
Because gut dysbiosis is capable of influencing the severity and progression of HF, the discovery and application of novel treatments could be effective against this pathology (Table 3). Intervention in the dysbiotic intestinal microbiome includes dietary modification, exercise training, as well as the use of antibiotics, pre/probiotics, and molecules binding various mediators (TMAO) and anti-toxins (anti-LPS).

Table 3.
Treatment strategies targeting gut microbiota.
5.1. Diet Modulation and Exercise Training
A Western diet rich in saturated fat but low in unsaturated fat is positively associated with anaerobic microorganisms, such as Bacteroides and Bilophila, highlighting the inflammatory potential of the intestinal microbiome in relation to cardiovascular risk and HF [157,158]. However, dietary modulation based on the Mediterranean regime remains the cornerstone of treatment of HF and amelioration of the gut’s microbial environment. The Mediterranean diet includes more whole grains, legumes, vegetables, fruits, and olive oil, accompanied by moderate proportions of fish, dairy products, and red wine and low consumption of red meat [159]. During a study period of 15 years, Strengers et al. have proven that compliance with the Mediterranean diet may lower the risk of HF, especially in the male population, measured using modified Mediterranean Diet Scores (mMDS) [160]. Accordingly, Kouvari et al. proposed that adherence to a Mediterranean diet had a beneficial effect on the prognosis of HF with preserved ejection fraction and C-reactive protein during a 10-year follow-up [152]. These findings imply that the Mediterranean diet is a safe and cardio-protective regime capable of ameliorating the inflammatory environment.
Exercise training is the second pillar of treatment of HF. According to the guidelines of the European Society of Cardiology (ESC), exercise is strongly advised, as it may ameliorate quality of life and functional status and reduce the risk of HF hospitalizations [1]. Engaging in sports activities, such as simple, daily, mild aerobic exercise, compared to a sedentary lifestyle, can have multiple benefits for intestinal health. A recent meta-analysis by Malandish et al. has proven the favorable influence of aerobic exercise on the overweight HF population by decreasing the inflammatory mediators TNF-α, IL-6, and high sensitivity CRP (hs-CRP) [161]. The study by Queipo-Ortuño et al. on animal models revealed that physical activity induced modification of the intestinal microflora through an increase in cardioprotective short-chain fatty acids and by affecting the hormones leptin and ghrelin [162]. Accordingly, Liu et al. discovered that exercise training not only had a protective effect on cardiac systolic function of post-myocardial infarction (MI) mice but also regulated certain bacterial populations, among which the most frequent were Butyricimonas and Akkermansia [163]. However, different forms of exercise and their multiple effects on the gut microbiome have not been adequately studied.
5.2. Probiotics and Prebiotics
Probiotics are defined as living microbes capable of modulating the gut microbiome and restoring intestinal balance. Clinical trials from the last decade have highlighted the protective effect of probiotics on HF. Treatment with probiotics containing Lactobacillus rhamnosus GR-1 reduced the progress of post-infarction left ventricular remodeling and ameliorated cardiac function in terms of systolic and diastolic levels in mice [164]. Similarly, Saccharomyces improved systolic dysfunction in an HF population as well as renal function and inflammation in the short term [165]. According to Moludi et al., probiotic therapy after myocardial infarction attenuated cardiac remodeling by affecting certain molecular and echocardiographic parameters [153]. Karim et al. have proven the benefits of multi-strain probiotics on HF-induced sarcopenia by enhancing the physical performance of these patients and restricting oxidative stress and inflammatory process [143].
Prebiotics are mostly fibers acting as nutrients for bacteria, promoting their growth and metabolic activity in favor of the organism. The consumption of prebiotics, which mostly includes fruits, vegetables, and whole grains, remains controversial. Marquez et al. revealed that a high-fiber diet limited pathologic blood pressure indices, myocardial fibrosis, and left ventricular hypertrophy [166]. Although similar favorable effects were observed by Komatsu et al. concerning the aforementioned pathologies and inflammation, inulin induced elevations in plasma triglycerides in mice with metabolic syndrome [167]. However, the use of fiber or acetate was not capable of restoring cardiac function in mice with genetic predisposition to HF [168]. The limited evidence from clinical studies along with the variety and complexity of gut microbiota render the use of probiotics and/or prebiotics supplementary.
5.3. Antibiotics and Potential Targeted Inflammatory Treatment
Antibiotics may moderate the biodiversity of the intestinal microbiome and affect the inflammatory response. Recent studies have started to reveal a possible anti-inflammatory and cardio-protective effect of antibiotics in HF. In a retrospective case control study during a follow up of almost 20 years, it was found that certain antibiotic therapies, especially sulfonamide, trimethoprim, and macrolides, considerably reduced the risk of HF, whereas cephalosporins had the opposite outcome [154]. In parallel, administration of polymyxin-β and tobramycin not only decreased the overproduction of pro-inflammatory cytokines by monocytes in patients with HF but also improved endothelial function [169]. Likewise, minocycline decreased the levels of pro-inflammatory cytokines in rats with ischemic-induced HF, leading to amelioration of the echocardiographic parameters of systolic function and depression [170]. Although some antibiotics have shown a beneficial effect on the inflammatory process, their administration in the control of the gut microbiome is restricted by side effects resulting from their chronic use, the creation of resistant microbial colonies, as well as the risk of cardiovascular events, mostly among the elderly [171]. Therefore, rational and careful application of antibiotic regimens is recommended depending on the infectious disease.
Over the last years, novel experimental treatments, which target certain metabolites or inflammatory mediators, have been shown to potentially be promising. Wang et al. have proven that 3,3-Dimethyl-1-butanol (DMB), a choline analogue, inhibited the plasma concentration of TMAO and prevented the progress of left ventricular hypertrophy, fibrosis, and HF in animal models [156]. Ursodeoxycholic acid (UDCA), a secondary bile acid, has shown evidence of anti-inflammatory action and may provide support in the maintenance of the intestinal barrier [172,173]. It should also be noted that UDCA has decreased the arrhythmic burden and the atherosclerotic process in animal studies [174,175].
Short-chain fatty acids (SCFAs) derived from the consumption of a diet rich in fiber have a favorable impact on metabolic syndrome by regulating glucose and lipid homeostasis. Apart from their anti-inflammatory properties and inhibition of left ventricular hypertrophy, butyrate alleviated cardiac systolic dysfunction by enhancing mitochondrial activity in rat models [176]. Similarly, Jiang et al. suggest a potential protection from ventricular arrhythmias after myocardial infarction induced in rats [177].
Empagliflozin, an antidiabetic sodium–glucose cotransporter-2 (SGLT2) inhibitor approved for the treatment of HF, modulated the intestinal flora by increasing the cardio-protective short-chain fatty acid producing bacteria and reduced the toxic concentrations of glycochenodeoxycholate, cis-aconitate, and uric acid [178]. Additionally, it also has anti-inflammatory properties by decreasing the IL-6 levels in HF patients and enhancing myocardial function by restricting oxidative stress [179,180].
5.4. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) refers to a method of administering bacteria through stool transfer from a healthy organism to the intestinal tract of a patient; it is mostly used for the treatment of Clostridium difficile infection. One study analyzed the benefits of stool transfer to individuals with recurrent infections from Cl. Difficile, resulting in improvement of intestinal homeostasis [181]. Fecal microbiome transplantation to overweight pre-HF with preserved ejection fraction (HFpEF) animal models had a protective effect on cardiac systolic and diastolic function. As stated by Hatahet et al., the possible mechanism may be the induction of the short-chain fatty acid butyrate, which contributes to the degradation of toxic branched-chain amino acids [155]. However, evidence for this technique is insufficient in order to be established in daily clinical practice.
6. Prognostic Models for HF Based on the Gut Microbiome
The bidirectional relationship between HF and the intestinal microbiome emphasizes the need for algorithms diagnosing HF early, non-invasively, and accurately. Firstly, the group of Aryal et al. in 2020 demonstrated the dynamics of artificial intelligence based on a supervised machine learning model in distinguishing various intestinal microbial data from the American Gut Project and predicting the risk of cardiovascular disease (CVD), including HF, in a sample of 951 individuals [182]. Similarly, Hodzic et al. developed a predictive algorithm of atherosclerotic cardiovascular disease based on the distinction of certain gut microbial signatures from stool samples, attaining an area under the curve (AUC) score of 0.926 [183]. Furthermore, Erawijantari et al. from Finland present the FINRISK Microbiome DREAM challenge as a potential model for the prognostic risk of HF through localization of certain gut microbiomes, especially those associated with inflammation, during a 10-year period. This program included numerous forms of clinical evidence and microbiome data in a population cohort of over 7000 adults, thus facilitating the prediction of HF and the identification of high-risk patients [184]. Despite the small number of studies, the discovery of novel HF predictive models remains fundamental.
7. Conclusions
Gut dysbiosis and HF are two interrelated factors severely impaired by the presence of inflammation. Patients with HF have demonstrated significant alterations in the composition of the normal gut microbiome depending on the severity of the left ventricular ejection fraction. HF may disrupt the integrity of the intestinal barrier, resulting in the translocation of microbes and their metabolites, thus enhancing the inflammatory environment. In addition, the intestinal microbiome and inflammation are capable of inducing numerous pathophysiological pathways in which cytokines and various metabolites may have a serious impact not only on the prognosis but also on the progression of HF. Apart from diet modulation and exercise training, the rest of the therapeutic strategies rely on clinical studies in human and animal models. Consequently, the regulation of the intestinal microflora has become a promising field of research for the treatment of HF, as the discovery of new metabolic pathways may unfold more concepts for novel therapeutic interventions depending on the genomic profile of each individual.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Vasan, R.S.; Enserro, D.M.; Beiser, A.S.; Xanthakis, V. Lifetime Risk of Heart Failure Among Participants in the Framingham Study. J. Am. Coll. Cardiol. 2022, 79, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef]
- Segiet, O.A.; Piecuch, A.; Mielanczyk, L.; Michalski, M.; Nowalany-Kozielska, E. Role of interleukins in heart failure with reduced ejection fraction. Anatol. J. Cardiol. 2019, 22, 287–299. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Wang, J. Metabolism and Chronic Inflammation: The Links Between Chronic Heart Failure and Comorbidities. Front. Cardiovasc. Med. 2021, 8, 650278. [Google Scholar] [CrossRef]
- Vlachakis, P.K.; Theofilis, P.; Kachrimanidis, I.; Giannakopoulos, K.; Drakopoulou, M.; Apostolos, A.; Kordalis, A.; Leontsinis, I.; Tsioufis, K.; Tousoulis, D. The Role of Inflammasomes in Heart Failure. Int. J. Mol. Sci. 2024, 25, 5372. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef]
- Sata, Y.; Marques, F.Z.; Kaye, D.M. The Emerging Role of Gut Dysbiosis in Cardio-metabolic Risk Factors for Heart Failure. Curr. Hypertens Rep. 2020, 22, 38. [Google Scholar] [CrossRef]
- Katsimichas, T.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. Gut Microbiota and Coronary Artery Disease: Current Therapeutic Perspectives. Metabolites 2023, 13, 256. [Google Scholar] [CrossRef]
- Theofilis, P.; Vlachakis, P.K.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Targeting the Gut Microbiome to Treat Cardiometabolic Disease. Curr. Atheroscler. Rep. 2024, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Fink, M.P. Leaky gut hypothesis: A historical perspective. Crit. Care Med. 1990, 18, 579–580. [Google Scholar] [PubMed]
- Francisqueti-Ferron, F.V.; Nakandakare-Maia, E.T.; Siqueira, J.S.; Ferron, A.J.T.; Vieira, T.A.; Bazan, S.G.Z.; Corrêa, C.R. The role of gut dysbiosis-associated inflammation in heart failure. Rev. Assoc. Med. Bras. 1992, 68, 1120–1124. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Ai, L.; Zhou, J.; Huang, J.; Xu, F.; Zeng, X.; Han, J.; Yin, F.; Zhu, Y.; et al. The Correlation Between Heart Failure and Gut Microbiome Metabolites. Infect. Microbes Dis. 2020, 2, 136–143. [Google Scholar] [CrossRef]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria Provide Genetic and Molecular Reporter Systems to Identify Specific Diseases. Int. J. Mol. Sci. 2024, 25, 4431. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Kastl, A.J., Jr.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Hajimohammad, A.; Fattahi, E.; Ardebili, A.; Kaboosi, H.; Ghaemi, E.A. The Frequency of Verrucomicrobia in the Intestinal Mucus of Patients with Cancer and Polyps Compared to Healthy Individuals. Jundishapur J. Microbiol. 2024, 17, 1–7. [Google Scholar] [CrossRef]
- Caetano, C.F.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A.; Rolo, J. The Role of Yeasts in Human Health: A Review. Life 2023, 13, 924. [Google Scholar] [CrossRef]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Dangerous Liaisons. Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef]
- Harkins, P.; Burke, E.; Swales, C.; Silman, A. ‘All disease begins in the gut’-the role of the intestinal microbiome in ankylosing spondylitis. Rheumatol. Adv. Pract. 2021, 5, rkab063. [Google Scholar] [CrossRef]
- Parkar, S.G.; Gopal, P.K. Gut Microbiota and Metabolism in Different Stages of Life and Health. Microorganisms 2022, 10, 474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantarel, B.L.; Lombard, V.; Henrissat, B. Complex carbohydrate utilization by the healthy human microbiome. PLoS ONE 2012, 7, e28742. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Devillard, E.; McIntosh, F.M.; Paillard, D.; Thomas, N.A.; Shingfield, K.J.; Wallace, R.J. Differences between human subjects in the composition of the faecal bacterial community and faecal metabolism of linoleic acid. Microbiology 2009, 155, 513–520. [Google Scholar] [CrossRef]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Anachad, O.; Taouil, A.; Taha, W.; Bennis, F.; Chegdani, F. The Implication of Short-Chain Fatty Acids in Obesity and Diabetes. Microbiol. Insights 2023, 16, 11786361231162720. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef] [PubMed]
- Fukiya, S.; Arata, M.; Kawashima, H.; Yoshida, D.; Kaneko, M.; Minamida, K.; Watanabe, J.; Ogura, Y.; Uchida, K.; Itoh, K.; et al. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. FEMS Microbiol. Lett. 2009, 293, 263–270. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Underwood, M.A.; Bevins, C.L. Paneth cells, defensins, and the commensal microbiota: A hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 2007, 19, 70–83. [Google Scholar] [CrossRef]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Colgan, S.P. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016, 73, 4203–4212. [Google Scholar] [CrossRef]
- Taghavi, M.; Khosravi, A.; Mortaz, E.; Nikaein, D.; Athari, S.S. Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. Eur. J. Pharmacol. 2017, 808, 8–13. [Google Scholar] [CrossRef]
- He, B.; Xi, F.; Zhang, X.; Zhang, J.; Guo, W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007, 26, 4053–4065. [Google Scholar] [CrossRef]
- Garg, S.; Nichols, J.R.; Esen, N.; Liu, S.; Phulwani, N.K.; Syed, M.M.; Wood, W.H.; Zhang, Y.; Becker, K.G.; Aldrich, A.; et al. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro 2009, 1, e00007. [Google Scholar] [CrossRef]
- Owaga, E.; Hsieh, R.H.; Mugendi, B.; Masuku, S.; Shih, C.K.; Chang, J.S. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2015, 16, 20841–20858. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Balabanova, L.; Bondarev, G.; Seitkalieva, A.; Son, O.; Tekutyeva, L. Insights into Alkaline Phosphatase Anti-Inflammatory Mechanisms. Biomedicines 2024, 12, 2502. [Google Scholar] [CrossRef]
- Lutgendorff, F.; Akkermans, L.M.; Söderholm, J.D. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Matar, A.; Damianos, J.A.; Jencks, K.J.; Camilleri, M. Intestinal Barrier Impairment, Preservation, and Repair: An Update. Nutrients 2024, 16, 3494. [Google Scholar] [CrossRef]
- Chittimalli, K.; Jahan, J.; Sakamuri, A.; McAdams, Z.L.; Ericsson, A.C.; Jarajapu, Y.P.R. Restoration of the gut barrier integrity and restructuring of the gut microbiome in aging by angiotensin-(1-7). Clin. Sci. 2023, 137, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhou, J.; Wang, C.; Yang, X.; Han, Y.; Zhou, Q.; Yao, R.; Sui, A. A dynamics association study of gut barrier and microbiota in hyperuricemia. Front. Microbiol. 2023, 14, 1287468. [Google Scholar] [CrossRef]
- Collins, S.L.; Patterson, A.D. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 6344. [Google Scholar] [CrossRef]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 14728–14733. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Roberts, A.B.; Pollet, R.M.; Ingle, J.D.; Biernat, K.A.; Pellock, S.J.; Venkatesh, M.K.; Guthrie, L.; O’Neal, S.K.; Robinson, S.J.; et al. Structure and Inhibition of Microbiome β-Glucuronidases Essential to the Alleviation of Cancer Drug Toxicity. Chem. Biol. 2015, 22, 1238–1249. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Lacy, B.E.; Wise, J.L.; Cangemi, D.J. Leaky Gut Syndrome: Myths and Management. Gastroenterol. Hepatol. 2024, 20, 264–272. [Google Scholar]
- Tang, W.H.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Tousoulis, D.; Guzik, T.; Padro, T.; Duncker, D.J.; De Luca, G.; Eringa, E.; Vavlukis, M.; Antonopoulos, A.S.; Katsimichas, T.; Cenko, E.; et al. Mechanisms, therapeutic implications, and methodological challenges of gut microbiota and cardiovascular diseases: A position paper by the ESC Working Group on Coronary Pathophysiology and Microcirculation. Cardiovasc. Res. 2022, 118, 3171–3182. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Y.; Ding, X.; Lin, S.; Li, X.; Yan, W.; Chen, M. Alteration of the gut microbiome in patients with heart failure: A systematic review and meta-analysis. Microb. Pathog. 2024, 192, 106647. [Google Scholar] [CrossRef]
- Desai, D.; Desai, A.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; et al. Re-defining the Gut Heart Axis: A Systematic Review of the Literature on the Role of Gut Microbial Dysbiosis in Patients With Heart Failure. Cureus 2023, 15, e34902. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mei, X.; Jiang, Y.; Chen, T.; Zhou, Y. Gut Microbiota in Heart Failure Patients With Preserved Ejection Fraction (GUMPTION Study). Front. Cardiovasc. Med. 2021, 8, 803744. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Bailón, M.; Jiménez-García, R.; Hernández-Barrera, V.; Miguel-Díez, J.; Miguel-Yanes, J.M.; Muñoz-Rivas, N.; Lorenzo-Villalba, N.; Carabantes-Alarcon, D.; Zamorano-León, J.J.; Astasio-Arbiza, P.; et al. Heart Failure Is a Risk Factor for Suffering and Dying of Clostridium difficile Infection. Results of a 15-Year Nationwide Study in Spain. J. Clin. Med. 2020, 9, 614. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Xanthopoulos, A.; Tsougos, E.; Triposkiadis, F. Human Gut Microbiota in Heart Failure: Trying to Unmask an Emerging Organ. Biomedicines 2023, 11, 2574. [Google Scholar] [CrossRef]
- Genth-Zotz, S.; von Haehling, S.; Blankenberg, S. [Immunactivation in chronic heart failure. Inflammatory mediators]. Z. Kardiol. 2004, 93, 004–1405. [Google Scholar]
- Seta, Y.; Shan, K.; Bozkurt, B.; Oral, H.; Mann, D.L. Basic mechanisms in heart failure: The cytokine hypothesis. J. Card. Fail. 1996, 2, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Parissis, J.T.; Adamopoulos, S.; Rigas, A.; Kostakis, G.; Karatzas, D.; Venetsanou, K.; Kremastinos, D.T. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am. J. Cardiol. 2004, 94, 1326–1328. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 018–1067. [Google Scholar] [CrossRef]
- Zhou, L.; Azfer, A.; Niu, J.; Graham, S.; Choudhury, M.; Adamski, F.M.; Younce, C.; Binkley, P.F.; Kolattukudy, P.E. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006, 98, 1177–1185. [Google Scholar] [CrossRef]
- Heymans, S.; González, A.; Pizard, A.; Papageorgiou, A.P.; López-Andrés, N.; Jaisser, F.; Thum, T.; Zannad, F.; Díez, J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015, 17, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, Y.; Xu, H.; Zhou, Y. The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines 2023, 11, 442. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Chen, X.-H.; Zhou, S.-J.; Lei, Y.-Q.; Chen, Q.; Cao, H. Relationship between heart failure and intestinal inflammation in infants with congenital heart disease. BMC Microbiol. 2024, 24, 98. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; Gallo, A.; Macerola, N.; Pero, E.; Ibba, F.; Camilli, S.; Riccardi, L.; Sarlo, F.; De Ninno, G.; Baroni, S.; et al. Role of Intestinal Inflammation and Permeability in Patients with Acute Heart Failure. Medicina 2023, 60, 8. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Bohn, B.; Nasiri, M.; Zuver, A.M.; Onat, D.D.; Royzman, E.A.; Nwokocha, J.; Mabasa, M.; Pinsino, A.; Brunjes, D.; et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J. Heart Lung Transpl. 2020, 39, 880–890. [Google Scholar] [CrossRef]
- Guivala, S.J.; Bode, K.A.; Okun, J.G.; Kartal, E.; Schwedhelm, E.; Pohl, L.V.; Werner, S.; Erbs, S.; Thiele, H.; Büttner, P. Interactions between the gut microbiome, associated metabolites and the manifestation and progression of heart failure with preserved ejection fraction in ZSF1 rats. Cardiovasc. Diabetol. 2024, 23, 024–02398. [Google Scholar] [CrossRef]
- Halade, G.V.; Mat, Y.; Gowda, S.G.B.; Jain, S.; Hui, S.P.; Yadav, H.; Kain, V. Sleep deprivation in obesogenic setting alters lipidome and microbiome toward suboptimal inflammation in acute heart failure. FASEB J. 2023, 37, e22899. [Google Scholar] [CrossRef]
- Akshay, A.; Gasim, R.; Ali, T.E.; Kumar, Y.S.; Hassan, A. Unlocking the Gut-Cardiac Axis: A Paradigm Shift in Cardiovascular Health. Cureus 2023, 15, e51039. [Google Scholar] [CrossRef]
- Jain, H.; Marsool, M.D.M.; Goyal, A.; Sulaiman, S.A.; Fatima, L.; Idrees, M.; Sharma, B.; Borra, V.; Gupta, P.; Nadeem, A.; et al. Unveiling the relationship between gut microbiota and heart failure: Recent understandings and insights. Curr. Probl. Cardiol. 2024, 49, 102179. [Google Scholar] [CrossRef]
- Nguyen, M.; Gautier, T.; Masson, D.; Bouhemad, B.; Guinot, P.-G. Endotoxemia in Acute Heart Failure and Cardiogenic Shock: Evidence, Mechanisms and Therapeutic Options. J. Clin. Med. 2023, 12, 2579. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Bargi, R.; Hosseini, M.; Farzadnia, M.; Khazaei, M. Cardiac and renal fibrosis and oxidative stress balance in lipopolysaccharide-induced inflammation in male rats. ARYA Atheroscler. 2018, 14, 71–77. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Hazen, S.L. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef]
- Ahmad, F.; Karim, A.; Khan, J.; Qaisar, R. Plasma zonulin correlates with cardiac dysfunction and poor physical performance in patients with chronic heart failure. Life Sci. 2022, 311, 121150. [Google Scholar] [CrossRef]
- Oliva, A.; Cammisotto, V.; Cangemi, R.; Ferro, D.; Miele, M.C.; De Angelis, M.; Cancelli, F.; Pignatelli, P.; Venditti, M.; Pugliese, F.; et al. Low-Grade Endotoxemia and Thrombosis in COVID-19. Clin. Transl. Gastroenterol. 2021, 12, e00348. [Google Scholar] [CrossRef]
- Ala, M.; Eftekhar, S.P. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int. J. Tryptophan Res. 2022, 15, 11786469221096643. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Wei, H.; Zhang, Y.; Wang, Y.; Wang, D.W. Increased Tryptophan Catabolism Provides Predictive Value to Chronic Heart Failure Patients with Low-Grade Inflammation. Inflammation, 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yang, Y.; Li, D. Lipopolysaccharide induced vascular smooth muscle cells proliferation: A new potential therapeutic target for proliferative vascular diseases. Cell Prolif. 2017, 50, e12332. [Google Scholar] [CrossRef]
- Ebner, N.; Földes, G.; Schomburg, L.; Renko, K.; Springer, J.; Jankowska, E.A.; Sharma, R.; Genth-Zotz, S.; Doehner, W.; Anker, S.D.; et al. Lipopolysaccharide responsiveness is an independent predictor of death in patients with chronic heart failure. J. Mol. Cell. Cardiol. 2015, 87, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.M.; Frigy, A.; Nagy, E.E. Targeting Mediators of Inflammation in Heart Failure: A Short Synthesis of Experimental and Clinical Results. Int. J. Mol. Sci. 2021, 22, 13053. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Yuzefpolskaya, M.; Colombo, P.C. The state of left ventricular device therapy for advanced heart failure: Midway upon the journey towards euvolemia and full functional recovery. Eur. J. Cardiothorac. Surg. 2022, 62, ezac173. [Google Scholar] [CrossRef]
- Reil, P.M.; Maghiar, T.T.; Vîlceanu, N.; Pascalau, A.; Judea Pusta, C.T.; Marcu, F.; Cavalu, S.; Pop, O. Assessing the Role of Lipopolysaccharide (LPS) Receptor (CD14) in Septic Cardiomyopathy: The Value of Immunohistochemical Diagnostics. Diagnostics 2022, 12, 781. [Google Scholar] [CrossRef]
- Katare, P.B.; Nizami, H.L.; Paramesha, B.; Dinda, A.K.; Banerjee, S.K. Activation of toll like receptor 4 (TLR4) promotes cardiomyocyte apoptosis through SIRT2 dependent p53 deacetylation. Sci. Rep. 2020, 10, 19232. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.D.; Surani, S.; Horseman, M.A. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr. Cardiol. Rev. 2017, 13, 86–93. [Google Scholar] [CrossRef]
- Pastori, D.; Carnevale, R.; Nocella, C.; Novo, M.; Santulli, M.; Cammisotto, V.; Menichelli, D.; Pignatelli, P.; Violi, F. Gut-Derived Serum Lipopolysaccharide is Associated With Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Hear. Assoc. 2017, 6, e005784. [Google Scholar] [CrossRef]
- Li, D.; Lu, Y.; Yuan, S.; Cai, X.; He, Y.; Chen, J.; Wu, Q.; He, D.; Fang, A.; Bo, Y.; et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 2022, 116, 230–243. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Salari, A.; Kheirkhah, J.; Ghorbani, Z. The Effects of Probiotics on Inflammation, Endothelial Dysfunction, and Atherosclerosis Progression: A Mechanistic Overview. Heart Lung Circ. 2022, 31, e45–e71. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Wijayasinghe, Y.S.; Khan, I.; El Samaloty, N.M.; Adnan, M.; Dar, T.A.; Poddar, N.K.; Singh, L.R.; Sharma, H.; Khan, S. Implications of trimethylamine N-oxide (TMAO) and Betaine in Human Health: Beyond Being Osmoprotective Compounds. Front. Mol. Biosci. 2022, 9, 964624. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, Z.; Cui, J.; Li, D.; Lu, J.; Cui, X.; Xie, L.; Wu, Y.; Lin, Q.; Li, Y. Trimethylamine N-Oxide in Heart Failure: A Meta-Analysis of Prognostic Value. Front. Cardiovasc. Med. 2022, 9, 817396. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, H.; Xiang, Q.; Hu, H.; Zhai, C.; Xu, S.; Tian, H. Role of Trimethylamine N-Oxide in Heart Failure. RCM 2024, 25, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Wang, Y.; Jiang, X.; Guo, M.; Yang, Z. NLRP3 inflammasome as a novel therapeutic target for heart failure. Anatol. J. Cardiol. 2022, 26, 15–22. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.H.; Li, F.; Yang, F.; Qian, L.L.; Wang, R.X. The Role of NLRP3 Inflammasome Signaling on Arrhythmias in Diabetes. J. Inflamm. Res. 2022, 15, 6883–6889. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Bresciani, L.; Falco, A.; Quaini, F.; Mena, P.; Brighenti, F.; Crozier, A.; Stilli, D.; Del Rio, D. Trimethylamine-N-Oxide (TMAO)-Induced Impairment of Cardiomyocyte Function and the Protective Role of Urolithin B-Glucuronide. Molecules 2018, 23, 549. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- McCrory, C.; Lenardon, M.; Traven, A. Bacteria-derived short-chain fatty acids as potential regulators of fungal commensalism and pathogenesis. Trends Microbiol. 2024, 32, 1106–1118. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Gandoy-Fieiras, N.; Gonzalez-Juanatey, J.R.; Eiras, S. Myocardium Metabolism in Physiological and Pathophysiological States: Implications of Epicardial Adipose Tissue and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 2641. [Google Scholar] [CrossRef] [PubMed]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure—Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Chen, A.-T.; Zhang, J.; Zhang, Y. Gut microbiota in heart failure and related interventions. iMeta 2023, 2, e125. [Google Scholar] [CrossRef] [PubMed]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut-Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.J.; Wang, Z.V.; Battiprolu, P.K.; Jiang, N.; Morales, C.R.; Kong, Y.; Rothermel, B.A.; Gillette, T.G.; Hill, J.A. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc. Natl. Acad. Sci. USA 2011, 108, 4123–4128. [Google Scholar] [CrossRef]
- Kaye, D.M.; Shihata, W.A.; Jama, H.A.; Tsyganov, K.; Ziemann, M.; Kiriazis, H.; Horlock, D.; Vijay, A.; Giam, B.; Vinh, A.; et al. Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite-Sensing Receptors Leads to Cardiovascular Disease. Circulation 2020, 141, 1393–1403. [Google Scholar] [CrossRef]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Yan, Q.; Jia, S.; Li, D.; Yang, J. The role and mechanism of action of microbiota-derived short-chain fatty acids in neutrophils: From the activation to becoming potential biomarkers. Biomed. Pharmacother. 2023, 169, 115821. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef]
- Jian, C.; Kanerva, S.; Qadri, S.; Yki-Järvinen, H.; Salonen, A. In vitro Effects of Bacterial Exposure on Secretion of Zonulin Family Peptides and Their Detection in Human Tissue Samples. Front. Microbiol. 2022, 13, 848128. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef]
- Murray, M.T.; Nowicki, J. 157—Celiac Disease. In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2020; pp. 1206–1212.e1. [Google Scholar]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Karim, A.; Khan, J.; Qaisar, R. Statin Therapy Induces Gut Leakage and Neuromuscular Disjunction in Patients With Chronic Heart Failure. J. Cardiovasc. Pharmacol. 2023, 82, 189–195. [Google Scholar] [CrossRef]
- Perticone, M.; Gigliotti, S.; Shehaj, E.; Maio, R.; Suraci, E.; Miceli, S.; Andreozzi, F.; Matera, G.; Perticone, F. Gut Permeability and Immune-Mediated Inflammation in Heart Failure. Biomedicines 2024, 12, 1217. [Google Scholar] [CrossRef]
- Karim, A.; Muhammad, T.; Shah, I.; Khan, J.; Qaisar, R. A multistrain probiotic reduces sarcopenia by modulating Wnt signaling biomarkers in patients with chronic heart failure. J. Cardiol. 2022, 80, 449–455. [Google Scholar] [CrossRef]
- Blachier, F. Amino Acid-Derived Bacterial Metabolites in the Colorectal Luminal Fluid: Effects on Microbial Communication, Metabolism, Physiology, and Growth. Microorganisms 2023, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; La Rovere, M.T.; Corbellini, D.; Pasini, E.; Verri, M.; Barbieri, A.; Condino, A.M.; Boschi, F. Plasma Amino Acid Abnormalities in Chronic Heart Failure. Mechanisms, Potential Risks and Targets in Human Myocardium Metabolism. Nutrients 2017, 9, 1251. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef]
- Teunis, C.J.; Stroes, E.S.G.; Boekholdt, S.M.; Wareham, N.J.; Murphy, A.J.; Nieuwdorp, M.; Hazen, S.L.; Hanssen, N.M.J. Tryptophan metabolites and incident cardiovascular disease: The EPIC-Norfolk prospective population study. Atherosclerosis 2023, 387, 117344. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Ebner, N.; Springer, J.; Schefold, J.C.; Doehner, W.; Dschietzig, T.B.; Anker, S.D.; von Haehling, S. Impact of Plasma Kynurenine Level on Functional Capacity and Outcome in Heart Failure—Results From Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Circ. J. 2016, 81, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Nordrehaug, J.E.; Slettom, G.; Solvang, S.H.; Pedersen, E.K.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Nygård, O.; Giil, L.M. Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE 2020, 15, e0227365. [Google Scholar] [CrossRef]
- Razquin, C.; Ruiz-Canela, M.; Toledo, E.; Hernández-Alonso, P.; Clish, C.B.; Guasch-Ferré, M.; Li, J.; Wittenbecher, C.; Dennis, C.; Alonso-Gómez, A.; et al. Metabolomics of the tryptophan-kynurenine degradation pathway and risk of atrial fibrillation and heart failure: Potential modification effect of Mediterranean diet. Am. J. Clin. Nutr. 2021, 114, 1646–1654. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yisireyili, M.; Goto, S.; Cheng, X.W.; Nakayama, T.; Matsushita, T.; Niwa, T.; Murohara, T.; Takeshita, K. Indoxyl Sulfate Activates NLRP3 Inflammasome to Induce Cardiac Contractile Dysfunction Accompanied by Myocardial Fibrosis and Hypertrophy. Cardiovasc. Toxicol. 2022, 22, 365–377. [Google Scholar] [CrossRef]
- Kouvari, M.; Chrysohoou, C.; Aggelopoulos, P.; Tsiamis, E.; Tsioufis, K.; Pitsavos, C.; Tousoulis, D. Mediterranean diet and prognosis of first-diagnosed Acute Coronary Syndrome patients according to heart failure phenotype: Hellenic Heart Failure Study. Eur. J. Clin. Nutr. 2017, 71, 1321–1328. [Google Scholar] [CrossRef]
- Moludi, J.; Saiedi, S.; Ebrahimi, B.; Alizadeh, M.; Khajebishak, Y.; Ghadimi, S.S. Probiotics Supplementation on Cardiac Remodeling Following Myocardial Infarction: A Single-Center Double-Blind Clinical Study. J. Cardiovasc. Transl. Res. 2021, 14, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Krieg, S.; Gaensbacher, J.; Doege, C.; Krieg, A.; Luedde, T.; Luedde, M.; Roderburg, C.; Kostev, K. The Association between Antibiotic Use and the Incidence of Heart Failure: A Retrospective Case-Control Study of 162,188 Outpatients. Biomedicines 2023, 11, 260. [Google Scholar] [CrossRef]
- Hatahet, J.; Cook, T.M.; Bonomo, R.R.; Elshareif, N.; Gavini, C.K.; White, C.R.; Jesse, J.; Mansuy-Aubert, V.; Aubert, G. Fecal microbiome transplantation and tributyrin improves early cardiac dysfunction and modifies the BCAA metabolic pathway in a diet induced pre-HFpEF mouse model. Front. Cardiovasc. Med. 2023, 10, 1105581. [Google Scholar] [CrossRef]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef]
- Maurya, S.K.; Carley, A.N.; Maurya, C.K.; Lewandowski, E.D. Western Diet Causes Heart Failure With Reduced Ejection Fraction and Metabolic Shifts After Diastolic Dysfunction and Novel Cardiac Lipid Derangements. JACC Basic Transl. Sci. 2023, 8, 422–435. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Strengers, J.G.; den Ruijter, H.M.; Boer, J.M.A.; Asselbergs, F.W.; Verschuren, W.M.M.; van der Schouw, Y.T.; Sluijs, I. The association of the Mediterranean diet with heart failure risk in a Dutch population. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Malandish, A.; Gulati, M. The impacts of exercise interventions on inflammaging markers in overweight/obesity patients with heart failure: A systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. Heart Vasc. 2023, 47, 101234. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.Y.; Zhou, H.; Zhan, Q.; Lai, W.; Zeng, Q.; Ren, H.; Xu, D. Moderate-Intensity Exercise Affects Gut Microbiome Composition and Influences Cardiac Function in Myocardial Infarction Mice. Front. Microbiol. 2017, 8, 1687. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.C.; Moscavitch, S.D.; Faria Neto, H.C.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Komatsu, Y.; Aoyama, K.; Yoneda, M.; Ashikawa, S.; Nakano, S.; Kawai, Y.; Cui, X.; Furukawa, N.; Ikeda, K.; Nagata, K. The prebiotic fiber inulin ameliorates cardiac, adipose tissue, and hepatic pathology, but exacerbates hypertriglyceridemia in rats with metabolic syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2021, 320, H281–H295. [Google Scholar] [CrossRef]
- Jama, H.A.; Fiedler, A.; Tsyganov, K.; Nelson, E.; Horlock, D.; Nakai, M.E.; Kiriazis, H.; Johnson, C.; Du, X.-J.; Mackay, C.R.; et al. Manipulation of the gut microbiota by the use of prebiotic fibre does not override a genetic predisposition to heart failure. Sci. Rep. 2020, 10, 17919. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.Y.; Hu, X.M.; Zhang, Y.; Zhang, S.Y. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin. Med. J. 2019, 132, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Ahmad, M.; Jadayel, R.; Najjar, F.; Lagace, D.; Leenen, F.H.H. Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction. PLoS ONE 2019, 14, e0217437. [Google Scholar] [CrossRef]
- Heianza, Y.; Zheng, Y.; Ma, W.; Rimm, E.B.; Albert, C.M.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Duration and life-stage of antibiotic use and risk of cardiovascular events in women. Eur. Heart J. 2019, 40, 3838–3845. [Google Scholar] [CrossRef]
- Ko, W.K.; Lee, S.H.; Kim, S.J.; Jo, M.J.; Kumar, H.; Han, I.B.; Sohn, S. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS ONE 2017, 12, e0180673. [Google Scholar] [CrossRef]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, E.; Pozhidaeva, L.; Pitcher, D.S.; Mansfield, C.; Koh, J.H.B.; Williamson, C.; Aslanidi, O.; Gorelik, J.; Ng, F.S. Prolonged ursodeoxycholic acid administration reduces acute ischaemia-induced arrhythmias in adult rat hearts. Sci. Rep. 2020, 10, 15284. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, C.; Peng, M.; Su, Q.; Liu, R.; Guo, Z.; Chen, S.; Li, Z.; Chang, G. Glycoursodeoxycholic Acid Ameliorates Atherosclerosis and Alters Gut Microbiota in Apolipoprotein E-Deficient Mice. J. Am. Heart Assoc. 2021, 10, e019820. [Google Scholar] [CrossRef] [PubMed]
- Panagia, M.; He, H.; Baka, T.; Pimentel, D.R.; Croteau, D.; Bachschmid, M.M.; Balschi, J.A.; Colucci, W.S.; Luptak, I. Increasing mitochondrial ATP synthesis with butyrate normalizes ADP and contractile function in metabolic heart disease. NMR Biomed. 2020, 33, e4258. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Tong, Y.; Gao, H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can. J. Physiol. Pharmacol. 2020, 98, 391–399. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, C.; Wang, P.; Wei, W.; Shi, X.; Wang, P.; Yang, J.; Wang, L.; Tang, S.; Fang, Y.; et al. Cardiovascular Benefits of Empagliflozin Are Associated With Gut Microbiota and Plasma Metabolites in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1888–1896. [Google Scholar] [CrossRef]
- Gotzmann, M.; Henk, P.; Stervbo, U.; Blázquez-Navarro, A.; Mügge, A.; Babel, N.; Westhoff, T.H. Empagliflozin Reduces Interleukin-6 Levels in Patients with Heart Failure. J. Clin. Med. 2023, 12, 4458. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Khoruts, A. Fecal microbiota transplantation-early steps on a long journey ahead. Gut Microbes 2017, 8, 199–204. [Google Scholar] [CrossRef]
- Aryal, S.; Alimadadi, A.; Manandhar, I.; Joe, B.; Cheng, X. Machine Learning Strategy for Gut Microbiome-Based Diagnostic Screening of Cardiovascular Disease. Hypertension 2020, 76, 1555–1562. [Google Scholar] [CrossRef]
- Hodzic, A.; Oudah, M. Microbiome Classification for Heart Disease Detection. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; pp. 237–242. [Google Scholar]
- Erawijantari, P.P.; Kartal, E.; Liñares-Blanco, J.; Laajala, T.D.; Feldman, L.E.; FINRISK Microbiome DREAM Challenge and ML4Microbiome Communities; Carmona-Saez, P.; Shigdel, R.; Claesson, M.J.; Bertelsen, R.J.; et al. Microbiome-based risk prediction in incident heart failure: A community challenge. medRxiv 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).