The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review
Abstract
1. Introduction
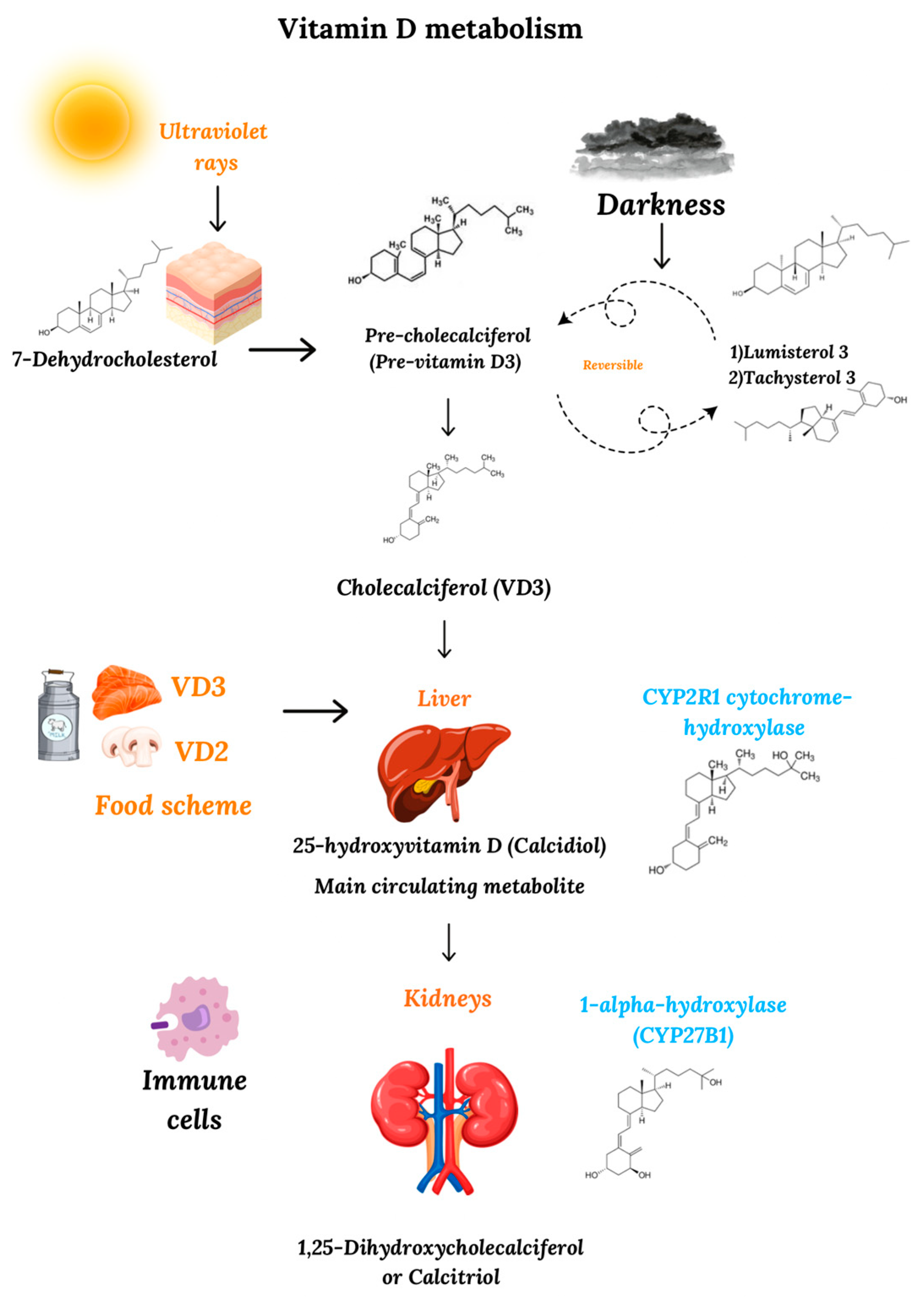
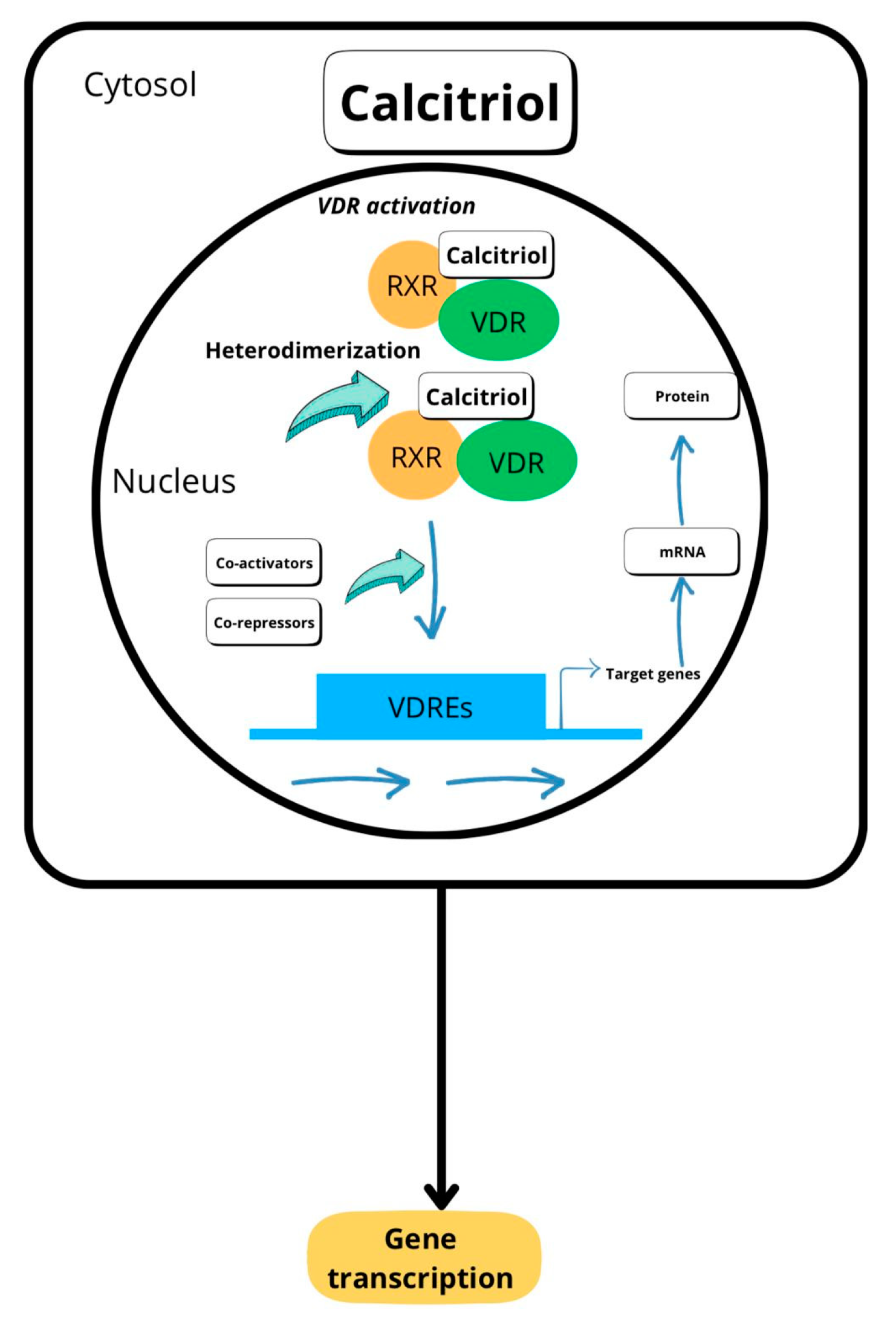
2. Metabolism and Mechanism of Action of Vitamin D
3. Current Situation Regarding Serum Levels of Vitamin D
4. Association of Vitamin D with Influenza and COVID-19
4.1. Effects of Vitamin D on the Prevention of Influenza Virus and SARS-CoV-2 Infections
4.2. Role of Vitamin D in the Prevention of Complications Caused by the Influenza Virus and SARS-CoV-2
5. Association of Vitamin D with Dengue
5.1. Role of Vitamin D in the Prevention of Dengue Infection
5.2. Role of Vitamin D in the Prevention of Complications Caused by Dengue
6. Future Research
6.1. Definition of Vitamin D Reference Values for a Healthy Immune System
6.2. Role of Vitamin D in Emerging Infectious Diseases
6.3. Long-Term Effects of Vitamin D Deficiency on Immune Function
6.4. Effect of Vitamin D on Vaccines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlberg, C. Vitamin D in the context of evolution. Nutrients 2022, 14, 3018. [Google Scholar] [CrossRef] [PubMed]
- Jacome, A. Calciferoles: Las hormonas del momento. Rev. Colomb. Endocrinol Diabetes Metab. 2019, 6, 318–324. [Google Scholar] [CrossRef]
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J. Biol. Chem. 1922, 53, 293–298. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes. Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Penna, G.; Amuchastegui, S.; Giarratana, N. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J. Immunol. 2007, 178, 145–153. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B. Cod-liver oil in phthisis. Lond. J. Med. 1849, 1, 1–18. [Google Scholar] [CrossRef]
- Shin, D.M.; Jo, E.K. Antimicrobial peptides in innate immunity against mycobacteria. Immune Netw. 2011, 11, 245–252. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Mirzaei, K.; Keshavarz, S.A.; Ansar, H.; Saboori, S.; Tootee, A. Evidences of dual role of vitamin D through cellular energy homeostasis and inflammation pathway in risk of cancer in obese subjects. Minerva Med. 2013, 104, 295–307. [Google Scholar] [PubMed]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Del Campo, J.A.; Ranchal, I.; Lampe, E.; Romero-Gomez, M. Association between vitamin D and hepatitis C virus infection: A meta-analysis. World J. Gastroenterol. 2013, 19, 5917–5924. [Google Scholar] [CrossRef]
- Papagni, R.; Pellegrino, C.; Di Gennaro, F.; Patti, G.; Ricciardi, A.; Novara, R.; Cotugno, S. Impact of vitamin D in prophylaxis and treatment in tuberculosis patients. Int. J. Mol. Sci. 2022, 23, 3860. [Google Scholar] [CrossRef]
- Pitman, M.C.; Meagher, N.; Price, D.J.; Rhodes, A.; Chang, J.J.; Scher, B.; Allan, B.; Street, A.; McMahon, J.H.; Rasmussen, T.A.; et al. Effect of high dose vitamin D(3) on the HIV-1 reservoir: A pilot randomised controlled trial. J. Virus Erad. 2023, 9, 100345. [Google Scholar] [CrossRef]
- Giraldo, D.M.; Cardona, A.; Urcuqui-Inchima, S. High-dose of vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: An exploratory study. Clin. Chim. Acta 2018, 478, 140–151. [Google Scholar] [CrossRef]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does vitamin D supplementation reduce COVID-19 severity? A systematic review. QJM 2022, 25, 665–672. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, X.; Gu, L.; Zhan, Y.; Chen, L.; Li, X. Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials. Front. Nutr. 2022, 7, 799709. [Google Scholar] [CrossRef]
- Secretaría de Salud, Gobierno de México. Panorama Epidemiológico de Dengue 2024. Available online: https://www.gob.mx/salud/documentos/panorama-epidemiologico-de-dengue-2024 (accessed on 10 January 2025).
- Malavige, G.N.; Jeewandara, C.; Ogg, G.S. Dengue and COVID-19: Two sides of the same coin. J. Biomed. Sci. 2022, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 20 November 2024).
- Dietary Guidelines for Americans. Food Sources of Vitamin D. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials/food-sources-select-nutrients/food-sources (accessed on 20 November 2024).
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Fronczek, M.; Strzelczyk, J.K.; Biernacki, K.; Salatino, S.; Osadnik, T.; Ostrowska, Z. New Variants of the Cytochrome P450 2R1 (CYP2R1) Gene in Individuals with Severe Vitamin D-Activating Enzyme 25(OH)D Deficiency. Biomolecules 2021, 11, 1867. [Google Scholar] [CrossRef]
- Guo, Y.D.; Strugnell, S.; Back, D.W.; Jones, G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc. Natl. Acad Sci. USA 1993, 90, 8668–8672. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin D3-1α-Hydroxylase. J. Clin. Endocrinol. Metab. 2001, 1, 888–894. [Google Scholar]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2018, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Boullion, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D-3-1 alpha-hydroxylase in human monocytes. J. Bone Min. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Seuter, J.K.; Virtanen, T.; Nurmi, J.; Pihlajamäki, J.; Voutilainen, T.P.; Tuomainen, A.; Neme, C.; Carlberg, C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017, 174, 314–321. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Food Nutr. 2023, 17, 1070808. [Google Scholar] [CrossRef]
- Clark, P.; Vivanco-Muñoz, N.; Piña, J.T.; Rivas-Ruiz, R.; Huitrón, G.; Chico-Barba, G.; Reza-Albarrán, A.A. High prevalence of hypovitaminosis D in Mexicans aged 14 years and older and its correlation with parathyroid hormone. Arch. Osteoporos. 2015, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Santos Araújo, E.P.D.; Queiroz, D.J.M.; Neves, J.P.R.; Lacerda, L.M.; Gonçalves, M.D.C.R.; Carvalho, A.T. Prevalence of hypovitaminosis D and associated factors in adolescent students of a capital of northeastern Brazil. Nutr. Hosp. 2017, 34, 1416–1423. [Google Scholar] [CrossRef]
- Kamboj, P.; Dwivedi, S.; Toteja, G.S. Prevalence of hypovitaminosis D in India & way forward. Indian J. Med. Res. 2018, 148, 548–556. [Google Scholar]
- Martínez-Torres, J.; Barajas-Lizarazo, M.A.; Cárdenas-Malpica, P.A.; Escobar-Velásquez, K.D.; Carvajal-Suárez, L.S.; Moreno-Bayona, J.A. Prevalencia de la deficiencia e insuficiencia de vitamina D y factores asociados en mujeres colombianas en 2015. Nutr. Hosp. 2022, 39, 843–851. [Google Scholar]
- Angeles-Agdeppa, I.; Perlas, L.A.; Capanzana, M.V. Vitamin D status of Filipino adults: Evidence from the 8th National Nutrition Survey 2013. Malays. J. Nutr. 2018, 24, 395–406. [Google Scholar]
- Gallego-González, D.; Mejía-Mesa, S.; Martínez-Sánchez, L.M.; Rendón-Diez, M. Hipovitaminosis D: Una Visión Desde la Clínica y la biología molecular. Rev. Médicas UIS 2017, 30, 45–56. [Google Scholar] [CrossRef]
- CDC. Preliminary Estimated Flu Disease Burden 2023–2024 Flu Season [Internet]. Flu Burden. 2025 [cited 2025 Mar 4]. Available online: https://espanol.cdc.gov/flu-burden/php/data-vis/2023-2024.html (accessed on 23 November 2024).
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Epidemiological Update—24 December 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---24-december-2024 (accessed on 24 December 2024).
- Laaksi, I.; Ruohola, J.P.; Mattila, V.; Auvinen, A.; Ylikomi, T.; Pihlajamäki, H. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish men. J. Infect. Dis. 2010, 202, 809–814. [Google Scholar] [CrossRef]
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 124–130. [Google Scholar] [CrossRef]
- GrassrootsHealth Nutrient Research Institute. Scientists’ call to D*action for Public Health. Available online: https://www.grassrootshealth.net/project/our-scientists/ (accessed on 21 November 2024).
- Lau, F.H.; Majumder, R.; Torabi, R.; Saeg, F.; Hoffman, R.; Cirillo, J.D.; Greiffeistein, P. Vitamin D Insufficiency is Prevalent in Severe COVID-19. BMJ Yale. Available online: https://www.medrxiv.org/content/10.1101/2020.04.24.20075838v1 (accessed on 20 November 2024).
- Ye, K.; Tang, F.; Liao, X.; Shaw, B.A.; Deng, M.; Huang, G.; Yang, J.; Fang, X.; Lin, S.; Yan, J. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?—A Case-Control Study. JANA 2020, 40, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. Vitamin D Supplements Don’t Reduce COVID-19 Risk. JAMA 2022, 328, 1581. [Google Scholar] [CrossRef]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; Santacruz-Tinoco, C.E.; Martínez-Miguel, B.; et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Bobbio, N.; Schinca, E.; Giribone, L.; Cristina, M.L. Preventive Vitamin D Supplementation and Risk for COVID-19 Infection: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 679. [Google Scholar] [CrossRef] [PubMed]
- Sif, H.; Monick, M.M.; Hinde, S.H.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar]
- Ferder, M.; Inserra, F.; Manucha, W.; Ferder, L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am. J. Physiol. Cell Physiol. 2013, 304, C1027–C1039. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- 58 Wang, C.; Wang, S.; Li, D.; Chen, P.; Han, S.; Zhao, G.; Chen, Y.; Zhao, J.; Xiong, J.; Qiu, J.; et al. Human Cathelicidin Inhibits SARS-CoV-2 Infection: Killing Two Birds with One Stone. ACS Infect Dis. 2021, 7, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-U.; Jeong, Y.-J.; Lee, P.; Lee, M.-S.; Park, J.-H.; Kim, Y.-S.; Kim, D.-J. Extracellular Nucleoprotein Exacerbates Influenza virus Pathogenesis by Activating Toll-like Receptor 4 and the NLRP3 Inflammasome. Cell Mol. Immunol. 2022, 19, 715–725. Available online: https://www.nature.com/articles/s41423-022-00862-5 (accessed on 15 December 2024). [CrossRef]
- Liu, Y.; Chen, H.; Sun, Y.; Chen, F. Antiviral role of Toll-like receptors and cytokines against the new 2009 H1N1 virus infection. Mol. Biol. Rep. 2012, 39, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Horova, H.; Landova, B.; Hodek, J.; Chalupsky, K.; Krafcikova, P.; Chalupska, D.; Duchoslav, V.; Weber, J.; Boura, E.; Klima, M. Localization of SARS-CoV-2 Capping Enzymes Revealed by an Antibody against the nsp10 Subunit. Viruses 2021, 13, 1487. [Google Scholar] [CrossRef]
- Li, Y.; Renner, D.M.; Comar, C.E.; Whelan, J.N.; Reyes, H.M.; Cardenas-Diaz, F.L.; Truitt, R.; Tan, L.H.; Dong, B.; Alysandratos, K.D.; et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2022643118. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. SARS-CoV-2 takes its Toll. Nat. Immunol. 2021, 22, 801–802. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J. Steroid. Biochem. Mol. Biol. 2010, 121, 234–238. [Google Scholar] [CrossRef]
- Kazemi, A.M.; Nosratabadi, R.; Asadikaram, G. Vitamin D and toll like receptors. Life Sci. 2018, 197, 123–130. [Google Scholar]
- Lang, P.O.; Samaras, D. Aging adults and seasonal influenza: Does the vitamin D status (H)arm the body? J. Aging Res. 2011, 2012, 806198. [Google Scholar] [CrossRef]
- Li, S.M.; Ouyang, L.H.; Zhou, D.G. Effects of vitamin D3 on expression of defensins, Toll-like receptors, and vitamin D receptor in liver, kidney, and spleen of Silky Fowl. Czech. J. Anim. Sci. 2013, 58, 1–7. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Recent Insights into the Molecular Mechanisms of the Toll-like Receptor Response to Influenza Virus Infection. Int. J. Mol. Sci. 2024, 25, 5909. [Google Scholar] [CrossRef] [PubMed]
- Alhabibi, A.M.; Hassan, A.S.; Abd Elbaky, N.M.; Eid, H.A.; Khalifa, M.A.A.A.; Wahab, M.A.; Althoqapy, A.A.; Abdou, A.E.; Zakaria, D.M.; Nassef, E.M.; et al. Impact of Toll-Like Receptor 2 and 9 Gene Polymorphisms on COVID-19: Susceptibility, Severity, and Thrombosis. J. Inflamm. Res. 2023, 16, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Saenz, R.A.; Quinlivan, M.; Elton, D.; Macrae, S.; Blunden, A.; Mumford, J.A.; Daly, J.M.; Digard, P.; Cullinane, A.; Grenfell, B.T.; et al. Dynamics of influenza virus infection and pathology. J. Virol. 2010, 84, 3974–3983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Z. Immune response in influenza virus infection and modulation of immune injury by viral neuraminidase. Virol. J. 2023, 28, 193. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The pathology of influenza virus infections. Annu. Rev. Pathol. 2008, 3, 499–522. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhao, B.; Wang, J.; Zhu, Z.; Teng, Z.; Shao, J.; Shen, J.; Gao, Y.; Yuan, Z.; et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS ONE 2011, 6, e28680. [Google Scholar] [CrossRef]
- Ling, L.; Chen, Z.; Lui, G.; Wong, C.K.; Wong, W.T.; Ng, R.W.Y.; Tso, E.Y.K.; Fung, K.S.C.; Chan, V.; Yeung, A.C.M.; et al. Longitudinal Cytokine Profile in Patients With Mild to Critical COVID-19. Front. Immunol. 2021, 12, 763292. [Google Scholar] [CrossRef]
- Pacheco-Hernández, L.M.; Ramírez-Noyola, J.A.; Gómez-García, I.A.; Ignacio-Cortés, S.; Zúñiga, J.; Choreño-Parra, J.A. Comparing the Cytokine Storms of COVID-19 and Pandemic Influenza. J. Interferon. Cytokine Res. 2022, 42, 369–392. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.A.; Belser, J.A.; Wadford, D.A.; Katz, J.M.; Tumpey, T.M. Inducible Nitric Oxide Contributes to Viral Pathogenesis Following Highly Pathogenic Influenza Virus Infection in Mice. J. Infect. Dis. 2013, 10, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Godbole, N.M.; Pawar, S.D.; Mohan, V.; Pandey, G.; Gupta, S.; Kumar, D.; Dhole, T.N.; Godbole, M.M. Calcitriol [1,25[OH]2D3] pre-and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur. J. Nutr. 2013, 52, 1405–1415. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1α,25-dihydroxyvitamin D3 has a direct effect on naïve CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef]
- Garcia, A.M.; Bishop, E.L.; Li, D.; Jeffery, L.E.; Garten, A.; Thakker, A.; Certo, M.; Mauro, C.; Tennant, D.A.; Dimeloe, S.; et al. Tolerogenic effects of 1,25-dihydroxyvitamin D on dendritic cells involve induction of fatty acid synthesis. J. Steroid. Biochem. Mol. Biol. 2021, 211, 105891. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-Dihydroxyvitamin D3 promotes high glucose-Induced M1 macrophage switching to M2 via the VDR-PPARγ signaling pathway. Biomed. Res. Int. 2015, 2015, 157834. [Google Scholar]
- Okeke, E.B.; Uzonna, J.E. The pivotal role of regulatory T cells in the regulation of innate immune cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.L.; Joosten, I.; Michels, M.; Woestenenk, R.; Preijers, F.; He, X.H.; Netea, M.G.; van der Ven, A.J.; Koenen, H.J. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology 2011, 134, 459–468. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L.; Zhang, Y.; Pu, L.; Liu, J.; Li, X.; Chen, Z.; Hao, Y.; Wang, B.; Han, J.; et al. High Level of Neutrophil Extracellular Traps Correlates With Poor Prognosis of Severe Influenza A Infection. J. Infect. Dis. 2018, 217, 428–437. [Google Scholar] [CrossRef]
- Kapoor, S.; Mihalovicová, L.; Pisareva, E.; Pastor, B.; Mirandola, A.; Roch, B.; Bryant, J.; Philip, P.; Chouaib, S.; Thierry, A.R. Association of vascular netosis with COVID-19 severity in asymptomatic and symptomatic patients. iScience 2024, 26, 109573. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir. Investig. 2020, 58, 419–420. [Google Scholar] [CrossRef]
- Handono, K.; Sidarta, Y.O.; Pradana, B.A.; Nugroho, R.A.; Hartono, I.A.; Kalim, H.; Endharti, A.T. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med. Indones. 2014, 46, 189–198. [Google Scholar]
- Basyreva, L.Y.; Shmeleva, E.V.; Ivanos, V.A.; Vakhrusheva, T.V.; Panasenko, O.M.; Ostrovsky, E.M.; Gusev, S.A.; Sergienko, V.I. The Effect of Vitamin D3 on Neutrophil Extracellular Trap Formation in High-Glucosa Conditions. Bull. Exp. Biol. Med. 2023, 176, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Kurian, S.J.; Bagchi, D.; Manu, M.K.; Saravu, K.; Unnikrishnan, M.K.; Mukhopadhyay, C.; Rao, M.; Miraj, S.S. Vitamin D in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. J. Am. Coll. Nutr. 2021, 40, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Dengue-Global Situation. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (accessed on 15 December 2024).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Iqtadar, S.; Khan, A.; Mumtaz, S.U.; Livingstone, S.; Chaudhry, M.N.A.; Raza, N.; Zahra, M.; Abaidullah, S. Vitamin D Deficiency (VDD) and Susceptibility towards Severe Dengue Fever—A Prospective Cross-Sectional Study of Hospitalized Dengue Fever Patients from Lahore, Pakistan. Trop. Med. Infect Dis. 2023, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Méndez, M.; Gómez-Pardo, A.; González-Bourguet, B.; Nicolás-Velasco, R.I. Low concentrations of serum vitamin D in patients with dengue without warning signs. Microbiol. Infect. Dis. 2020, 4, 1–5. [Google Scholar]
- Zaman, S.; Mahmud, M.R.; Khalid, M.A.; Zahid, A.; Khalid, S.; Kabir, I.; Manzoor, S.; Baqai, H.Z. Effectiveness of Vitamin D in Prevention of Dengue Haemorrhagic Fever and Dengue Shock Syndrome. JRMC 2017, 21, 205–207. [Google Scholar]
- Arboleda-Alzate, J.F.; Rodenhuis-Zybert, I.A.; Hernández, J.C.; Smit, J.M.; Urcuqui-Inchim, S. Human macrophages differentiated in the presence of vitamin D3 restrict dengue virus infection and innate responses by downregulating mannose receptor expression. PLoS Negl. Trop. Dis. 2017, 11, e0005904. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kuo, S.H.; Lin, C.Y.; Fu, P.J.; Lin, Y.S.; Yeh, T.M.; Liu, H.S. Dengue virus-induced ER stress is required for autophagy activation, viral replication, and pathogenesis both in vitro and in vivo. Sci. Rep. 2018, 8, 489. [Google Scholar] [CrossRef]
- Ceballos-Olvera, I.; Chavez-Salinas, S.; Medina, F.; Ludert, J.E.; del Angel, R.M. JNK phosphorylation, induced during dengue virus infection, is important for viral infection and requires the presence of cholesterol. Virology 2010, 396, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Patil, P.S.; Shil, P.; Seervi, M.; Kakade, M.B.; Tillu, H.; Salunke, A. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 2017, 92, 23–30. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef]
- Sánchez-Vargas, L.A.; Hernández-Flores, K.G.; Thomas-Dupont, P.; Izaguirre-Hernández, I.Y.; Sánchez-Marce, E.E.; Remes-Ruiz, R.; Fonseca-Coronado, S.; Hernández-Romano, P.A.; Flores-Collins, M.E.; Vivanco-Cid, H. Characterization of the IL-17 and CD4+ Th17 cells in the clinical course of dengue virus infections. Viruses 2020, 12, 1435. [Google Scholar] [CrossRef]
- Hou, W.; Jin, Y.H.; Kang, H.S.; Kim, B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014, 88, 8479–8489. [Google Scholar] [CrossRef]
- Guabiraba, R.; Besnard, A.-G.; Marques, R.E.; Maillet, I.; Fagundes, C.T.; Conceição, T.M.; Rust, N.M.; Charreau, S.; Paris, I.; Lecron, J.C.; et al. IL-22 modulates IL-17A production and controls inflammation and tissue damage in experimental dengue infection. Eur. J. Immunol. 2013, 43, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.F.; Kaufusi, P.H.; Nerurkar, V.R. Dengue hemorrhagic fever-associated immunomediators induced via maturation of dengue virus nonstructural 4B protein in monocytes modulate endothelial cell adhesion molecules and human microvascular endothelial cells permeability. Virology 2012, 422, 326–337. [Google Scholar] [CrossRef]
- Medin, C.L.; Fitzgerald, K.A.; Rothman, A.L. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J. Virol. 2005, 79, 11053–11061. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Medina, F.; De la Cruz Hernández, S.I.; Rosales, V.H.; Ludert, J.E.; del Angel, R.M. The 1α,25-dihydroxy-vitamin D3 reduces dengue virus infection in human myelomonocyte (U937). Antivir. Res. 2012, 94, 57–61. [Google Scholar] [CrossRef]
- Chang, S.H.; Chung, Y.; Dong, C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J. Biol. Chem. 2010, 285, 38751–38755. [Google Scholar] [CrossRef]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Damas, N.D.; Weinert, B.T.; Pus, U.; Torres-Rusillo, S.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; et al. Vitamin D Inhibits IL-22 Production Through a Repressive Vitamin D Response Element in the il22 Promoter. Front. Immunol. 2021, 12, 715059. [Google Scholar] [CrossRef]
- Martínez-Moreno, J.; Hernandez, J.C.; Urcuqui-Inchima, S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol. Cell Biochem. 2019, 464, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Suttitheptumrong, A.; Mahutchariyakul, T.; Rawarak, N. Altered moesin and actin cytoskeleton protein rearrangements affect transendothelial permeability in human endothelial cells upon dengue virus infection and tnf-α treatment. Viruses 2021, 13, 2042. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D modulates inflammatory response of DENV-2-infected macrophages by inhibiting the expression of inflammatory-liked miRNAs. Pathog. Glob. Health 2023, 117, 167–180. [Google Scholar] [CrossRef]
- Sann, S.; Heng, B.; Vo, H.T.M.; Arroyo Hornero, R.; Lay, S.; Sorn, S. Increased frequencies of highly activated regulatory T cells skewed to a T helper 1-like phenotype with reduced suppressive capacity in dengue patients. MBio 2024, 15, e0006324. [Google Scholar] [CrossRef]
- Jayaratne, H.E.; Wijeratne, D.; Fernando, S.; Kamaladasa, A.; Gomes, L.; Wijewickrama, A.; Ogg, G.S.; Malavige, G.N. Regulatory T-cells in acute dengue viral infection. Immunology 2018, 154, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Mader, J.K.; Hoeller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M.; Pieber, T.R. High-dose cholecalciferol supplementation significantly increases peripheral CD4⁺ Tregs in healthy adults without negatively affecting the frequency of other immune cells. Eur. J. Nutr. 2014, 53, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, X.; Chen, W.; Jiu, J.; Gao, C.; Gao, T. Vitamin D3 attenuates autoimmune thyroiditis by regulating Th17/Treg cell differentiation via YAP/JAK1/STAT1 axis. Immunol. Lett. 2024, 269, 106890. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galindo-Méndez, M.; Galindo-Ruiz, M.; Concheso-Venegas, M.F.; Mendoza-Molina, S.U.; Orozco-Cruz, D.; Weintraub-Benzion, E. The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review. Biomedicines 2025, 13, 927. https://doi.org/10.3390/biomedicines13040927
Galindo-Méndez M, Galindo-Ruiz M, Concheso-Venegas MF, Mendoza-Molina SU, Orozco-Cruz D, Weintraub-Benzion E. The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review. Biomedicines. 2025; 13(4):927. https://doi.org/10.3390/biomedicines13040927
Chicago/Turabian StyleGalindo-Méndez, Mario, Mario Galindo-Ruiz, María Florencia Concheso-Venegas, Sebastián Uriel Mendoza-Molina, David Orozco-Cruz, and Efraín Weintraub-Benzion. 2025. "The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review" Biomedicines 13, no. 4: 927. https://doi.org/10.3390/biomedicines13040927
APA StyleGalindo-Méndez, M., Galindo-Ruiz, M., Concheso-Venegas, M. F., Mendoza-Molina, S. U., Orozco-Cruz, D., & Weintraub-Benzion, E. (2025). The Impact of Vitamin D in the Prevention of Influenza, COVID-19, and Dengue: A Review. Biomedicines, 13(4), 927. https://doi.org/10.3390/biomedicines13040927





