Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution
Abstract
1. Introduction
2. MD and Atherosclerosis Is the Path to a New Paradigm of Approaches to the Treatment of CVDs
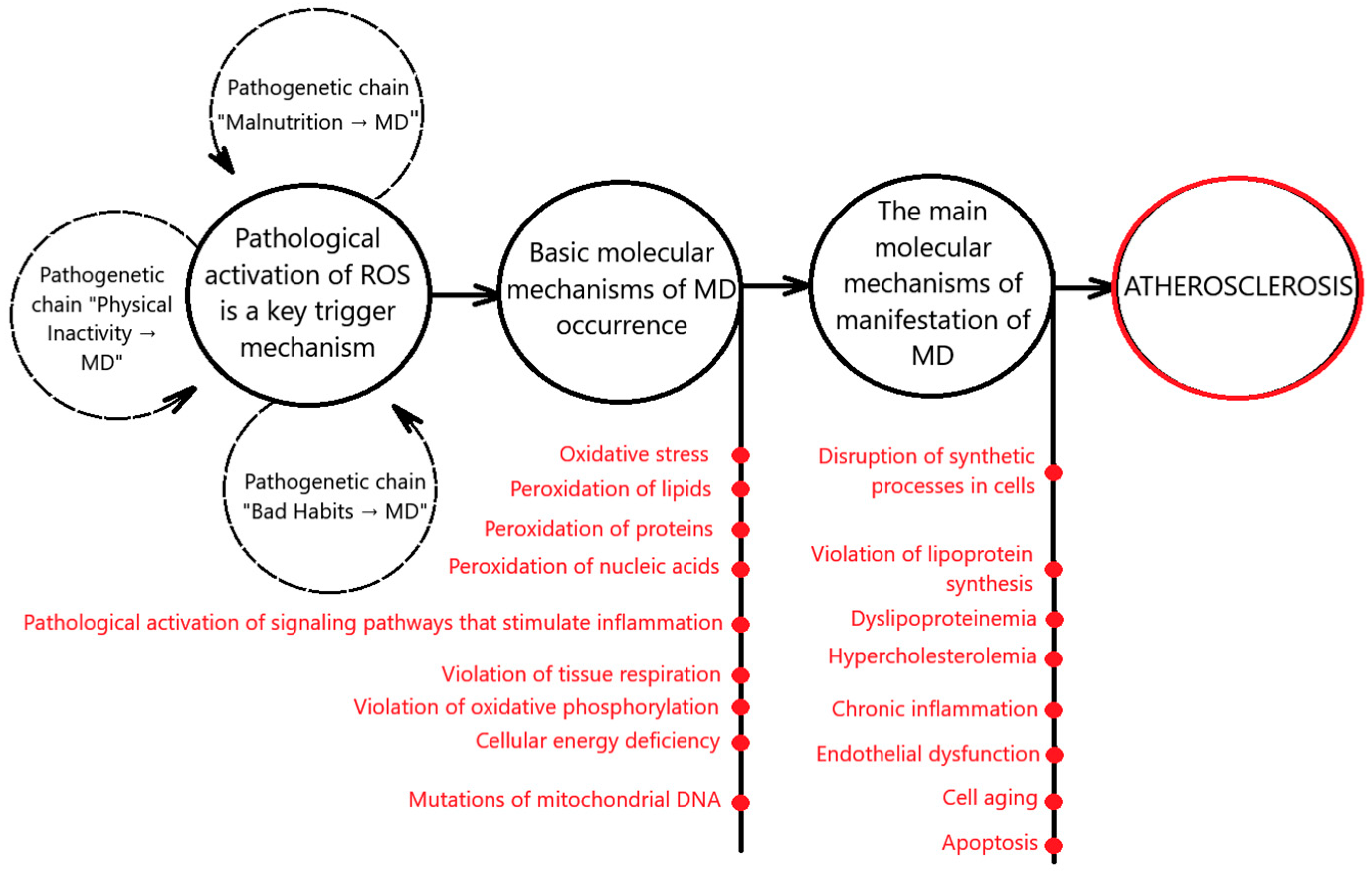
3. Pathogenetic Connections Between MD and Atherosclerosis
3.1. Pathogenetic Chain “Malnutrition → MD → Atherosclerosis”
3.1.1. The Influence of Overnutrition with the Absence of Periods of Fasting of Sufficient Duration on the Molecular Mechanisms of MD That Induce Atherosclerosis
3.1.2. Impact of Nutrient Deficiencies on the Molecular Mechanisms of MD That Induce Atherosclerosis
3.1.3. Microbiome and Molecular Mechanisms of MD That Induce Atherosclerosis
3.1.4. The Influence of Toxic and Potentially Toxic Food Components on the Molecular Mechanisms of MD That Induce Atherosclerosis
3.2. Pathogenetic Chain “Physical Inactivity → MD → Atherosclerosis”
3.3. Pathogenetic Chain “Bad Habits → MD → Atherosclerosis”
3.3.1. Pathogenetic Chain “Alcohol Abuse → MD → Atherosclerosis”
3.3.2. Pathogenetic Chain “Smoking → MD → Atherosclerosis”
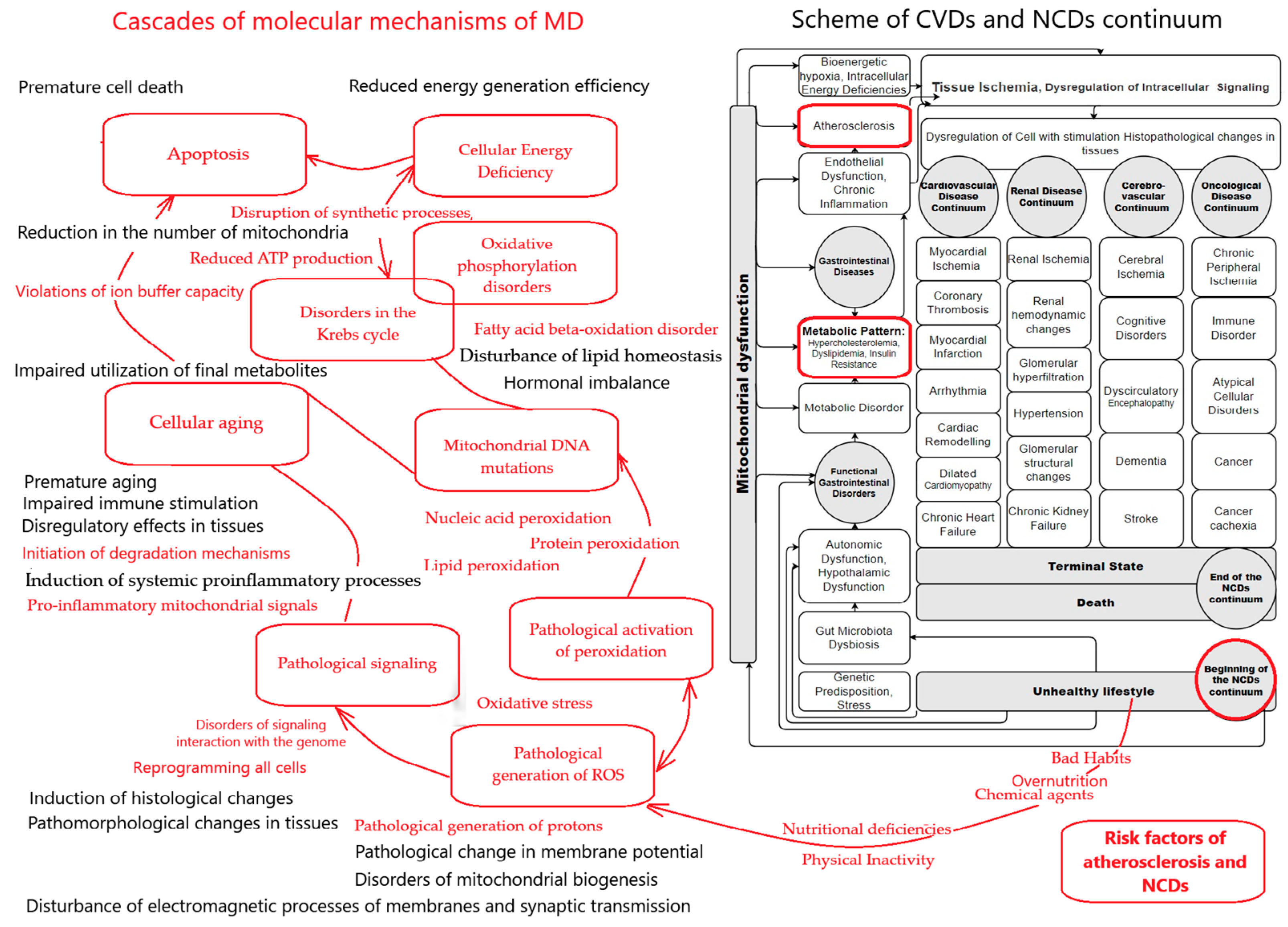
4. Molecular Mechanisms of MD and Atherosclerosis
4.1. Pathological Generation of ROS, Oxidative Stress, and Atherosclerosis
4.2. Mitochondrial Lipoprotein Synthesis and Atherosclerosis
4.3. MD and Endothelial Dysfunction
4.4. Mitochondrial DNA Mutations and Atherosclerosis
4.5. MD, Chronic Inflammation, and Atherosclerosis
4.6. MD, Cellular Energy Deficiency, and Atherosclerosis
4.7. MD, Aging, and Atherosclerosis
5. Problems of Studying the Progression of MD and Atherosclerosis During the Follow-Up of the Disease
6. Problems in Assessing MD in Atherosclerosis
6.1. Methods for Assessing Tissue Respiration of Mitochondria
6.2. Methods for Measuring Mitochondrial Enzymes
6.3. Other Methods for Measuring Mitochondrial Function
7. Discussion and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Alliance. Cardiovascular Diseases. Available online: https://ncdalliance.org/why-ncds/ncds/cardiovascular-diseases (accessed on 22 March 2024).
- Mendis, S.; Graham, I.; Narula, J. Addressing the Global Burden of Cardiovascular Diseases; Need for Scalable and Sustainable Frameworks. Glob. Heart 2022, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Dorresteijn, J.A.; Janssen, A.; Lambrinou, E.; Scherrenberg, M.; Bonnefoy-Cudraz, E.; Cobain, M.; Piepoli, M.F.; Visseren, F.L.; Dendale, P.; et al. Risk predic-tion tools in cardiovascular disease prevention: A report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collabo-ration with the Acute Car-diovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur. J. Prev. Cardiol. 2019, 26, 1534–1544. [Google Scholar] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 pop-ulation-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Van-denbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- The Lancet. Non-communicable diseases: What now? Lancet 2022, 399, 1201. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Noncommunicable Diseases. Available online: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1 (accessed on 22 March 2024).
- Hyder, A.A.; Rylance, S.; Al Saegh, A.; Feigin, V.L.; Kataria, I.; Laatikainen, T.; Lee, L.; Mahendradhata, Y.; Marten, R.; Mikkelsen, B.; et al. Strengthening evidence to inform health systems: Opportunities for the WHO and partners to accelerate progress on non-communicable diseases. BMJ Glob. Health 2023, 8, e013994. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva. 2013. Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 14 July 2024).
- Gassner, L.; Zechmeister-Koss, I.; Reinsperger, I. National Strategies for Preventing and Managing Non-communicable Diseases in Selected Countries. Front. Public Health 2022, 10, 838051. [Google Scholar] [CrossRef]
- Kostova, D.; Richter, P.; Van Vliet, G.; Mahar, M.; Moolenaar, R.L. The Role of Non-communicable Diseases in the Pursuit of Global Health Security. Health Secur. 2021, 19, 288–301. [Google Scholar] [CrossRef]
- Mikkelsen, B.; Williams, J.; Rakovac, I.; Wickramasinghe, K.; Hennis, A.; Shin, H.R.; Farmer, M.; Weber, M.; Berdzuli, N.; Bor-ges, C.; et al. Life course approach to prevention and control of non-communicable diseases. BMJ 2019, 364, l257. [Google Scholar] [CrossRef]
- Kundu, J.; Chakraborty, R. Socio-economic inequalities in burden of communicable and non-communicable diseases among older adults in India: Evidence from Longitudi-nal Ageing Study in India, 2017–2018. PLoS ONE 2023, 18, e0283385. [Google Scholar] [CrossRef]
- Andrade, C.A.S.; Mahrouseh, N.; Gabrani, J.; Charalampous, P.; Cuschieri, S.; Grad, D.A.; Unim, B.; Mechili, E.A.; Chen-Xu, J.; Devleesschauwer, B.; et al. Inequalities in the burden of non-communicable diseases across European countries: A systematic analysis of the Global Burden of Disease 2019 study. Int. J. Equity Health 2023, 22, 140. [Google Scholar] [CrossRef]
- Hambleton, I.R.; Caixeta, R.; Jeyaseelan, S.M.; Luciani, S.; Hennis, A.J.M. The rising burden of non-communicable diseases in the Americas and the impact of population aging: A secondary analysis of available data. Lancet Reg. Health Am. 2023, 21, 100483. [Google Scholar] [CrossRef] [PubMed]
- Nevoit, G.; Jarusevicius, G.; Potyazhenko, M.; Mintser, O.; Bumblyte, I.A.; Vainoras, A. Mitochondrial Dysfunction and Risk Factors for Noncommunicable Diseases: From Basic Concepts to Future Prospective. Diseases 2024, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, W.; Cheng, J.; Qu, H.; Xu, M.; Wang, L. Effects of mitochondrial dysfunction on cellular function: Role in atherosclerosis. Biomed. Pharmacother. 2024, 174, 116587. [Google Scholar] [CrossRef]
- Glanz, V.Y.; So-benin, I.A.; Grechko, A.V.; Yet, S.F.; Orekhov, A.N. The role of mitochondria in cardiovascular diseases related to atherosclerosis. Front. Biosci. Elite 2020, 12, 102–112. [Google Scholar]
- El-Hafidi, M.; Correa, F.; Zazueta, C. Mitochondrial dys-function in metabolic and cardiovascular diseases associated with cardiolipin remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165744. [Google Scholar] [CrossRef]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Sanchez-Aguilera, P.; Krycer, J.R.; Morales, P.E.; Monsalves-Alvarez, M.; Cifuentes, M.; Rothermel, B.A.; La-vandero, S. Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases? Endocr. Rev. 2020, 41, bnaa005. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Zhu, X.; Wei, Y.; Zhao, W.; Si, S.; Li, Y. A Mitochondrial Perspec-tive on Noncommunicable Diseases. Biomedicines 2023, 11, 647. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Bomer, N.; Pavez-Giani, M.G.; Grote Beverborg, N.; Cleland, J.G.F.; van Veldhuisen, D.J.; van der Meer, P. Micronutrient deficiencies in heart failure: Mitochondrial dysfunction as a common pathophysiological mechanism? J. Intern. Med. 2022, 291, 713–731. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial function as a therapeutic target in heart failure: Expert consensus document. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Bisaccia, G.; Ricci, F.; Gallina, S.; Di Baldassarre, A.; Ghinassi, B. Mitochondrial dysfunction and heart disease: Critical ap-praisal of an overlooked asso-ciation. Int. J. Mol. Sci. 2021, 22, 614. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Patial, S.; Sharma, A.; Raj, K.; Shukla, G. Atherosclerosis: Progression, risk factors, diagnosis, treatment, probiotics and synbiotics as a new prophylactic hope. Microbe 2024, 5, 100212. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Binder, C.J. Prevention and Treatment of Atherosclerosis. Improving State-of-the-Art Management and Search for Novel Targets; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Gupta, K.K.; Ali, S.; Sanghera, R.S. Pharmacological Options in Atherosclerosis: A Review of the Existing Evidence. Cardiol. Ther. 2019, 8, 5–20. [Google Scholar] [CrossRef]
- van Boven, A.J.; Jukema, J.W.; Paoletti, R. Endothelial dysfunction and dyslipidemia: Possible effects of lipid lowering and lipid modifying therapy. Pharmacol. Res. 1994, 29, 261–272. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mito-chondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef]
- Kyriazis, I.D.; Vassi, E.; Alvanou, M.; Angelakis, C.; Skaperda, Z.; Tekos, F.; Garikipati, V.N.S.; Spandidos, D.A.; Kouretas, D. The impact of diet upon mitochondrial physiology. Int. J. Mol. Med. 2022, 50, 135. [Google Scholar] [CrossRef]
- Mehrabani, S.; Bagherniya, M.; Askari, G.; Read, M.I.; Sahebkar, A. The effect of fasting or calorie restriction on mitophagy induction: A literature review. J. Cachexia Sarcopenia Muscle 2020, 11, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, S.; Wu, J.; Wang, Y. Mitochondrial metabolic dysfunction and non-alcoholic fatty liver disease: New insights from pathogenic mechanisms to clinically targeted therapy. J. Transl. Med. 2023, 21, 510. [Google Scholar] [CrossRef]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Standley, R.A.; Zhang, X.; Carnero, E.A.; Yi, F.; Cornnell, H.H.; Coen, P.M. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental Chemical Exposures and Mitochondrial Dysfunction: A Review of Recent Literature. Curr. Environ. Health Rep. 2022, 9, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leung, M.C.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B.; Herrington, D.M. Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women. Am. J. Clin. Nutr. 2004, 80, 1175–1184. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Christensen, J.J.; Arnesen, E.K.; Rundblad, A.; Telle-Hansen, V.H.; Narverud, I.; Blomhoff, R.; Bogsrud, M.P.; Retterstøl, K.; Ulven, S.M.; Holven, K.B. Dietary fat quality, plasma atherogenic lipoproteins, and atherosclerotic cardiovascular disease: An overview of the rationale for dietary recommendations for fat intake. Atherosclerosis 2024, 389, 117433. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, H.Y. Effect of Glycemic Index and Glycemic Load on Atherosclerotic Steno-sis and Stroke. J. Atheroscler. Thromb. 2020, 27, 1243–1244. [Google Scholar] [CrossRef]
- Sieri, S.; Agnoli, C.; Grioni, S.; Weiderpass, E.; Mattiello, A.; Sluijs, I.; Sanchez, M.J.; Jakobsen, M.U.; Sweeting, M.; van der Schouw, Y.T.; et al. Glycemic index, glycemic load, and risk of coronary heart disease: A pan-European cohort study. Am. J. Clin. Nutr. 2020, 112, 631–643. [Google Scholar] [CrossRef]
- Costabile, G.; Bergia, R.E.; Vitale, M.; Hjorth, T.; Campbell, W.; Landberg, R.; Riccardi, G.; Giacco, R. Effects on cardiovascular risk factors of a low- vs high-glycemic index Mediterranean diet in high cardiometabolic risk individuals: The MEDGI-Carb study. Eur. J. Clin. Nutr. 2024, 78, 384–390. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Willett, W.C.; Yusuf, S.; Hu, F.B.; Glenn, A.J.; Liu, S.; Mente, A.; Miller, V.; Bang-diwala, S.I.; Gerstein, H.C.; et al. Clinical Nutrition & Risk Factor Modification Centre Collaborators. Association of glycaemic index and glycaemic load with type 2 diabetes, cardiovascular disease, cancer, and all-cause mortality: A meta-analysis of mega cohorts of more than 100,000 participants. Lancet Diabetes Endocrinol. 2024, 12, 107–118. [Google Scholar]
- Schrepfer, E.; Scorrano, L. Mitofusins, from Mitochondria to Metabolism. Mol. Cell 2016, 61, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Killilea, A.N. Mineral requirements for mitochondrial function: A connection to redox balance and cellular differentiation. Free Radic. Biol. Med. 2022, 182, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular Nutrition and Mitochondria; Elsevier Inc.: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and Iron: Current questions. Expert. Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron defi-ciency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gale, J.R.; Reynolds, I.J.; Weiss, J.H.; Aizenman, E. The Multifaceted Roles of Zinc in Neuronal Mitochondrial Dysfunction. Biomedicines 2021, 9, 489. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Interplay between Zn2+ Homeostasis and Mitochondrial Functions in Cardiovascular Diseases and Heart Ageing. Int. J. Mol. Sci. 2022, 23, 6890. [Google Scholar] [CrossRef]
- Mehta, S.L.; Kumari, S.; Mendelev, N.; Li, P.A. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, L.T.; Semanchik, P.L.; White-Springer, S.H. Beyond antioxidants: Selenium and skeletal muscle mitochondria. Front. Vet. Sci. 2022, 9, 1011159. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiu, M.L.; Badiu, C. Selenium involvement in mitochondrial function in thyroid dis-orders. Hormones 2020, 19, 25–30. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Q.; Chen, T.; Zhou, Z.; Li, S.; Li, Z.; Zhang, D. The possible association of mitochondrial fusion and fission in copper deficiency-induced oxidative damage and mitochondrial dysfunction of the heart. J. Trace Elem. Med. Biol. 2024, 85, 127483. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cupropto-sis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. Magnesium (Mg2+) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cell Physiol. Biochem. 2023, 57 (Suppl. 1), 1–23. [Google Scholar]
- Fujita, K.; Shindo, Y.; Katsuta, Y.; Goto, M.; Hotta, K.; Oka, K. Intracellular Mg2+ protects mitochondria from oxidative stress in human keratinocytes. Commun. Biol. 2023, 6, 868. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P.; Domingo, J.C.; Castro-Marrero, J. Mitochondrial Dysfunction and Coenzyme Q10 Supplementation in Post-Viral Fatigue Syndrome: An Overview. Int. J. Mol. Sci. 2024, 25, 574. [Google Scholar] [CrossRef]
- Millichap, L.; Turton, N.; Damiani, E.; Marcheggiani, F.; Orlando, P.; Silvestri, S.; Tiano, L.; Hargreaves, I.P. The Effect of Neuronal CoQ10 Deficiency and Mitochondrial Dysfunction on a Rote-none-Induced Neuronal Cell Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 6622. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.J.; Fischman, D.A.; Hammerling, U. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprivation. FASEB J. 2008, 22, 3878–3887. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Matta Reddy, A.; Iqbal, M.; Chopra, H.; Urmi, S.; Junapudi, S.; Bibi, S.; Kumar, G.S.; Nirmala Pangi, V.; Singh, I.; Abdel-Daim, M.M. Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction. EXCLI J. 2022, 21, 967–990. [Google Scholar]
- Li, Q.; Hoppe, T. Role of amino acid metabolism in mitochondrial homeostasis. Front. Cell Dev. Biol. 2023, 11, 1127618. [Google Scholar] [CrossRef]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Modi, H.R.; Katyare, S.S. Effect of treatment with cadmium on structure-function rela-tionships in rat liver mitochondria: Studies on oxidative energy metabolism and lipid/phospholipids profiles. J. Membr. Biol. 2009, 232, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Richard, T.H.; Gohil, V.M. Mitochondrial phospholipid metabolism in health and disease. J. Cell Sci. 2023, 136, jcs260857. [Google Scholar] [CrossRef]
- Wajner, M.; Amaral, A.U. Mitochondrial dysfunction in fatty acid oxidation disorders: Insights from human and animal studies. Biosci. Rep. 2015, 36, e00281. [Google Scholar] [CrossRef]
- Guerra, I.M.S.; Ferreira, H.B.; Melo, T.; Rocha, H.; Moreira, S.; Diogo, L.; Domingues, M.R.; Moreira, A.S.P. Mitochondrial Fatty Acid β-Oxidation Disorders: From Disease to Lipidomic Studies—A Critical Review. Int. J. Mol. Sci. 2022, 23, 13933. [Google Scholar] [CrossRef]
- Martínez, A.; Velázquez, L.; Díaz, R.; Huaiquipán, R.; Pérez, I.; Muñoz, A.; Valdés, M.; Sepúlveda, N.; Paz, E.; Quiñones, J. Impact of Novel Foods on the Human Gut Microbiome: Current Status. Microorganisms 2024, 12, 1750. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.V.; Marimán, A.; Ramos, B.; Silva, M.J.; del Campo, A. Standpoints in mitochondrial dysfunction: Underlying mechanisms in search of therapeutic strategies. Mitochondrion 2022, 63, 9–22. [Google Scholar]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport. Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Nibali, L.; Henderson, B. (Eds.) The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; 544p. [Google Scholar]
- Zachos, K.A.; Gamboa, J.A.; Dewji, A.S.; Lee, J.; Brijbassi, S.; Andreazza, A.C. The interplay between mitochondria, the gut microbiome and metabolites and their therapeutic potential in primary mitochondrial disease. Front. Pharmacol. 2024, 15, 1428242. [Google Scholar] [CrossRef]
- Qiao, L.; Yang, G.; Wang, P.; Xu, C. The potential role of mitochondria in the microbiota-gut-brain axis: Implications for brain health. Pharmacol. Res. 2024, 209, 107434. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Bwiza, C.P.; Lee, C. Mitonuclear genomics and aging. Hum. Genet. 2020, 139, 381–399. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Munshi, A. Epigenetic reprogramming of mtDNA and its etiology in mitochondrial diseases. J. Physiol. Biochem. 2024, 80, 727–741. [Google Scholar] [CrossRef]
- Stoccoro, A.; Coppedè, F. Mitochondrial DNA Methylation and Human Diseases. Int. J. Mol. Sci. 2021, 22, 4594. [Google Scholar] [CrossRef]
- Falkenberg, M.; Larsson, N.G.; Gustafsson, C.M. Replication and Transcription of Human Mitochondrial DNA. Annu. Rev. Biochem. 2024, 93, 47–77. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. ROS in gastrointestinal inflammation: Rescue or Sabotage? Br. J. Pharmacol 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Yangpeng, L.; Chenw, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A revie. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota–mitochondria inter-talk: Conse-quence for microbiota–host interaction. Pathog. Dis. 2016, 74, ftv096. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neuro-degeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Mottawea, W.; Chiang, C.-K.; Méhlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef]
- Frye, G.J.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal dysfunction in autism spectrum disorder: The role of the mitochondria and the enteric microbiome. Microb. Ecol. Health Dis. 2015, 26, 27458. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A systematic framework for understanding the micro-biome in human health and disease: From basic principles to clinical translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Khan, I.M.; Nassar, N.; Chang, H.; Khan, S.; Cheng, M.; Wang, Z.; Xiang, X. The microbiota: A key regulator of health, productivity, and reproductive success in mammals. Front. Microbiol. 2024, 15, 1480811. [Google Scholar] [CrossRef]
- Coppedè, F. Mitochondrial DNA methylation and mitochondria-related epigenetics in neurodegeneration. Neural Regen. Res. 2024, 19, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Imdad, S.; Lim, W.; Kim, J.H.; Kang, C. Intertwined Relationship of Mitochondrial Metabolism, Gut Microbiome and Exercise Potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef]
- Shenderov, B.A.; Midtvedt, T. Epigenomic programing: A future way to health? Microb. Ecol. Health Dis. 2014, 25, 24145. [Google Scholar] [CrossRef] [PubMed]
- Kozjak-Pavlovic, V.; Ross, K.; Rudel, T. Import of bacterial pathogenicity factors into mitochondria. Curr. Opin. Microbiol. 2008, 11, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lobet, E.; Letesson, J.J.; Arnould, T. Mitochondria: A target for bacteria. Biochem. Pharmacol. 2015, 94, 173–185. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltran, D.; Luna, M.C.; Romo-Vaquero, M.; Garcia-Villalba, R.; Mira, A.; Espin, J.C.; Tomas-Barberian, F.A. Isolation of human Intestinal Bacteria Capable of producing the bioactive metabolite isourolithin A from Ellagic Acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- Franco-Obregon, A.; Gilbert, J.A. The Microbiome-Mitochondrion connection: Common Ancestries, Common Mechanisms, Common Goals. mSystems 2017, 2, e00018-17. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Gultekin, F.; Oner, M.E.; Savas, H.B.; Dogan, B. Food additives and microbiota. North Clin. Istanb. 2019, 7, 192–200. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M.; Wu, T.; Song, Y.; Li, Y.; Huang, X.; Lu, H.; Xu, Z.Z. Systematic evaluation of antimicrobial food preservatives on glucose metabolism and gut microbiota in healthy mice. NPJ Sci. Food 2022, 6, 42. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mito-chondrial function. Best Pr. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Hama, Y.; Noda, N.N. Mechanisms of mitochondrial reorganization. J. Biochem. 2024, 175, 167–178. [Google Scholar] [CrossRef]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Siggins, R.W.; McTernan, P.M.; Simon, L.; Souza-Smith, F.M.; Molina, P.E. Mitochon-drial Dysfunction: At the Nexus between Alcohol-Associated Immunometabolic Dysreg-ulation and Tissue Injury. Int. J. Mol. Sci. 2023, 24, 8650. [Google Scholar] [CrossRef]
- Steinberg, D.; Pearson, T.A.; Kuller, L.H. Alcohol and atherosclerosis. Ann. Intern. Med. 1991, 114, 967–976. [Google Scholar] [CrossRef]
- Mantle, D.; Preedy, V.R. Free radicals as mediators of alcohol toxicity. Advers. Drug React. Toxicol. Rev. 1999, 18, 235–252. [Google Scholar]
- French, S.W. Mechanisms of alcoholic liver injury. Can. J. Gastroenterol. 2000, 14, 327–332. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Introduction-Serial review: Alcohol, oxidative stress and cell injury. Free Radic. Biol. Med. 2001, 31, 1524–1526. [Google Scholar] [CrossRef]
- Bailey, S.M.; Pietsch, E.C.; Cunningham, C.C. Ethanol stimulates the production of reac-tive oxygen species at mitochondrial complexes I and III. Free Radic. Biol. Med. 1999, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.G.; Denton, R.M. The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. Biochim. Biophys. Acta 1990, 1018, 287–291. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Up-take. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Pagel-Langenickel, I.; Bao, J.; Pang, L.; Sack, M.N. The role of mitochondria in the pathophysi-ology of skeletal muscle insulin resistance. Endocr. Rev. 2010, 31, 25–51. [Google Scholar] [CrossRef]
- Dong, H.; Tsai, S.Y. Mitochondrial Properties in Skeletal Muscle Fiber. Cells 2023, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Nevoit, G.V. Evaluation of the clinical effectiveness of the method for determining the personalized correction of the patient’s lifestyle and new promising predictors. Ukr. Ther. J. 2021, 1, 20–25. (In Ukrainian) [Google Scholar]
- Walter, H.; Moos, D.V.; Faller, I.P.; Glavas, D.N.; Harpp, N.; Kamperi, I.; Kanara, K.; Kodukula, A.N.; Mavrakis, J.; Pernokas, M.; et al. Pathogenic mitochondrial dys-function and metabolic abnormalities. Biochem. Pharmacol. 2021, 193, 114809. [Google Scholar]
- Khan, M.S.; Butler, J. Targeting mitochondrial function in heart failure: Makes sense but will it work? JACC Basic Transl. Sci. 2019, 4, 158–160. [Google Scholar] [CrossRef]
- Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports-Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Powers, S.K. Mitochondrial Dysfunction Is a Common Denominator Linking Skeletal Muscle Wasting Due to Disease, Aging, and Prolonged Inactivity. Antioxidants 2021, 10, 588. [Google Scholar] [CrossRef]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R. The Role of Mitochondria in Mediation of Skeletal Muscle Repair. Muscles 2023, 2, 119–163. [Google Scholar] [CrossRef]
- Nevoit, G.V.; Potiazhenko, M.; Mintser, O. Systemic dependences of changes in body composition with the progression of non-communicable diseases. World Med. Biol. 2021, 3, 132–137. [Google Scholar] [CrossRef]
- Lee, J.; Cooke, J.P. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis 2011, 215, 281–283. [Google Scholar] [CrossRef]
- Prefontaine, D.; Morin, A.; Jumarie, C.; Porter, A. In vitro bioactivity of combustion products from 12 tobacco constituents. Food Chem. Toxicol. 2006, 44, 724–738. [Google Scholar] [CrossRef]
- Benowitz, N.L. Cigarette smoking and cardiovascular disease: Pathophysiology and im-plications for treatment. Prog. Cardiovasc. Dis. 2003, 46, 91–111. [Google Scholar] [CrossRef]
- Heiss, C.; Amabile, N.; Lee, A.C.; Real, W.M.; Schick, S.F.; Lao, D.; Wong, M.L.; Jahn, S.; Angeli, F.S.; Minasi, P.; et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide pro-duction. J. Am. Coll. Cardiol. 2008, 51, 1760–1771. [Google Scholar] [CrossRef]
- Flouris, A.D.; Vardavas, C.I.; Metsios, G.S.; Tsatsakis, A.M.; Koutedakis, Y. Biological evidence for the acute health effects of secondhand smoke exposure. Am. J. Physiol. 2009, 298, L3–L12. [Google Scholar] [CrossRef]
- Yang, Z.; Harrison, C.M.; Chuang, G.C.; Ballinger, S.W. The role of tobacco smoke induced mi-tochondrial damage in vascular dysfunction and atherosclerosis. Mutat. Res. 2007, 621, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.L.; Sammy, M.J.; Ballinger, S.W. Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology 2017, 391, 18–33. [Google Scholar] [CrossRef]
- van Jaarsveld, H.; Kuyl, J.M.; Alberts, D.W. Exposure of rats to low concentrations of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res. Cardiol. 1992, 87, 393–399. [Google Scholar] [CrossRef]
- Gvozdjak, J.; Gvozdjakova, A.; Kucharska, J.; Bada, V. The effect of smoking on myocardial metabolism. Czech. Med. 1987, 10, 47–53. [Google Scholar] [PubMed]
- Gvozdjakova, A.; Kucharska, J.; Gvozdjak, J. Effect of smoking on the oxidative processes of cardiomyocytes. Cardiology 1992, 81, 81–84. [Google Scholar] [PubMed]
- van Jaarsveld, H.; Kuyl, J.M.; Alberts, D.W. Antioxidant vitamin supplementation of smoke exposed rats partially protects against myocardial ischemic/reperfusion injury. Free Radic. Res. Commun. 1992, 17, 263–269. [Google Scholar] [CrossRef]
- Davis, J.; Shelton, L.; Watnabe, I.; Arnold, J. Passive smoking affects endothelium and platelets. Arch. Intern. Med. 1989, 149, 386–389. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Bada, V.; Sany, L.; Kucharska, J.; Kruty, F.; Bozek, P.; Trstanski, L.; Gvozdjak, J. Smoke cardiomyopathy: Disturbance of oxidative processes in myocardial mitochondria. Cardiovasc. Res. 1984, 18, 229–232. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Simko, F.; Kucharska, J.; Braunova, Z.; Psenek, P.; Kyselovic, J. Captopril increased mitochondrial coen-zyme Q10 level, improved respiratory chain function and energy production in the left ventricle in rabbits with smoke mitochondrial cardiomyopathy. BioFactors 1999, 10, 61–65. [Google Scholar] [CrossRef]
- Knight-Lozano, C.A.; Young, C.G.; Burow, D.L.; Hu, Z.; Uyeminami, D.; Pinkerton, K.; Ischiropou-los, H.; Ballinger, S.W. Cigarette Smoke Exposure and Hypercholesterolemia increase mito-chondrial damage in cardiovascular tissues. Circulation 2002, 105, 849–854. [Google Scholar] [CrossRef]
- Penn, A.; Chen, L.C.; Synder, C.A. Inhalation of steady-state sidestream smoke from one cigarette promotes atherosclerotic plaque development. Circulation 1994, 90, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Vayssier-Taussat, M.; Camilli, T.; Aron, Y.; Meplan, C.; Hainaut, P.; Polla, B.S.; Weksler, B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1293–H1300. [Google Scholar] [CrossRef]
- van der Toorn, M.; Slebos, D.J.; de Bruin, H.G.; Leuvenink, H.G.; Bakker, S.J.; Gans, R.O.; Koeter, G.H.; van Oosterhout, A.J.; Kauffman, H.F. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1211–L1218. [Google Scholar] [CrossRef]
- Agarwal, A.R.; Yin, F.; Cadenas, E. Metabolic shift in lung alveolar cell mitochondria follow-ing acrolein exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L764–L773. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.R.; Zhao, L.; Sancheti, H.; Sundar, I.K.; Rahman, I.; Cadenas, E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox sta-tus independent of inflammatory responses in mouse lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L889–L898. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Zarrintan, S.; Brandenburg, S.M.; Kol, A.; de Bruin, H.G.; Jafari, S.; Dijk, F.; Kalicharan, D.; Kelders, M.; Gosker, H.R.; et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 2013, 14, 97. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Kucharska, J.; Sany, L.; Gvozdjak, J. The effect of cigarette smoke on cyto-chrome-oxidase of the heart muscle. Cor. Vasa 1984, 26, 466–468. [Google Scholar] [PubMed]
- Alonso, J.R.; Cardellach, F.; Lopez, S.; Casademont, J.; Miro, O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol. 2003, 93, 142–146. [Google Scholar] [CrossRef]
- Miro, O.; Alonso, J.R.; Jarreta, D.; Casademont, J.; Urbano-Marquez, A.; Cardellach, F. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis 1999, 20, 1331–1336. [Google Scholar] [CrossRef]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L. Carbon monoxide and mitochon-dria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Bouhours-Nouet, N.; May-Panloup, P.; Coutant, R.; de Casson, F.B.; Descamps, P.; Douay, O.; Reynier, P.; Ritz, P.; Malthiery, Y.; Simard, G. Maternal smoking is associated with mitochon-drial DNA depletion and respiratory chain complex III deficiency in placenta. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E171–E177. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.M.; Pompilius, M.; Pinkerton, K.E.; Ballinger, S.W. Mitochondrial oxidative stress significantly influences atherogenic risk and cytokine-induced oxidant production. Environ. Health Perspect. 2011, 119, 676–681. [Google Scholar] [CrossRef]
- van der Toorn, M.; Rezayat, D.; Kauffman, H.F.; Bakker, S.J.; Gans, R.O.; Koeter, G.H.; Choi, A.M.; van Oosterhout, A.J.; Slebos, D.J. Lipid-soluble components in cigarette smoke induce mitochon-drial production of reactive oxygen species in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L109–L114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Knight, C.A.; Mamerow, M.M.; Vickers, K.; Penn, A.; Postlethwait, E.M.; Ballinger, S.W. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mito-chondrial damage in apolipoprotein E−/− mice fed a chow diet. Circulation 2004, 110, 3715–3720. [Google Scholar] [CrossRef]
- Westbrook, D.G.; Anderson, P.G.; Pinkerton, K.E.; Ballinger, S.W. Perinatal tobacco smoke expo-sure increases vascular oxidative stress and mitochondrial damage in non-human primates. Cardiovasc. Toxicol. 2010, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Bertuccio, M.P.; Picerno, I.; Spataro, P.; Di Pietro, A. Mitochondrial dysfunction by prooxidant vanadium: Ex vivo assessment of individual susceptibility. Environ. Toxicol. Pharmacol. 2015, 39, 93–101. [Google Scholar] [CrossRef]
- Fetterman, J.L.; Pompilius, M.; Westbrook, D.G.; Uyeminami, D.; Brown, J.; Pinkerton, K.E.; Ballinger, S.W. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE(−/−) mice. PLoS ONE 2013, 8, e66835. [Google Scholar] [CrossRef]
- Ballinger, S.W.; Bouder, T.G.; Davis, G.S.; Judice, S.A.; Nicklas, J.A.; Albertini, R.J. Mitochondrial genome damage associated with cigarette smoking. Cancer Res. 1996, 56, 5692–5697. [Google Scholar]
- Hosgood, H.D.; Liu, C.S.; Rothman, N.; Weinstein, S.J.; Bonner, M.R.; Shen, M.; Lim, U.; Vir-tamo, J.; Cheng, W.L.; Albanes, D.; et al. Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis 2010, 31, 847–849. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Bonner, M.R.; Liu, C.S.; Li, G.; Vermeulen, R.; Dosemeci, M.; Yin, S.; Lan, Q. Association between mitochondrial DNA copy number, blood cell counts, and occupa-tional benzene exposure. Environ. Mol. Mutagen. 2008, 49, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Araya, J.; Ito, S.; Kobayashi, K.; Takasaka, N.; Yoshii, Y.; Wakui, H.; Kojima, J.; Shimizu, K.; Numata, T.; et al. Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L737–L746. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, K.; Thomsen, H.K.; Astrup, P. Effects of carbon monoxide on myocardium. Ultra-structural changes in rabbits after moderate, chronic exposure. Circ. Res. 1974, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Araya, J.; Kurita, Y.; Kobayashi, K.; Takasaka, N.; Yoshida, M.; Hara, H.; Minagawa, S.; Wakui, H.; Fujii, S.; et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 2015, 11, 547–559. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Jing, Z.; Liu, D.; Yan, S.; Wang, Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: A systemic review and meta-analysis of cohort studies. Eur. J. Endocrinol. 2014, 171, R173–R182. [Google Scholar] [CrossRef]
- Coogan, P.F.; White, L.F.; Jerrett, M.; Brook, R.D.; Su, J.G.; Seto, E.; Burnett, R.; Palmer, J.R.; Rosenberg, L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation 2012, 125, 767–772. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Raaschou-Nielsen, O.; Ketzel, M.; Jensen, S.S.; Hvidberg, M.; Loft, S.; Tjonneland, A.; Overvad, K.; Sorensen, M. Diabetes incidence and long-term exposure to air pollution: A cohort study. Diabetes Care 2012, 35, 92–98. [Google Scholar] [CrossRef]
- Brook, R.D.; Cakmak, S.; Turner, M.C.; Brook, J.R.; Crouse, D.L.; Peters, P.A.; van Donkelaar, A.; Villeneuve, P.J.; Brion, O.; Jerrett, M.; et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013, 36, 3313–3320. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, R.T.; Kwong, J.C.; Villeneuve, P.J.; Goldberg, M.S.; Brook, R.D.; van Donkelaar, A.; Jerrett, M.; Martin, R.V.; Brook, J.R.; et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ. Health Perspect. 2013, 121, 804–810. [Google Scholar] [CrossRef]
- Brook, R.D.; Jerrett, M.; Brook, J.R.; Bard, R.L.; Finkelstein, M.M. The relationship between diabetes mellitus and traffic-related air pollution. J. Occup. Environ. Med. 2008, 50, 32–38. [Google Scholar] [CrossRef]
- Kramer, U.; Herder, C.; Sugiri, D.; Strassburger, K.; Schikowski, T.; Ranft, U.; Rathmann, W. Traf-fic-related air pollution and incident type 2 diabetes: Results from the SALIA cohort study. Environ. Health Perspect. 2010, 118, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Eze, I.C.; Schaffner, E.; Fischer, E.; Schikowski, T.; Adam, M.; Imboden, M.; Tsai, M.; Carballo, D.; von Eckardstein, A.; Kunzli, N.; et al. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ. Int. 2014, 70, 95–105. [Google Scholar] [CrossRef]
- Boffetta, P.; Dosemeci, M.; Gridley, G.; Bath, H.; Moradi, T.; Silverman, D. Occupational exposure to diesel engine emissions and risk of cancer in Swedish men and women. Cancer Causes Control 2001, 12, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Sorensen, M.; Hansen, J.; Loft, S.; Overvad, K.; Tjonneland, A. Air pollution from traffic and cancer incidence: A Danish cohort study. Environ. Health A Glob. Access Sci. Source 2011, 10, 67. [Google Scholar] [CrossRef]
- Soll-Johanning, H.; Bach, E.; Olsen, J.H.; Tuchsen, F. Cancer incidence in urban bus drivers and tramway employees: A retrospective cohort study. Occup. Environ. Med. 1998, 55, 594–598. [Google Scholar] [CrossRef]
- Boeglin, M.L.; Wessels, D.; Henshel, D. An investigation of the relationship between air emis-sions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ. Res. 2006, 100, 242–254. [Google Scholar] [CrossRef]
- Castano-Vinyals, G.; Cantor, K.P.; Malats, N.; Tardon, A.; Garcia-Closas, R.; Serra, C.; Carrato, A.; Rothman, N.; Vermeulen, R.; Silverman, D.; et al. Air pollution and risk of urinary bladder cancer in a case-control study in Spain. Occup. Environ. Med. 2008, 65, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kauppinen, T.; Kyyronen, P.; Heikkila, P.; Lindbohm, M.L.; Pukkala, E. Risk of esophageal, ovarian, testicular, kidney and bladder cancers and leukemia among finnish workers exposed to diesel or gasoline engine exhaust. Int. J. Cancer 2004, 111, 286–292. [Google Scholar] [CrossRef]
- Ji, J.; Granstrom, C.; Hemminki, K. Occupational risk factors for kidney cancer: A cohort study in Sweden. World J. Urol. 2005, 23, 271–278. [Google Scholar] [CrossRef]
- Kogevinas, M.; Mannetje, A.; Cordier, S.; Ranft, U.; Gonzalez, C.A.; Vineis, P.; Chang-Claude, J.; Lynge, E.; Wahrendorf, J.; Tzonou, A.; et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Control 2003, 14, 907–914. [Google Scholar] [CrossRef]
- Liu, C.C.; Tsai, S.S.; Chiu, H.F.; Wu, T.N.; Chen, C.C.; Yang, C.Y. Ambient exposure to criteria air pollutants and risk of death from bladder cancer in Taiwan. Inhal. Toxicol. 2009, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Hoover, R.N.; Mason, T.J.; Swanson, G.M. Motor exhaust-related occupations and bladder cancer. Cancer Res. 1986, 46, 2113–2116. [Google Scholar]
- Barregard, L.; Holmberg, E.; Sallsten, G. Leukaemia incidence in people living close to an oil refinery. Environ. Res. 2009, 109, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.L.; Goldberg, M.S.; Ross, N.A.; Chen, H.; Labreche, F. Postmenopausal breast cancer is associated with exposure to traffic-related air pollution in Montreal, Canada: A case-control study. Environ. Health Perspect. 2010, 118, 1578–1583. [Google Scholar] [CrossRef]
- Hystad, P.; Villeneuve, P.J.; Goldberg, M.S.; Crouse, D.L.; Johnson, K. Exposure to traffic-related air pollution and the risk of developing breast cancer among women in eight Canadian provinces: A case-control study. Environ. Int. 2015, 74, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.E.; Goldberg, M.S.; Crouse, D.L.; Ross, N.A.; Chen, H.; Valois, M.F.; Liautaud, A. Trafficrelated air pollution and prostate cancer risk: A case-control study in Montreal, Canada. Occup. Environ. Med. 2013, 70, 511–518. [Google Scholar] [CrossRef]
- Lewtas, J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res. 2007, 636, 95–133. [Google Scholar] [CrossRef]
- Jacobs, M.; Zhang, G.; Chen, S.; Mullins, B.; Bell, M.; Jin, L.; Guo, Y.; Huxley, R.; Pereira, G. The association between ambient air pollution and selected adverse pregnancy outcomes in China: A systematic review. Sci. Total Environ. 2017, 579, 1179–1192. [Google Scholar] [CrossRef]
- Šrám, R.J.; Binková, B.; Dejmek, J.; Bobak, M. Ambient Air Pollution and Pregnancy Outcomes: A Review of the Literature. Environ. Health Perspect. 2005, 113, 375–382. [Google Scholar] [CrossRef]
- Padula, A.M.; Mortimer, K.M.; Tager, I.B.; Hammond, S.K.; Lurmann, F.W.; Yang, W.; Stevenson, D.K.; Shaw, G.M. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann. Epidemiol. 2014, 24, 888–895.e4. [Google Scholar] [CrossRef]
- Qian, Z.; Liang, S.; Yang, S.; Trevathan, E.; Huang, Z.; Yang, R.; Wang, J.; Hu, K.; Zhang, Y.; Vaughn, M.; et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int. J. Hyg. Environ. Health 2016, 219, 195–203. [Google Scholar] [CrossRef]
- Ritz, B.; Yu, F.; Fruin, S.; Chapa, G.; Shaw, G.M.; Harris, J.A. Ambient air pollution and risk of birth defects in Southern California. Am. J. Epidemiol. 2002, 155, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vascul. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef]
- Hulsmans, M.; Van Dooren, E.; Holvoet, P. Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 264–276. [Google Scholar] [CrossRef]
- Shemiakova, T.; Ivanova, E.; Grechko, A.V.; Gerasimova, E.V.; Sobenin, I.A.; Orekhov, A.N. Mitochondrial Dysfunction and DNA Damage in the Context of Pathogenesis of Atherosclerosis. Biomedicines 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and endothelial function. Circ. Res. 2013, 112, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II–mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Khan, N.A.; Govindaraj, P.; Meena, A.K.; Thangaraj, K. Mitochondrial disorders: Chal-lenges in diagnosis & treatment. Indian J. Med. Res. 2015, 141, 13–26. [Google Scholar]
- Mintser, O.P.; Nevoit, G.V.; Potyazhenko, M.M. Mitochondrial dysfunction in the general continuum of non-communicable diseases from the position of systemic medicine. Part, I. Literature review and results of theoretical research. Ukranial Med. J. 2022, 1, 67–74. (In Ukrainian) [Google Scholar]
- Peng, W.; Cai, G.; Xia, Y.; Chen, J.; Wu, P.; Wang, Z.; Li, G.; Wei, D. Mitochondrial dysfunc-tion in atherosclerosis. DNA Cell Biol. 2019, 38, 597–606. [Google Scholar] [CrossRef]
- Widlansky, M.; Gutterman, D. Regulation of Endothelial Function by Mitochondrial Reac-tive Oxygen Species. Antioxid. Redox Signal. 2011, 15, 1517–1530. [Google Scholar] [CrossRef]
- Burtenshaw, D.; Kitching, M.; Redmond, E.M.; Megson, I.L.; Cahill, P.A. Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front. Cardiovasc. Med. 2019, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. Landmark 2022, 27, 105. [Google Scholar] [CrossRef]
- Lee, W.E.; Genetzakis, E.; Figtree, G.A. Novel Strategies in the Early Detection and Treat-ment of Endothelial Cell-Specific Mitochondrial Dysfunction in Coronary Artery Disease. Antioxidants 2023, 12, 1359. [Google Scholar] [CrossRef]
- Tomic Naglic, D.; Manojlovic, M.; Pejakovic, S.; Stepanovic, K.; Prodanovic Simeunovic, J. Lipoprotein(a): Role in atherosclerosis and new treatment options. Biomol. Biomed. 2023, 23, 575–583. [Google Scholar] [CrossRef]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2023, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol han-dling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. 14 January 2024. In Endotext [Internet]; Feingold, K.R., Ana-walt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com Inc.: South Dartmouth, MA, USA, 2000; Available online: https://www.binasss.sa.cr/bibliotecas/bhm/set22/3.pdf (accessed on 22 March 2024).
- Nevoit, G.; Potyazhenko, M. Clinical-pathogenetic features of the course of noncommunicable diseases depending on the degree of comorbidity, the stage of the cardiovascular continuum. Bukovyna Med. Her. 2022, 1, 13–22. (In Ukrainian) [Google Scholar] [CrossRef]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and antioxidized LDL antibodies in atherosclerosis—Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.L.; Scherer, P.E.; Lodish, H.F.; Krieger, M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994, 269, 21003–21009. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabo-lism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [PubMed]
- Kosswig, N.; Rice, S.; Daugherty, A.; Post, S.R. Class A scavenger receptor-mediated adhesion and internalization require distinct cytoplasmic domains. J. Biol. Chem. 2003, 278, 34219–34225. [Google Scholar] [CrossRef]
- Krieger, M. Scavenger receptor class B type I is a multiligand HDL receptor that in-fluences diverse physiologic systems. J. Clin. Investig. 2001, 108, 793–797. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low density lipoprotein-containing circulating immune complexes: Role in atherosclerosis and diagnostic value. BioMed Res. Int. 2014, 2014, 205697. [Google Scholar] [CrossRef]
- Cimmino, G.; Cirillo, P.; Conte, S.; Pellegrino, G.; Barra, G.; Maresca, L.; Morello, A.; Calì, G.; Loffredo, F.; De Palma, R. Oxidized low-density lipoproteins induce tissue factor expression in T-lymphocytes via activation of lectin-like oxidized low-density lipoprotein receptor-1. Cardiovasc. Res. 2020, 116, 1125–1135. [Google Scholar] [CrossRef]
- Esper, R.J.; Nordaby, R.A.; Vilariño, J.O.; Paragano, A.; Cacharrón, J.L.; Machado, R.A. Endothelial Dysfunction: A Comprehensive Appraisal. Cardiovasc. Diabetol. 2006, 5, 4. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial Dysfunction: The Early Predictor of Atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Barthelmes, J.; Nägele, M.P.; Ludovici, V.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Endothelial Dysfunction in Cardiovascular Disease and Flammer Syndrome—Similarities and Differences. EPMA J. 2017, 8, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Widder, J.D.; Fraccarollo, D.; Galuppo, P.; Hansen, J.M.; Jones, D.P.; Ertl, G.; Bauer-sachs, J. Attenuation of Angiotensin II-Induced Vascular Dysfunction and Hypertension by Overexpression of Thioredoxin-2. Hypertension 2009, 54, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, W.; Tohme, S.; Chen, M.; Fu, Y.; Tian, D.; Lotze, M.; Tang, D.; Tsung, A. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol. 2015, 63, 114–121. [Google Scholar] [CrossRef]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Hojlund, K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Baker, L.; Harrison, J.; Figg, N.; Mercer, J.; Calvert, P.; Vidal-Puig, A.; Murphy, M.; Bennett, M. Mitochondrial DNA Damage Promotes Atherosclerosis and Is Associated with Vulnerable Plaque. Lancet 2013, 381, S117. [Google Scholar] [CrossRef]
- Suzuki, T.; Nagao, A.; Suzuki, T. Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Ann. Rev. Genet. 2011, 45, 299–329. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Ambrosini, S.; Lüscher, T.; Paneni, F.; Costantino, S. Epigenetic control of mitochondrial function in the vasculature. Front. Cardiovasc. Med. 2020, 7, 28. [Google Scholar] [CrossRef]
- Sinyov, V.V.; Sazonova, M.A.; Ryzhkova, A.I.; Galitsyna, E.V.; Melnichenko, A.A.; Postnov, A.Y.; Orekhov, A.N.; Grechko, A.V.; Sobenin, I.A. Potential use of buccal epithelium for genetic diagnosis of atherosclerosis using mtDNA mutations. Vessel Plus 2017, 1, 145–150. [Google Scholar] [CrossRef][Green Version]
- Yu, E.; Calvert, P.A.; Mercer, J.R.; Harrison, J.; Baker, L.; Figg, N.L.; Kumar, S.; Wang, J.C.; Hurst, L.A.; Obaid, D.R. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation 2013, 128, 702–712. [Google Scholar] [CrossRef]
- Ibanez, B.; Vilahur, G.; Badimon, J.J. Plaque Progression and Regression in Atherothrombosis. J. Thromb. Haemost. 2007, 5, 292–299. [Google Scholar] [CrossRef]
- Markin, A.M.; Khotina, V.A.; Zabudskaya, X.G.; Bogatyreva, A.I.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. Disturbance of Mitochondrial Dynamics and Mitochondrial Therapies in Atherosclerosis. Life 2021, 11, 165. [Google Scholar] [CrossRef]
- Markin, A.M.; Sobenin, I.A.; Grechko, A.V.; Zhang, D.; Orekhov, A.N. Cellular Mechanisms of Human Atherogenesis: Focus on Chronification of Inflammation and Mitochondrial Mutations. Front. Pharmacol. 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Bogatyreva, A.I.; Gerasimova, E.V.; Kirichenko, T.V.; Markina, Y.V.; Tolstik, T.V.; Kiseleva, D.G.; Popkova, T.V.; Markin, A.M. Mitochondrial DNA copy number in patients with systemic sclerosis. Front. Mol. Biosci. 2023, 10, 1313426. [Google Scholar] [CrossRef] [PubMed]
- Tolstik, T.V.; Kirichenko, T.V.; Markin, A.M.; Bogatyreva, A.I.; Markina, Y.V.; Kiseleva, D.G.; Shaposhnikova, N.N.; Starodubova, A.V.; Orekhov, A.N. The association of TNF-alpha secretion and mtDNA copy number in CD14+ monocytes of patients with obesity and CHD. Front. Mol. Biosci. 2024, 11, 1362955. [Google Scholar] [CrossRef] [PubMed]
- Volobueva, A.; Grechko, A.; Yet, S.F.; Sobenin, I.; Orekhov, A. Changes in Mitochondrial Genome Associated with Predisposition to Atherosclerosis and Related Disease. Biomolecules 2019, 9, 377. [Google Scholar] [CrossRef]
- Bray, A.W.; Ballinger, S.W. Mitochondrial DNA mutations and cardiovascular disease. Curr. Opin. Cardiol. 2017, 32, 267–274. [Google Scholar] [CrossRef]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, Platelets and Thrombosis in Acute Ischaemic Heart Disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar]
- Farmer, J.A.; Torre-Amione, G. Atherosclerosis and inflammation. Curr. Atheroscl. Rep. 2002, 4, 92–98. [Google Scholar] [CrossRef]
- Pelisek, J.; Wendorff, H.; Wendorff, C.; Kuehnl, A.; Eckstein, H. Age-associated changes in human carotid atherosclerotic plaques. Ann. Med. 2016, 48, 541–551. [Google Scholar] [CrossRef]
- Rosenfeld, M.E.; Ylä-Herttuala, S.; Lipton, B.A.; Ord, V.A.; Witztum, J.L.; Steinberg, D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am. J. Pathol. 1992, 140, 291. [Google Scholar]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Obas, V.; Vasan, R.S. The aging heart. Clin. Sci. 2018, 132, 1367–1382. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Mitochondrial oxidative stress and dysfunction in myocardial remodeling. Cardiovasc. Res. 2009, 81, 449–456. [Google Scholar] [CrossRef]
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weng, J.; Huan, L.; Sheng, S.; Xu, F. Mitophagy in atherosclerosis: From mechanism to therapy. Front. Immunol. 2023, 14, 1165507. [Google Scholar] [CrossRef]
- Li, Y.; Berliocchi, L.; Li, Z.; Rasmussen, L.J. Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age. Aging Cell 2024, 23, e13942. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Fielder, E.; Wan, T.; Alimohammadiha, G.; Ishaq, A.; Low, E.; Weigand, B.M.; Kelly, G.; Parker, C.; Griffin, B.; Jurk, D.; et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. eLife 2022, 11, e75492. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Goldstein, D.R. Aging Impairs Mitochondrial Function and Mitophagy and Elevates Interleukin 6 Within the Cerebral Vasculature. J. Am. Heart. Assoc. 2020, 9, e017820. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Lesnefsky, E.J.; Hoppel, C.L. Oxidative phosphorylation and aging. Ageing Res. Rev. 2006, 5, 402–433. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Goodell, S.; Cortopassi, G. Analysis of oxygen consumption and mitochondrial permeability with age in mice. Mech. Ageing Dev. 1998, 101, 245–256. [Google Scholar] [CrossRef]
- Dzau, V.; Braunwald, E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: A workshop consensus statement. Am. Heart J. 1991, 121, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Antman, E.M.; Black, H.R.; Hayes, D.L.; Manson, J.E.; Plutzky, J.; Popma, J.J.; Stevenson, W. The cardiovascular disease continuum validated: Clinical evidence of improved patient outcomes: Part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006, 114, 2850–2870. [Google Scholar] [CrossRef]
- Stevenson, W. The Cardiovascular Disease Continuum Validated: Clinical Evidence of Improved Patient Outcomes. Part II: Clinical Trial Evidence (Acute Coronary Syndromes Through Renal Disease) and Future Directions. Circulation 2006, 25, 2871–2891. [Google Scholar]
- O’Rourke, M.; Safar, M.; Dzau, V. The Cardiovascular Continuum extended: Aging effects on the aorta and microvasculature. Vasc. Med. 2010, 15, 461–468. [Google Scholar] [CrossRef]
- Nerpin, E. The Kidney in Different Stages of the Cardiovascular Continuum. Digital Com-Prehensive Summaries of Uppsala Dissertations from the Faculty of Medicine Uppsala; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2013; pp. 1–72. [Google Scholar]
- Chrysant, S.G. Stopping the cardiovascular disease continuum: Focus on prevention. World J. Cardiol. 2010, 2, 43–49. [Google Scholar] [CrossRef]
- Kim, S.-A.; Park, J.B.; O’Rourke, M.F. Vasculopathy of Aging and the Revised Cardiovascular Continuum. Pulse 2015, 3, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of Mitochondrial Dysfunction and Monoamine Oxidase Contribution to Oxidative Stress in Human Diabetic Hearts. Oxidative Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef] [PubMed]
- Avram, V.F.; Merce, A.P.; Hâncu, I.M.; Bătrân, A.D.; Kennedy, G.; Rosca, M.G.; Muntean, D.M. Impairment of Mitochondrial Respiration in Metabolic Diseases: An Overview. Int. J. Mol. Sci. 2022, 23, 8852. [Google Scholar] [CrossRef]
- Long, Q.; Huang, L.; Huang, K.; Yang, Q. Assessing Mitochondrial Bioenergetics in Isolat-ed Mitochondria from Mouse Heart Tissues Using Oroboros 2k–Oxygraph. Methods Mol. Biol. 2019, 1966, 237–246. [Google Scholar]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Boutagy, N.E.; Rogers, G.W.; Pyne, E.S.; Ali, M.M.; Hulver, M.W.; Frisard, M.I. Using Isolated Mitochondria from Minimal Quantities of Mouse Skeletal Muscle for High throughput Microplate Respiratory Measurements. J. Vis. Exp. 2015, 104, e53216. [Google Scholar]
- Bhatia, S.; Thompson, E.W.; Gunter, J.H. Studying the Metabolism of Epithelial-Mesenchymal Plasticity Using the Sea-horse XFe96 Extracellular Flux Analyzer. Methods Mol. Biol. 2021, 2179, 327–340. [Google Scholar] [PubMed]
- Wei, Y.; Yang, C.; Jiang, H.; Li, Q.; Che, F.; Wan, S.; Yao, S.; Gao, F.; Zhang, T.; Wang, J.; et al. Multi-nuclear magnetic resonance spectroscopy: State of the art and future directions. Insights Imaging 2022, 13, 135. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Car-bohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less–Fit Individuals. Sport Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- de Boer, E.; Petrache, I.; Goldstein, N.M.; Olin, J.T.; Keith, R.C.; Modena, B.; Mohning, M.P.; Yunt, Z.X.; San-Millán, I.; Swigris, J.J. Decreased Fatty Oxidation and Altered Lactate Production During Exercise in Post–Acute COVID-19 Patients. Am. J. Respir. Crit. Care Med. 2021, 205, 126–129. [Google Scholar] [CrossRef]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post–Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Benfatto, M.; Pace, E.; Davoli, I.; Francini, R.; De Matteis, F.; Scordo, A.; Clozza, A.; De Paolis, L.; Curceanu, C.; Grigo-lini, P. Biophotons: New Experimental Data and Analysis. Entropy 2023, 25, 1431. [Google Scholar] [CrossRef]
- Palmeira, C.M.; Moreno, A.J. Mitochondrial Bioenergetics: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; pp. 25–58. [Google Scholar]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Watanabe, R.; Hilhorst, M.; Zhang, H.; Zeisbrich, M.; Berry, G.J.; Wallis, B.B.; Harrison, D.G.; Giacomini, J.C.; Goronzy, J.J.; Weyand, C.M. Glucose Metabolism Controls Disease-Specific Signatures of Macrophage Effector Functions. JCI Insight 2018, 3, e123047. [Google Scholar] [CrossRef] [PubMed]
- Owada, T.; Yamauchi, H.; Saitoh, S.; Miura, S.; Machii, H.; Takeishi, Y. Resolution of Mitochondrial Oxidant Stress Improves Aged-Cardiovascular Performance. Coron. Artery Dis. 2017, 28, 33. [Google Scholar] [CrossRef]
- Shchepinova, M.M.; Cairns, A.G.; Prime, T.A.; Logan, A.; James, A.M.; Hall, A.R.; Vidoni, S.; Arndt, S.; Caldwell, S.T.; Prag, H.A.; et al. MitoNeoD: A Mitochondria-Targeted Superoxide Probe. Cell Chem. Biol. 2017, 24, 1285–1298.e12. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W.; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure with Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e003190. [Google Scholar] [CrossRef] [PubMed]
- Nevoit, G.; Bumblyte, I.A.; Potyazhenko, M.; Minser, O.; Vainoras, A. Modern biophysical view of electromagnetic processes of the phenomenon of life of living biological systems as a promising basis for the development of complex medi-cine: The role of biophotons. J. Complex. Health Sci. 2023, 6, 1–15. [Google Scholar] [CrossRef]
- Popp, F.A. Properties of biophotons and their theoretical implications. Indian J. Exp. Biol. 2003, 41, 391–402. [Google Scholar]
- Du, J.; Deng, T.; Cao, B.; Wang, Z.; Yang, M.; Han, J. The application and trend of ultra-weak photon emission in biol-ogy and medicine. Front. Chem. 2023, 11, 1140128. [Google Scholar] [CrossRef]
- Zapata, F.; Pastor-Ruiz, V.; Ortega-Ojeda, F.; Montalvo, G.; Ruiz-Zolle, A.V.; García-Ruiz, C. Human ultra-weak pho-ton emission as non-invasive spectroscopic tool for diagnosis of internal states—A review. J. Photochem. Photobiol. B 2021, 216, 112141. [Google Scholar] [CrossRef]
- Yang, M.; Ding, W.; Liu, Y.; Fan, H.; Bajpai, R.P.; Fu, J.; Pang, J.; Zhao, X.; Han, J. Ultra-weak photon emission in healthy subjects and patients with type 2 diabetes: Evidence for a non-invasive diagnostic tool. Photochem. Photobiol. Sci. 2017, 16, 736–743. [Google Scholar] [CrossRef]
- Nevoit, G.; Filyunova, O.; Kitura, O.; Mintser, O.; Potyazenko, M.; Bumblyte, I.A.; Vainoras, A. Biophotonics and reflexology: Conceptualization of the role of biophotonic signaling. Fitoter. Chasopys Phytother. J. 2024, 3, 62–78. [Google Scholar] [CrossRef]
- Nevoit, G.; Bumblyte, I.A.; Korpan, A.; Minser, O.; Potyazhenko, M.; Iliev, M.T.; Vainoras, A.; Ignatov, I. The biophoton emission in biotechnological research—Part 1. Ukr. J. Phys. 2024, 3, 190–206. [Google Scholar] [CrossRef]
- Nevoit, G.; Minser, O.; Potiazhenko, M.; Babintseva, L. Electro-photonic emission analysis in functionally health re-spondents and patients with non-communicable diseases. Wiad. Lek. 2021, 6, 1439–1444. [Google Scholar] [CrossRef]
- Nevoit, G. Evaluation of Electro-Photonic Emission Analysis indicators in patients with Noncommunicable Diseases Ishemic Heart Disease. Med. Ecol. Probl. 2021, 251–252, 16–21. [Google Scholar]
- Hirano, M.; Emmanuele, V.; Quinzii, C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018, 62, 467–481. [Google Scholar]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Nevoit, G.; Jarusevicius, G.; Filyunova, O.; Danylchenko, S.; Potyazhenko, M.; Mintser, O.; Bumblyte, I.A.; Vainoras, A. Magnetoelectrochemical theory of metabolism: Electromagnetic communication of cells and the role of the extracellular matrix. Biol. J 2025, 1. accepted for publication. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevoit, G.; Jarusevicius, G.; Potyazhenko, M.; Mintser, O.; Bumblyte, I.A.; Vainoras, A. Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution. Biomedicines 2025, 13, 963. https://doi.org/10.3390/biomedicines13040963
Nevoit G, Jarusevicius G, Potyazhenko M, Mintser O, Bumblyte IA, Vainoras A. Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution. Biomedicines. 2025; 13(4):963. https://doi.org/10.3390/biomedicines13040963
Chicago/Turabian StyleNevoit, Ganna, Gediminas Jarusevicius, Maksim Potyazhenko, Ozar Mintser, Inga Arune Bumblyte, and Alfonsas Vainoras. 2025. "Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution" Biomedicines 13, no. 4: 963. https://doi.org/10.3390/biomedicines13040963
APA StyleNevoit, G., Jarusevicius, G., Potyazhenko, M., Mintser, O., Bumblyte, I. A., & Vainoras, A. (2025). Mitochondrial Dysfunction and Atherosclerosis: The Problem and the Search for Its Solution. Biomedicines, 13(4), 963. https://doi.org/10.3390/biomedicines13040963







