A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cytokine Profile and Analysis
2.3. Statistical Analysis
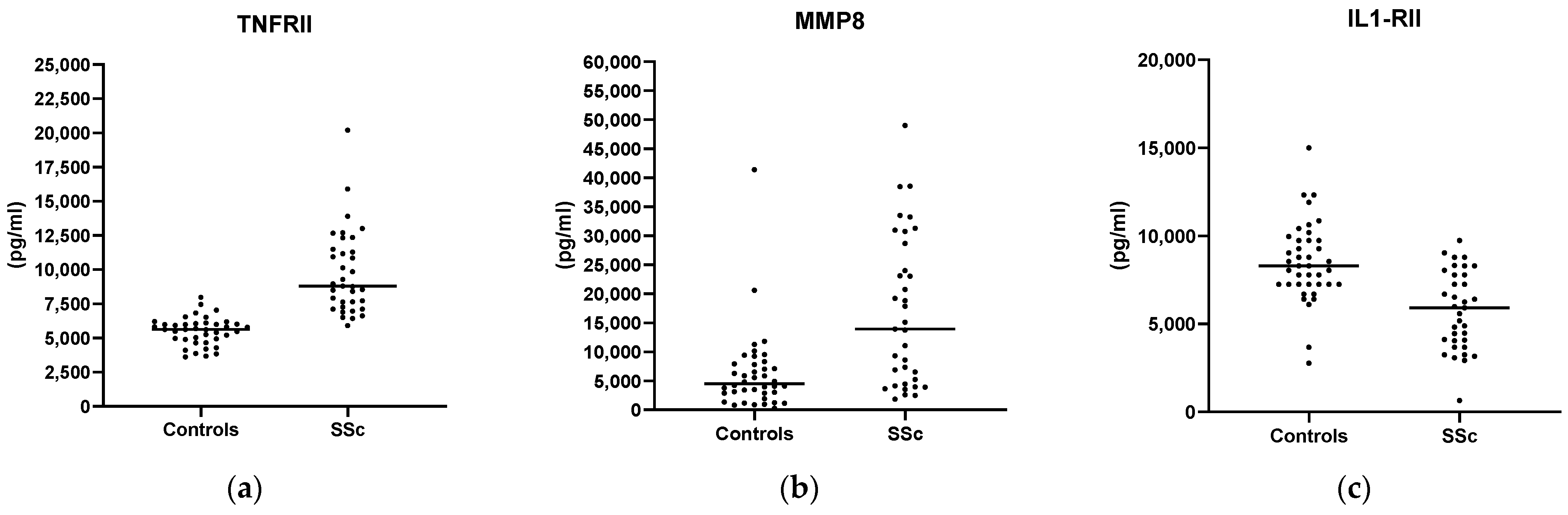
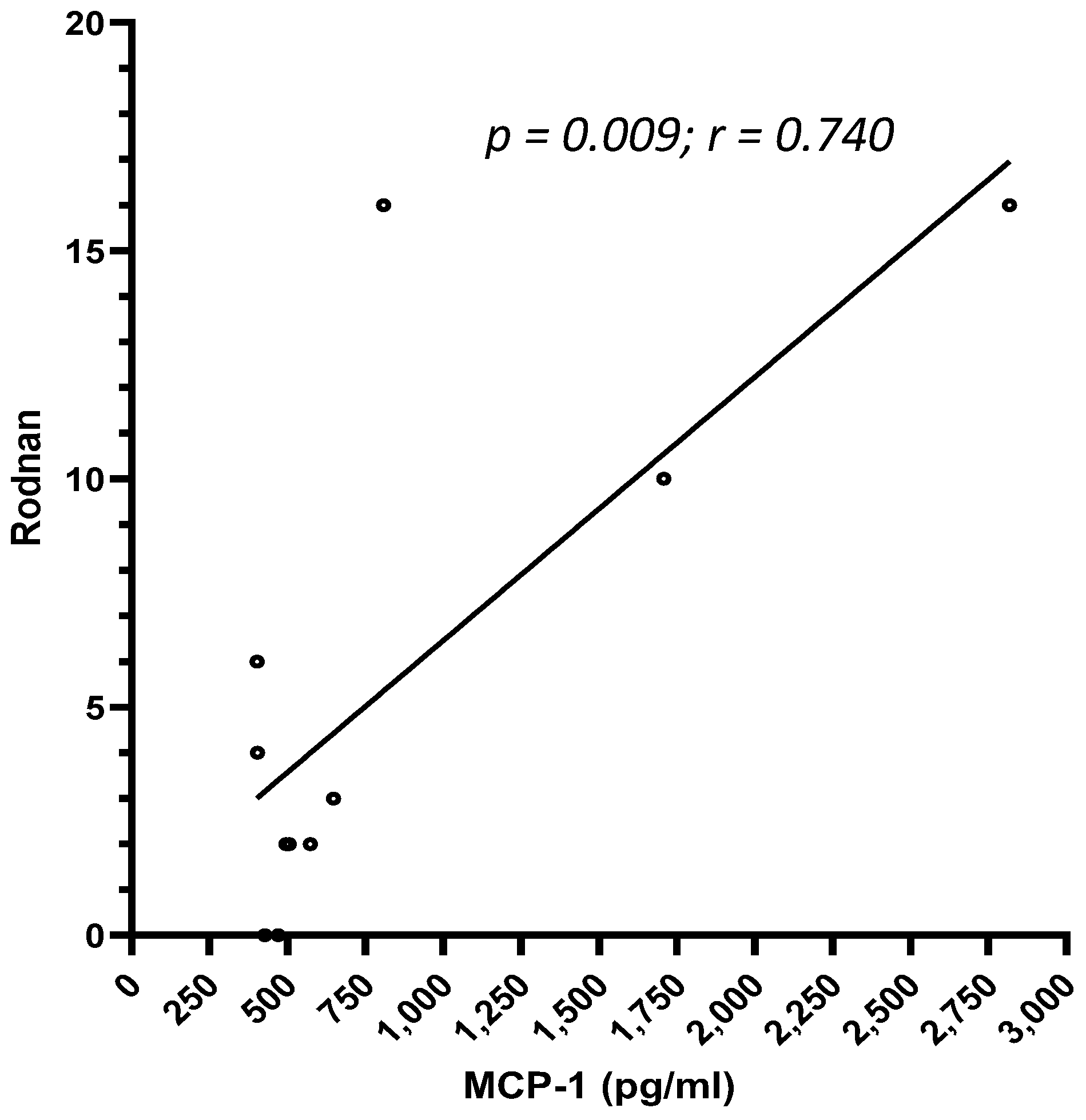
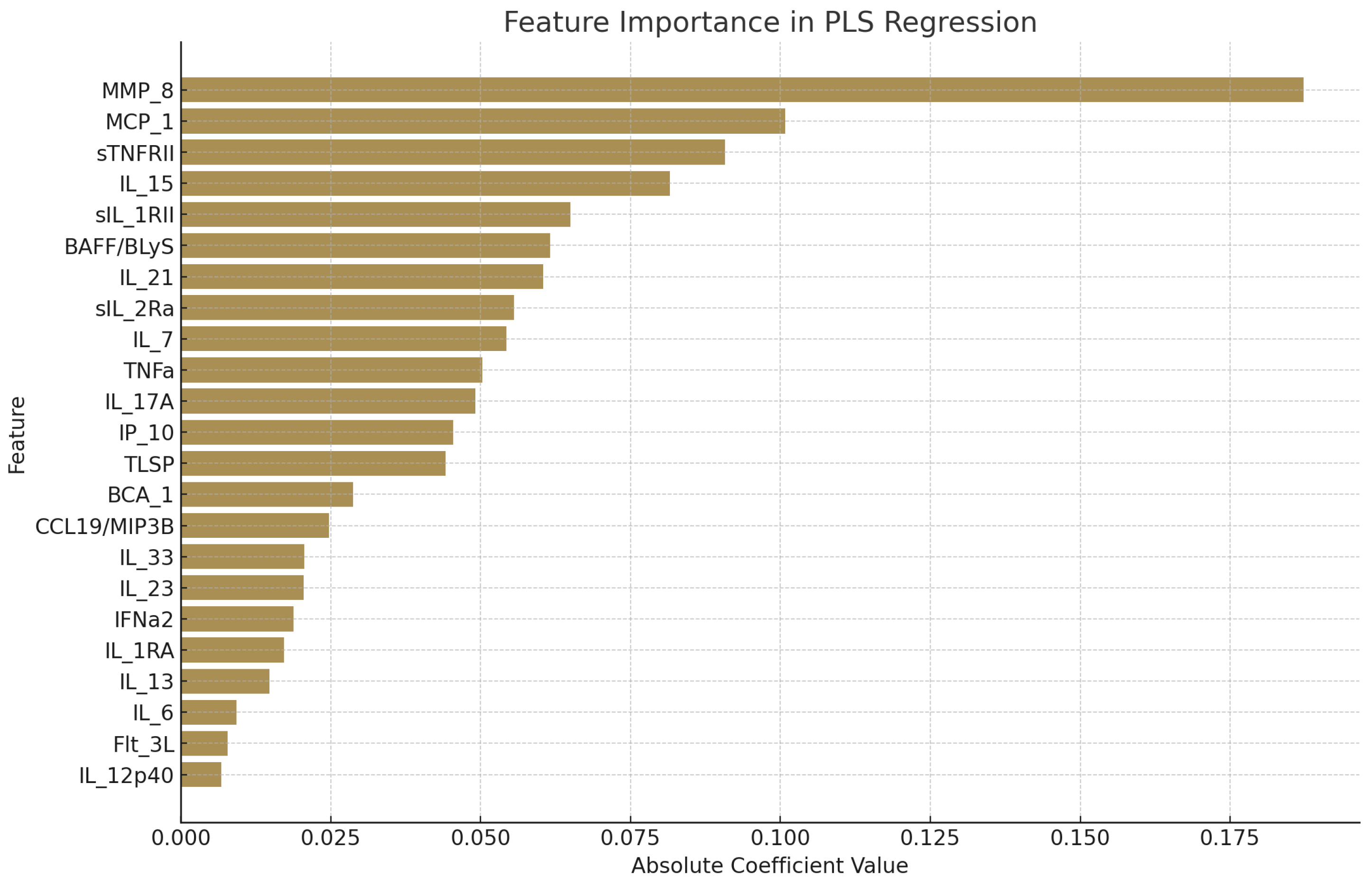
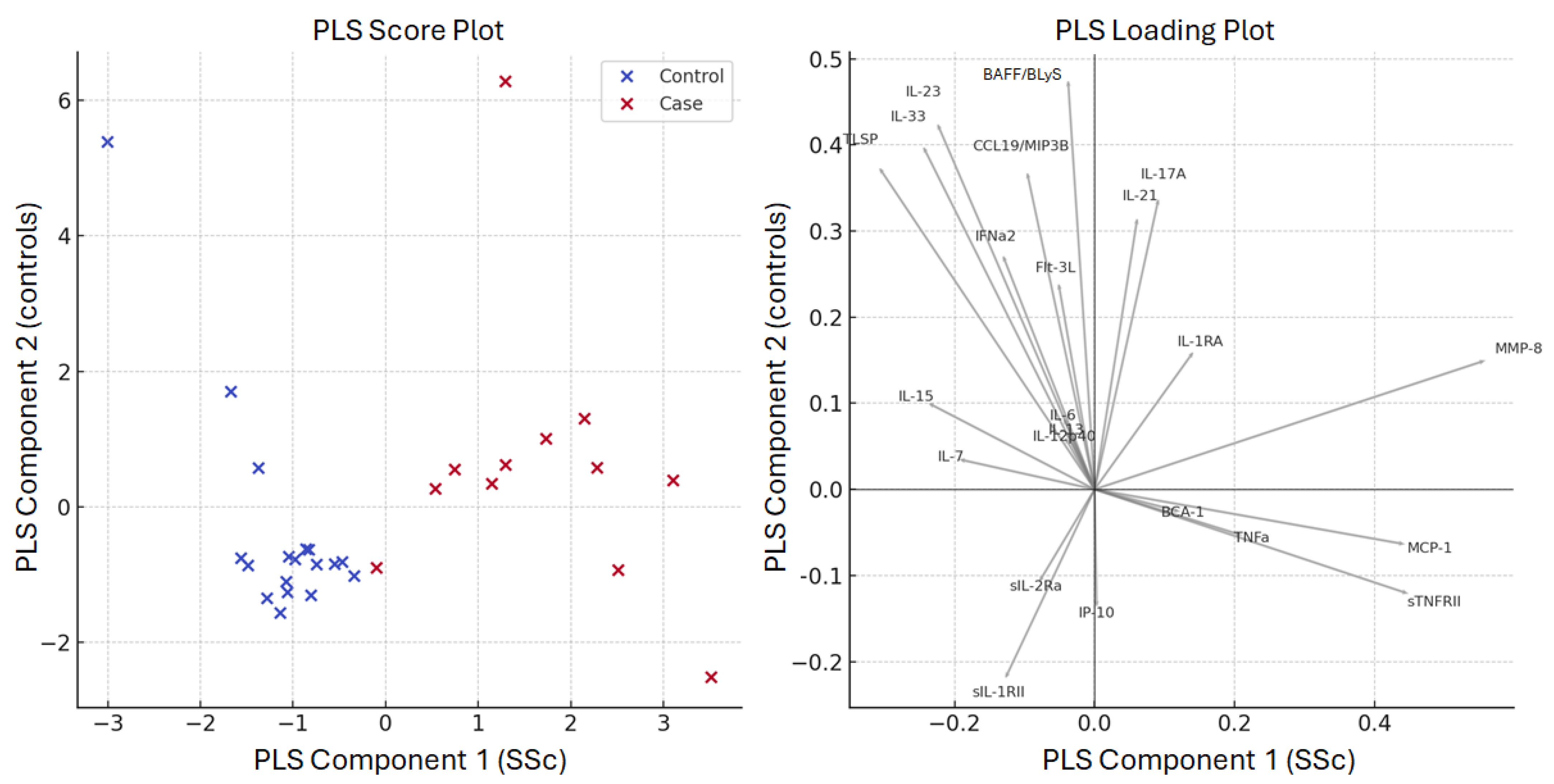
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| SSc | Systemic sclerosis |
| CCL2/MCP-1 | Monocyte chemotactic protein 1 |
| IP-10/CXCL10 | Interferon (IFN)-γ-induced protein-10 |
| Flt3 Ligand | FMS-like tyrosine kinase 3 |
| IFNa2 | Interferon Alpha 2 |
| IL | Interleukin |
| TNFa | Tumor necrosis factor alpha |
| CXCL13 | CXC motif chemokine ligand 13 |
| TSLP | Thymic stromal lymphopoietin |
| TNFRII | Tumor necrosis factor-RII |
| BAFF | B-cell activating factor |
| CCL19/MIP-3B | Macrophage Inflammatory Protein-3-beta |
| MMP-8 | Matrix metalloproteinase-8 |
| mRSS | Modified Rodnan skin score |
| DLCO | diffusing capacity for carbon monoxide |
| SPAS | Systolic pulmonary artery pressure |
| ANA | Antinuclear antibodies |
| ESR | Rrythrocytes sedimentation rate |
| CRP | C-reactive protein |
| BMI | Body mass index |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| HCQ | Hydroxychloroquine |
| MTX | Methotrexate |
| AZA | Azathioprine |
| CyA | Cyclosporin-A |
| MMF | Mycophenolate mofetil |
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.C.; Cacoub, P. Systemic sclerosis: An update in 2016. Autoimmun. Rev. 2016, 15, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, P.M. Lung involvement in systemic sclerosis. Presse Med. 2011, 40, e3–e17. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Wise, R.A.; Hochberg, M.C.; Stevens, M.B.; Wigley, F.M. Carbon monoxide diffusing capacity as predictor of outcome in systemic sclerosis. Am. J. Med. 1984, 77, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M. Biomarkers in systemic sclerosis: Their potential to predict clinical courses. J. Dermatol. 2016, 43, 29–38. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weisman, M.; Mayes, M.; Collier, D.; et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar] [PubMed]
- Pedregosa, F.; Varoquau, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, E. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Nishimagi, E.; Tochimoto, A.; Kawamoto, M.; Katsumata, Y.; Soejima, M.; Kanno, T.; Kamatani, N.; Hara, M. Intracellular IL-1α-binding proteins contribute to biological functions of endogenous IL-1α in systemic sclerosis fibroblasts. Proc. Natl. Acad. Sci. USA 2006, 103, 14501–14506. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.G.; Dave, M.N.; Leung, M.Y.; Cipolletta, C.; Meseck, M.; Woo, S.L.C.; Amin, A.R. Functional Genomic Analysis of Type II IL-1β Decoy Receptor: Potential for Gene Therapy in Human Arthritis and Inflammation. J. Immunol. 2002, 168, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, H.Y.; Cho, S.; Yoo, S.J.; Kim, W.J.; Yeon, H.R.; Choi, K.; Choi, J.M.; Kang, S.W.; Lee, W.W. Induction of the IL-1RII decoy receptor by NFAT/FOXP3 blocks IL-1β-dependent response of Th17 cells. elife 2021, 10, e61841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Craig, V.J.; Quintero, P.A.; Fyfe, S.E.; Patel, A.S.; Knolle, M.D.; Kobzik, L.; Owen, C.A. Pro-fibrotic Activities for Matrix Metalloproteinase-8 during Bleomycin-Mediated Lung Injury. J. Immunol. 2013, 190, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Forteza, J.; López-Otin, C.; Gómez-Reino, J.J.; González, A.; Conde, C. Matrix metalloproteinase-8 deficiency increases joint inflammation and bone erosion in the K/BxN serum-transfer arthritis model. Arthritis Res. Ther. 2010, 12, R224. [Google Scholar] [CrossRef] [PubMed]
- Tolusso, B.; Fabris, M.; Caporali, R.; Cuomo, G.; Isola, M.; Soldano, F.; Montecucco, C.; Valentini, G.; Ferraccioli, G. −238 and +489 TNF-α along with TNF-RII gene polymorphisms associate with the diffuse phenotype in patients with Systemic Sclerosis. Immunol. Lett. 2005, 96, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gruschwitz, M.S.; Albrecht, M.; Vieth, G.; Haustein, U.F. In situ expression and serum levels of tumor necrosis factor-alpha receptors in patients with early stages of systemic sclerosis. J. Rheumatol. 1997, 24, 1936–1943. [Google Scholar] [PubMed]
- Wuttge, D.M.; Wildt, M.; Geborek, P.; Wollheim, F.A.; Scheja, A.; Åkesson, A. Serum IL-15 in patients with early systemic sclerosis: A potential novel marker of lung disease. Arthritis Res. Ther. 2007, 9, R85. [Google Scholar] [CrossRef] [PubMed]
- Wuttge, D.M.; Wildt, M.; Scheja, A.; Westergren-Thorsson, G. Interleukin-15 attenuates transforming growth factor-β1-induced myofibroblast differentiation in human fetal lung fibroblasts. Eur. Cytokine Netw. 2010, 21, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Morimoto, S.; Amano, H.; Tokano, Y.; Takasaki, Y.; Hashimoto, H. Serum levels of interleukin 15 in patients with rheumatic diseases. J. Rheumatol. 2001, 28, 2389–2391. [Google Scholar] [PubMed]
- Karrer, S.; Bosserhoff, A.K.; Weiderer, P.; Distler, O.; Landthaler, M.; Szeimies, R.M.; Müller-Ladner, U.; Schölmerich, J.; Hellerbrand, C. The -2518 promotor polymorphism in the MCP-1 gene is associated with systemic sclerosis. J. Investig. Dermatol. 2005, 124, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sato, S.; Takehara, K. Augmented production of chemokines (monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1alpha (MIP-1alpha) and MIP-1beta) in patients with systemic sclerosis: MCP-1 and MIP-1alpha may be involved in the development of pulmonary fibrosis. Clin. Exp. Immunol. 1999, 117, 159–165. [Google Scholar] [PubMed]
- Yamamoto, T.; Eckes, B.; Hartmann, K.; Krieg, T. Expression of monocyte chemoattractant protein-1 in the lesional skin of systemic sclerosis. J. Dermatol. Sci. 2001, 26, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.; Santiago, B.; Rivero, M.; Rullas, J.; Alcami, J.; Pablos, J.L. Chemokine expression by systemic sclerosis fibroblasts: Abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 2001, 44, 1382–1386. [Google Scholar] [CrossRef] [PubMed]


| SSc (n = 35) | Controls (n = 40) | ||
|---|---|---|---|
| Sex (F/M) | 33 (94.3%)/2 (5.7%) | 40 F (100%) | p = ns |
| Age (years, mean ± SD) | 60.5 ± 13.8 | 57 ± 12.8 | p = ns |
| Disease duration (months, mean ± SD) | 96.5 ± 97.22 | ||
| Smoking | 13 (37.4%) | ||
| Raynaud’s phenomenon | 34 (97.1%) | ||
| Skin involvement | 27 (77.1%) | ||
| Digital ulcers | 6 (17.1%) | ||
| mRSS (mean ± SD) | 5.5 ± 5.8 | ||
| Joint involvement | 8 (22.8%) | ||
| Cardiac involvement | 0 | ||
| sPAP (mmHg, mean ± SD) | 30.5 ± 8.3 | ||
| Skin involvement | 15 (42.7%) | ||
| DLCO (%, mean ± SD) | 78 ± 22 | ||
| Gastrointestinal involvement | 7 (20%) | ||
| SSc Patients (n = 35) | |
|---|---|
| ANA | 34 (97.1%) |
| Rheumatoid factor | 7 (20%) |
| Anti-Scl70 (U/mL) | 29.3 ± 61.2 |
| Anti-CENP B (U/mL) | 55.9 ± 47.3 |
| Anti-SSA | 7 (20%) |
| Anti-SSB | 1 (2.8%) |
| Low C3 | 1 (2.8%) |
| Low C4 | 1 (2.8%) |
| Elevated ESR | 7 (20%) |
| Elevated CRP | 5 (14.3%) |
| Anemia | 7 (20%) |
| Leukopenia | 3 (8.6%) |
| Thrombocytopenia | 0 |
| Hypergammaglobulinemia | 4 (11.4%) |
| Dose of glucocorticoids (equivalent dose of prednisone, mg/day) | 2.2 ± 5.4 |
| Current DMARDs therapy | 12 (34.3%) |
| HCQ | 11 (31.4%) |
| MTX | 10 (28.6%) |
| AZA | 2 (5.7%) |
| CyA | 1 (2.8%) |
| MMF | 3 (8.6%) |
| Iloprost | 6 (17.1%) |
| Calcium channel blockers (nifedipine/amlodipine) | 12 (34.3%) |
| Variable | Beta Estimate | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| MMP-8 | 1.466 | 0.859 | 2.073 | 0.508 |
| MCP-1 | 0.728 | 0.142 | 1.314 | |
| IL-21 | 0.501 | −0.007 | 1.009 | 0.907 |
| sTNFRII | 0.468 | 0.021 | 0.914 | |
| IFNa2 | 0.326 | −0.211 | 0.864 | |
| IL-13 | 0.251 | −0.337 | 0.838 | 0.912 |
| BAFF/BLyS | 0.222 | −0.836 | 1.281 | |
| TNFa | 0.221 | −0.782 | 1.223 | |
| BCA-1 | 0.144 | −0.801 | 1.088 | |
| IL-17A | 0.139 | −0.419 | 0.698 | |
| CCL19/MIP3B | 0.057 | −0.411 | 0.526 | 0.958 |
| IL-6 | 0.053 | −0.549 | 0.654 | |
| Flt-3L | 0.021 | −0.734 | 0.775 | |
| IL-12p40 | −0.078 | −0.811 | 0.655 | 0.982 |
| IL-33 | −0.238 | −2.302 | 1.825 | |
| IL-1RA | −0.261 | −0.750 | 0.228 | |
| sIL-1RII | −0.290 | −0.589 | 0.008 | |
| IL-23 | −0.327 | −2.215 | 1.561 | 0.868 |
| IP-10 | −0.380 | −0.785 | 0.025 | |
| sIL-2Ra | −0.396 | −1.074 | 0.281 | 0.953 |
| TLSP | −0.400 | −1.311 | 0.511 | |
| IL-7 | −0.404 | −0.802 | −0.005 | 1.000 |
| IL-15 | −0.697 | −1.431 | 0.038 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, C.; Cafaro, G.; Pozzolo, R.d.; Bruno, L.; Sasso, N.; Cecchetti, R.; Antonucci, M.; Topini, F.; Bistoni, O.; Mecocci, P.; et al. A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis. Biomedicines 2025, 13, 967. https://doi.org/10.3390/biomedicines13040967
Perricone C, Cafaro G, Pozzolo Rd, Bruno L, Sasso N, Cecchetti R, Antonucci M, Topini F, Bistoni O, Mecocci P, et al. A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis. Biomedicines. 2025; 13(4):967. https://doi.org/10.3390/biomedicines13040967
Chicago/Turabian StylePerricone, Carlo, Giacomo Cafaro, Roberto dal Pozzolo, Lorenza Bruno, Nicole Sasso, Roberta Cecchetti, Matteo Antonucci, Fabiana Topini, Onelia Bistoni, Patrizia Mecocci, and et al. 2025. "A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis" Biomedicines 13, no. 4: 967. https://doi.org/10.3390/biomedicines13040967
APA StylePerricone, C., Cafaro, G., Pozzolo, R. d., Bruno, L., Sasso, N., Cecchetti, R., Antonucci, M., Topini, F., Bistoni, O., Mecocci, P., Gerli, R., & Bartoloni, E. (2025). A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis. Biomedicines, 13(4), 967. https://doi.org/10.3390/biomedicines13040967







