A Nexus of Biomolecular Complexities in Pituitary Neuroendocrine Tumors: Insights into Key Molecular Drivers
Abstract
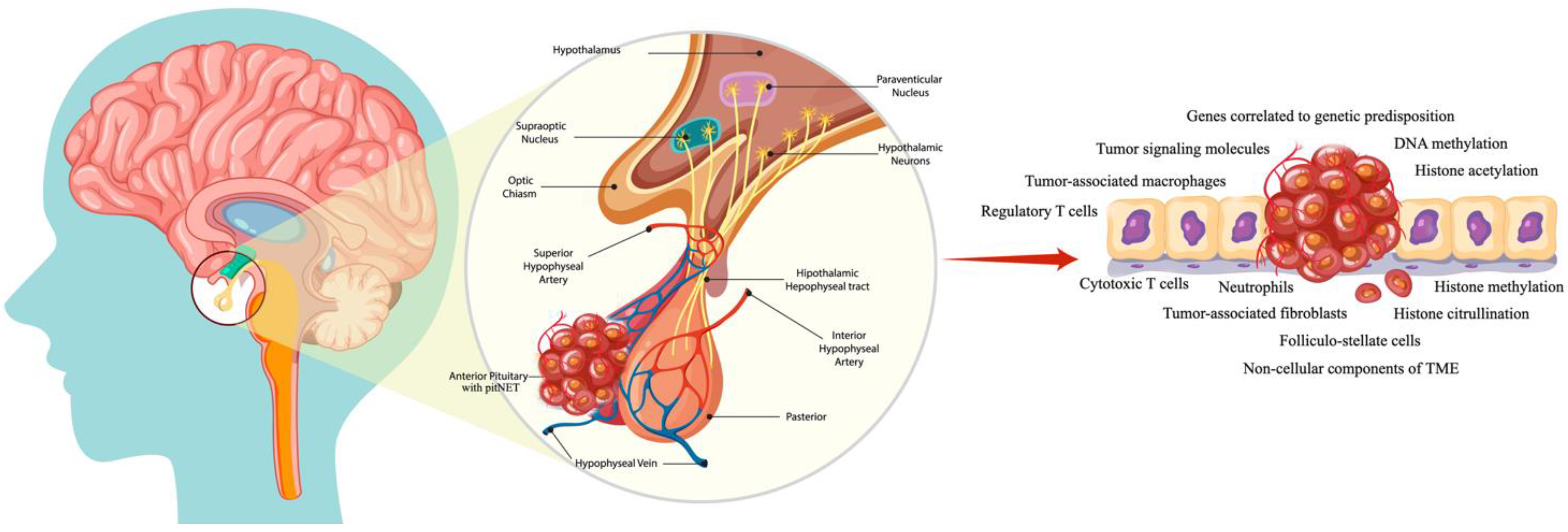
1. Introduction
2. Biomolecular Drivers
2.1. A Brief Overview of Tumor-Signaling Molecules and Genes Correlated with Genetic Predisposition in PitNETs
2.2. The Landscape of Molecular Events in Pituitary Tumor Apoplexy
2.3. Non-Invasive Circulating Biomarkers in PitNETs
2.3.1. Circulating Tumor DNA (ctDNA)
2.3.2. Cell-Free RNA (Long Non-Coding RNAs, Messenger RNA, and Micro-RNAs)
2.3.3. Epigenetic Factors
2.3.4. Circulating Tumor Cells
3. Tumoral Microenvironment
3.1. Immune and Stromal Cells
3.1.1. Macrophages
3.1.2. Lymphocytes
3.1.3. Neutrophils
3.1.4. Tumor-Associated Fibroblasts
3.1.5. Folliculo-Stellate Cells
3.2. Non-Cellular Components of the TME
3.2.1. Extracellular Matrix
3.2.2. Exosomes
4. Translational Impact of Therapeutic Interventions
5. Future Perspectives Regarding Therapeutic Agents for the TME
6. Conclusions
Funding
Conflicts of Interest
References
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Cannarile, L.; Delfino, D.V.; Riccardi, C. A dual role for glucocorticoid-induced leucine zipper in glucocorticoid function: Tumor growth promotion or suppression? Cell Death Dis. 2018, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, X.; Yao, K.; Yang, Y.; Zhang, L.; Liu, N.; Yan, C.; Qi, X.; Han, S. Targeting the Tumor Immune Microenvironment Could Become a Potential Therapeutic Modality for Aggressive Pituitary Adenoma. Brain Sci. 2023, 13, 164. [Google Scholar] [CrossRef]
- Ilie, M.D.; Vasiljevic, A.; Raverot, G.; Bertolino, P. The Microenvironment of Pituitary Tumors—Biological and Therapeutic Implications. Cancers 2019, 11, 1605. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Greenwald, N.F.; Du, Z.; Agar, N.Y.; Kaiser, U.B.; Woodmansee, W.W.; Reardon, D.A.; Freeman, G.J.; Fecci, P.E.; et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget 2016, 7, 76565–76576. [Google Scholar] [CrossRef]
- Wang, P.F.; Wang, T.J.; Yang, Y.K.; Yao, K.; Li, Z.; Li, Y.M.; Yan, C.X. The expression profile of PD-L1 and CD8(+) lymphocyte in pituitary adenomas indicating for immunotherapy. J. Neurooncol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Lin, A.L.; Jonsson, P.; Tabar, V.; Yang, T.J.; Cuaron, J.; Beal, K.; Cohen, M.; Postow, M.; Rosenblum, M.; Shia, J.; et al. Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J. Clin. Endocrinol. Metab. 2018, 103, 3925–3930. [Google Scholar] [CrossRef]
- Voellger, B.; Zhang, Z.; Benzel, J.; Wang, J.; Lei, T.; Nimsky, C.; Bartsch, J.W. Targeting Aggressive Pituitary Adenomas at the Molecular Level-A Review. J. Clin. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Dai, C.; Liang, S.; Sun, B.; Kang, J. The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas. Front. Endocrinol. 2020, 11, 608422. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.-D.; De Alcubierre, D.; Carretti, A.L.; Jouanneau, E.; Raverot, G. Therapeutic targeting of the pituitary tumor microenvironment. Pharmacol. Ther. 2023, 250, 108506. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S.; Sim, H.W.; McCormack, A.I. Exploring the Role of Novel Medical Therapies for Aggressive Pituitary Tumors: A Review of the Literature—“Are We There Yet?”. Cancers 2020, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Deng, K.; Lian, W.; Bao, X.; Feng, M.; Duan, L.; Zhu, H.; Xing, B. The Immune Profile of Pituitary Adenomas and a Novel Immune Classification for Predicting Immunotherapy Responsiveness. J. Clin. Endocrinol. Metab. 2020, 105, e3207–e3223. [Google Scholar] [CrossRef]
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef]
- Wang, A.R.; Gill, J.R. The Pituitary Gland: An Infrequent but Multifaceted Contributor to Death. Acad. Forensic Pathol. 2016, 6, 206–216. [Google Scholar] [CrossRef]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Florio, T. Adult pituitary stem cells: From pituitary plasticity to adenoma development. Neuroendocrinology 2011, 94, 265–277. [Google Scholar] [CrossRef]
- Dworakowska, D.; Grossman, A.B. The pathophysiology of pituitary adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Kameda-Smith, M.M.; Lu, J. The Pituitary Tumors and Their Tumor-Specific Microenvironment. Adv. Exp. Med. Biol. 2020, 1296, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Yang, C.; Bao, X.; Wang, R. Genetic and Epigenetic Causes of Pituitary Adenomas. Front. Endocrinol. 2021, 11, 596554. [Google Scholar] [CrossRef]
- Pepe, S.; Korbonits, M.; Iacovazzo, D. Germline and mosaic mutations causing pituitary tumours: Genetic and molecular aspects. J. Endocrinol. 2019, 240, R21–R45. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Butman, J.A.; Kiringoda, R.; Song, D.; Oldfield, E.H. Pituitary stalk hemangioblastomas in von Hippel-Lindau disease. J. Neurosurg. 2009, 110, 350–353. [Google Scholar] [CrossRef]
- de Kock, L.; Sabbaghian, N.; Plourde, F.; Srivastava, A.; Weber, E.; Bouron-Dal Soglio, D.; Hamel, N.; Choi, J.H.; Park, S.H.; Deal, C.L.; et al. Pituitary blastoma: A pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014, 128, 111–122. [Google Scholar] [CrossRef]
- Marques, P.; Caimari, F.; Hernández-Ramírez, L.C.; Collier, D.; Iacovazzo, D.; Ronaldson, A.; Magid, K.; Lim, C.T.; Stals, K.; Ellard, S.; et al. Significant Benefits of AIP Testing and Clinical Screening in Familial Isolated and Young-onset Pituitary Tumors. J. Clin. Endocrinol. Metab. 2020, 105, e2247–e2260. [Google Scholar] [CrossRef]
- Xekouki, P.; Pacak, K.; Almeida, M.; Wassif, C.A.; Rustin, P.; Nesterova, M.; de la Luz Sierra, M.; Matro, J.; Ball, E.; Azevedo, M.; et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: A new association for SDH? J. Clin. Endocrinol. Metab. 2012, 97, E357–E366. [Google Scholar] [CrossRef]
- Gossing, W.; Frohme, M.; Radke, L. Biomarkers for Liquid Biopsies of Pituitary Neuroendocrine Tumors. Biomedicines 2020, 8, 148. [Google Scholar] [CrossRef]
- Vlotides, G.; Eigler, T.; Melmed, S. Pituitary tumor-transforming gene: Physiology and implications for tumorigenesis. Endocr. Rev. 2007, 28, 165–186. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Jiao, Y.; Wang, R.; Ren, S.G.; Wawrowsky, K.; Melmed, S. STAT3 upregulation in pituitary somatotroph adenomas induces growth hormone hypersecretion. J. Clin. Investig. 2015, 125, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, C.; Song, J.; Zhang, Y.; Chen, J.; Song, Z.; Shou, X.; Ma, Z.; Peng, H.; Jian, X.; et al. Germline Mutations in CDH23, Encoding Cadherin-Related 23, Are Associated with Both Familial and Sporadic Pituitary Adenomas. Am. J. Hum. Genet. 2017, 100, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Faucz, F.R.; Horvath, A.D.; Azevedo, M.F.; Levy, I.; Bak, B.; Wang, Y.; Xekouki, P.; Szarek, E.; Gourgari, E.; Manning, A.D.; et al. Is IGSF1 involved in human pituitary tumor formation? Endocr. Relat. Cancer 2015, 22, 47–54. [Google Scholar] [CrossRef]
- Wei, D.; Yiyuan, C.; Qian, L.; Jianhua, L.; Yazhuo, Z.; Hua, G. The absence of PRDM2 involved the tumorigenesis of somatotroph adenomas through regulating c-Myc. Gene 2020, 737, 144456. [Google Scholar] [CrossRef]
- Li, J.; Dong, W.; Li, Z.; Wang, H.; Gao, H.; Zhang, Y. Impact of SLC20A1 on the Wnt/β-catenin signaling pathway in somatotroph adenomas. Mol. Med. Rep. 2019, 20, 3276–3284. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Liu, N.A.; Melmed, S. Somatostatin and dopamine receptor regulation of pituitary somatotroph adenomas. Pituitary 2017, 20, 93–99. [Google Scholar] [CrossRef]
- Srirangam Nadhamuni, V.; Korbonits, M. Novel Insights into Pituitary Tumorigenesis: Genetic and Epigenetic Mechanisms. Endocr. Rev. 2020, 41, 821–846. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Yoshimoto, K.; Yamada, S.; Ishizuka, A.; Mizusawa, N.; Horiguchi, H.; Hirokawa, M.; Asa, S.L. Inactivation of RASSF1A tumor suppressor gene by aberrant promoter hypermethylation in human pituitary adenomas. Lab. Investig. 2005, 85, 464–473. [Google Scholar] [CrossRef]
- Righi, A.; Jin, L.; Zhang, S.; Stilling, G.; Scheithauer, B.W.; Kovacs, K.; Lloyd, R.V. Identification and consequences of galectin-3 expression in pituitary tumors. Mol. Cell Endocrinol. 2010, 326, 8–14. [Google Scholar] [CrossRef]
- Ren, J.; Jian, F.; Jiang, H.; Sun, Y.; Pan, S.; Gu, C.; Chen, X.; Wang, W.; Ning, G.; Bian, L.; et al. Decreased expression of SFRP2 promotes development of the pituitary corticotroph adenoma by upregulating Wnt signaling. Int. J. Oncol. 2018, 52, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jian, X.; Deng, S.; Ma, Z.; Shou, X.; Shen, Y.; Zhang, Q.; Song, Z.; Li, Z.; Peng, H.; et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 2018, 9, 3171. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, C.; Xie, W.; Wang, Z.; Gao, H.; Cao, L.; Hao, L.; Zhang, Y. Long non-coding RNA C5orf66-AS1 is downregulated in pituitary null cell adenomas and is associated with their invasiveness. Oncol. Rep. 2017, 38, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hong, L.; Wu, Y.; Li, C.; Wan, H.; Li, G.; Sun, Y.; Yu, S.; Chittiboina, P.; Montgomery, B.; et al. Identification of a subtype-specific ENC1 gene related to invasiveness in human pituitary null cell adenoma and oncocytomas. J. Neurooncol. 2014, 119, 307–315. [Google Scholar] [CrossRef]
- Cheng, S.; Li, C.; Xie, W.; Miao, Y.; Guo, J.; Wang, J.; Zhang, Y. Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma. Aging 2020, 12, 2408–2427. [Google Scholar] [CrossRef]
- Feng, J.; Yu, S.Y.; Li, C.Z.; Li, Z.Y.; Zhang, Y.Z. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol. Cell Endocrinol. 2016, 436, 195–203. [Google Scholar] [CrossRef]
- Pease, M.; Ling, C.; Mack, W.J.; Wang, K.; Zada, G. The role of epigenetic modification in tumorigenesis and progression of pituitary adenomas: A systematic review of the literature. PLoS ONE 2013, 8, e82619. [Google Scholar] [CrossRef]
- Seemann, N.; Kuhn, D.; Wrocklage, C.; Keyvani, K.; Hackl, W.; Buchfelder, M.; Fahlbusch, R.; Paulus, W. CDKN2A/p16 inactivation is related to pituitary adenoma type and size. J. Pathol. 2001, 193, 491–497. [Google Scholar] [CrossRef]
- Burman, P.; Trouillas, J.; Losa, M.; McCormack, A.; Petersenn, S.; Popovic, V.; Theodoropoulou, M.; Raverot, G.; Dekkers, O.M.; Guenego, A.; et al. Aggressive pituitary tumours and carcinomas, characteristics and management of 171 patients. Eur. J. Endocrinol. 2022, 187, 593–605. [Google Scholar] [CrossRef]
- Loughrey, P.B.; Baker, G.; Herron, B.; Cooke, S.; Iacovazzo, D.; Lindsay, J.R.; Korbonits, M. Invasive ACTH-producing pituitary gland neoplasm secondary to MSH2 mutation. Cancer Genet. 2021, 256–257, 36–39. [Google Scholar] [CrossRef]
- Iglesias, P. Pituitary Apoplexy: An Updated Review. J. Clin. Med. 2024, 13, 2508. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Simò, R. Pituitary Apoplexy: Risk Factors and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 8721. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dutta, P. Landscape of Molecular Events in Pituitary Apoplexy. Front. Endocrinol. 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.; Abak, A.; Shoorei, H.; Taheri, M.; Ghafouri-Fard, S. MicroRNAs as regulators of ERK/MAPK pathway: A comprehensive review. Biomed. Pharmacother. 2020, 132, 110853. [Google Scholar] [CrossRef]
- Mou, C.; Han, T.; Zhao, H.; Wang, S.; Qu, Y. Clinical features and immunohistochemical changes of pituitary apoplexy. J. Clin. Neurosci. 2009, 16, 64–68. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef]
- García-Casas, A.; García-Olmo, D.C.; García-Olmo, D. Further the liquid biopsy: Gathering pieces of the puzzle of genometastasis theory. World J. Clin. Oncol. 2017, 8, 378–388. [Google Scholar] [CrossRef]
- Di Ieva, A.; Grizzi, F.; Gaetani, P.; Goglia, U.; Tschabitscher, M.; Mortini, P.; Rodriguez y Baena, R. Euclidean and fractal geometry of microvascular networks in normal and neoplastic pituitary tissue. Neurosurg. Rev. 2008, 31, 271–281. [Google Scholar] [CrossRef]
- Ushijima, T.; Asada, K. Aberrant DNA methylation in contrast with mutations. Cancer Sci. 2010, 101, 300–305. [Google Scholar] [CrossRef]
- Chan, K.C.; Jiang, P.; Zheng, Y.W.; Liao, G.J.; Sun, H.; Wong, J.; Siu, S.S.; Chan, W.C.; Chan, S.L.; Chan, A.T.; et al. Cancer genome scanning in plasma: Detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin. Chem. 2013, 59, 211–224. [Google Scholar] [CrossRef]
- Shapiro, B.; Chakrabarty, M.; Cohn, E.M.; Leon, S.A. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer 1983, 51, 2116–2120. [Google Scholar] [CrossRef] [PubMed]
- Mouliere, F.; Robert, B.; Arnau Peyrotte, E.; Del Rio, M.; Ychou, M.; Molina, F.; Gongora, C.; Thierry, A.R. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE 2011, 6, e23418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.T.; Tang, H.; Xie, W.Q.; Yao, H.; Gu, W.T.; Zheng, Y.Z.; Shang, H.B.; Wang, Y.; Wei, Y.X.; et al. Exosome-Transmitted lncRNA H19 Inhibits the Growth of Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2019, 104, 6345–6356. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Darvasi, O.; Likó, I.; Szücs, N.; Czirják, S.; Reiniger, L.; Szabó, B.; Krokker, L.; Pállinger, É.; Igaz, P.; et al. Comprehensive Analysis of Circulating miRNAs in the Plasma of Patients with Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 4151–4168. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, Y.; Fan, F.; Zeng, Y.; Li, C.; Zhou, G.; Hu, Z.; Zhang, L.; Liu, Z. Exosomal hsa-miR-21-5p derived from growth hormone-secreting pituitary adenoma promotes abnormal bone formation in acromegaly. Transl. Res. 2020, 215, 1–16. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.S.; Cao, K.C.; Bao, X.J.; Yu, J. Identification of CDK6 and RHOU in Serum Exosome as Biomarkers for the Invasiveness of Non-functioning Pituitary Adenoma. Chin. Med. Sci. J. 2019, 34, 168–176. [Google Scholar] [CrossRef]
- Wu, Z.R.; Yan, L.; Liu, Y.T.; Cao, L.; Guo, Y.H.; Zhang, Y.; Yao, H.; Cai, L.; Shang, H.B.; Rui, W.W.; et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018, 9, 4624. [Google Scholar] [CrossRef]
- Lu, T.; Yu, C.; Ni, H.; Liang, W.; Yan, H.; Jin, W. Expression of the long non-coding RNA H19 and MALAT-1 in growth hormone-secreting pituitary adenomas and its relationship to tumor behavior. Int. J. Dev. Neurosci. 2018, 67, 46–50. [Google Scholar] [CrossRef]
- Bahn, J.H.; Zhang, Q.; Li, F.; Chan, T.M.; Lin, X.; Kim, Y.; Wong, D.T.; Xiao, X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015, 61, 221–230. [Google Scholar] [CrossRef]
- Hauser, B.M.; Lau, A.; Gupta, S.; Bi, W.L.; Dunn, I.F. The Epigenomics of Pituitary Adenoma. Front. Endocrinol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Ma, H.S.; Wang, E.L.; Xu, W.F.; Yamada, S.; Yoshimoto, K.; Qian, Z.R.; Shi, L.; Liu, L.L.; Li, X.H. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med. Sci. Monit. 2018, 24, 4841–4850. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, R.; Du, W.; Yang, F.; Wei, X. RIZ1 and histone methylation status in pituitary adenomas. Tumour Biol. 2017, 39, 1010428317711794. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Yanjiao, H.; Qian, L.; Jichao, W.; Yazhuo, Z. Detection of circulating tumor cells in patients with pituitary tumors. BMC Cancer 2018, 18, 336. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Marques, P.; Grossman, A.B.; Korbonits, M. The tumour microenvironment of pituitary neuroendocrine tumours. Front. Neuroendocrinol. 2020, 58, 100852. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Barry, S.; Carlsen, E.; Marques, P.; Stiles, C.E.; Gadaleta, E.; Berney, D.M.; Roncaroli, F.; Chelala, C.; Solomou, A.; Herincs, M.; et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene 2019, 38, 5381–5395. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Yagnik, G.; Rutowski, M.J.; Shah, S.S.; Aghi, M.K. Stratifying nonfunctional pituitary adenomas into two groups distinguished by macrophage subtypes. Oncotarget 2019, 10, 2212–2223. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, Y.; Xu, H.; Ren, J.; Meng, T.; Ni, Y.; Zhu, Q.; Zhang, W.B.; Pan, Y.B.; Jin, J.; et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics 2021, 11, 3839–3852. [Google Scholar] [CrossRef]
- Principe, M.; Chanal, M.; Ilie, M.D.; Ziverec, A.; Vasiljevic, A.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bertolino, P. Immune Landscape of Pituitary Tumors Reveals Association Between Macrophages and Gonadotroph Tumor Invasion. J. Clin. Endocrinol. Metab. 2020, 105, dgaa520. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cheng, Y.; Ye, J.; Cai, P.; Zhang, J.; Li, R.; Yang, Y.; Wang, Z.; Zhang, H.; Lin, C.; et al. bFGF Promotes the Migration of Human Dermal Fibroblasts under Diabetic Conditions through Reactive Oxygen Species Production via the PI3K/Akt-Rac1- JNK Pathways. Int. J. Biol. Sci. 2015, 11, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Adam, B.; Jack, A.S.; Lam, A.; Broad, R.W.; Chik, C.L. Immune Cell Infiltrates in Pituitary Adenomas: More Macrophages in Larger Adenomas and More T Cells in Growth Hormone Adenomas. Endocr. Pathol. 2015, 26, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, X.; Gao, W.; Cai, R.; Wu, J.; Liu, T.; Chen, X.; Jiang, D.; Chen, C.; Cheng, Q.; et al. The biological behavior and clinical outcome of pituitary adenoma are affected by the microenvironment. CNS Neurosci. Ther. 2024, 30, e14729. [Google Scholar] [CrossRef]
- Wu, X.; Han, X.; Zhu, H.; Li, M.; Gong, L.; Jing, S.; Xie, W.; Liu, Z.; Li, C.; Zhang, Y. Single-cell transcriptomics identify a novel macrophage population associated with bone invasion in pituitary neuroendocrine tumors. J. Exp. Clin. Cancer Res. 2025, 44, 27. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Rossi, M.L.; Jones, N.R.; Esiri, M.M.; Havas, L.; al Izzi, M.; Coakham, H.B. Mononuclear cell infiltrate and HLA-Dr expression in 28 pituitary adenomas. Tumori 1990, 76, 543–547. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Idema, A.J.; Bol, K.F.; Nierkens, S.; Grauer, O.M.; Wesseling, P.; Grotenhuis, J.A.; Hoogerbrugge, P.M.; de Vries, I.J.; Adema, G.J. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009, 11, 394–402. [Google Scholar] [CrossRef]
- Han, S.; Ma, E.; Jiang, W.; Lu, Y.; Sun, X.; Feng, S. Overrepresentation of highly functional T regulatory cells in patients with nonfunctioning pituitary adenoma. Hum. Immunol. 2020, 81, 314–319. [Google Scholar] [CrossRef]
- Huang, X.; Xu, J.; Wu, Y.; Sheng, L.; Li, Y.; Zha, B.; Sun, T.; Yang, J.; Zang, S.; Liu, J. Alterations in CD8(+) Tregs, CD56(+) Natural Killer Cells and IL-10 Are Associated with Invasiveness of Nonfunctioning Pituitary Adenomas (NFPAs). Pathol. Oncol. Res. 2021, 27, 598887. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.E.; Shen, Z.J.; Kanchwala, M.; Xing, C.; Filatenkov, A.; Shang, P.; Barnett, S.; Abedin, Z.; Malter, J.S.; Raisanen, J.M.; et al. Aggressive Behavior in Silent Subtype III Pituitary Adenomas May Depend on Suppression of Local Immune Response: A Whole Transcriptome Analysis. J. Neuropathol. Exp. Neurol. 2017, 76, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Iacovazzo, D.; Chiloiro, S.; Carlsen, E.; Bianchi, A.; Giampietro, A.; Tartaglione, T.; Bima, C.; Bracaccia, M.E.; Lugli, F.; Lauretti, L.; et al. Tumour-infiltrating cytotoxic T lymphocytes in somatotroph pituitary neuroendocrine tumours. Endocrine 2020, 67, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, T.; Yang, Y.; Yu, C.; Liu, N.; Yan, C. Detection of programmed death ligand 1 protein and CD8+ lymphocyte infiltration in plurihormonal pituitary adenomas: A case report and review of the literatures. Medicine 2017, 96, e9056. [Google Scholar] [CrossRef]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Demers, M.; Wong, S.L.; Martinod, K.; Gallant, M.; Cabral, J.E.; Wang, Y.; Wagner, D.D. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 2016, 5, e1134073. [Google Scholar] [CrossRef]
- He, M.; Peng, A.; Huang, X.Z.; Shi, D.C.; Wang, J.C.; Zhao, Q.; Lin, H.; Kuang, D.M.; Ke, P.F.; Lao, X.M. Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. Oncoimmunology 2016, 5, e1219828. [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: A diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012, 31, 195–208. [Google Scholar] [CrossRef]
- Orimo, A.; Weinberg, R.A. Heterogeneity of stromal fibroblasts in tumors. Cancer Biol. Ther. 2007, 6, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Tofrizal, A.; Fujiwara, K.; Yashiro, T.; Yamada, S. Alterations of collagen-producing cells in human pituitary adenomas. Med. Mol. Morphol. 2016, 49, 224–232. [Google Scholar] [CrossRef]
- Zhou, K.; Fan, Y.D.; Duysenbi, S.; Wu, P.F.; Feng, Z.H.; Qian, Z.; Zhang, T.R. siRNA-mediated silencing of bFGF gene inhibits the proliferation, migration, and invasion of human pituitary adenoma cells. Tumour Biol. 2017, 39, 1010428317704805. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, S.; Hu, Y.; Zhou, P.; Gao, L.; Wang, M.; Sun, Z.; Chen, C.; Yin, S.; Wang, X.; et al. Invasive Pituitary Adenoma-Derived Tumor-Associated Fibroblasts Promote Tumor Progression both In Vitro and In Vivo. Exp. Clin. Endocrinol. Diabetes 2018, 126, 213–221. [Google Scholar] [CrossRef]
- Allaerts, W.; Vankelecom, H. History and perspectives of pituitary folliculo-stellate cell research. Eur. J. Endocrinol. 2005, 153, 1–12. [Google Scholar] [CrossRef]
- Tapoi, D.A.; Popa, M.L.; Tanase, C.; Derewicz, D.; Gheorghișan-Gălățeanu, A.A. Role of Tumor Microenvironment in Pituitary Neuroendocrine Tumors: New Approaches in Classification, Diagnosis and Therapy. Cancers 2023, 15, 5301. [Google Scholar] [CrossRef]
- Herkenham, M. Folliculo-stellate (FS) cells of the anterior pituitary mediate interactions between the endocrine and immune systems. Endocrinology 2005, 146, 33–34. [Google Scholar] [CrossRef]
- Claudius, L.; Yoshimi, Y.; Yoichiro, H.; Gabriel, M.; Koichi, M. Phagocytotic removal of apoptotic endocrine cells by folliculostellate cells and its functional implications in clusterin accumulation in pituitary colloids in helmeted guinea fowl (Numida meleagris). Acta Histochem. 2006, 108, 69–80. [Google Scholar] [CrossRef]
- Devnath, S.; Inoue, K. An insight to pituitary folliculo-stellate cells. J. Neuroendocrinol. 2008, 20, 687–691. [Google Scholar] [CrossRef]
- Voit, D.; Saeger, W.; Lüdecke, D.K. Folliculo-stellate cells in pituitary adenomas of patients with acromegaly. Pathol. Res. Pract. 1999, 195, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.D.; Vasiljevic, A.; Chanal, M.; Gadot, N.; Chinezu, L.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bertolino, P. Intratumoural spatial distribution of S100B + folliculostellate cells is associated with proliferation and expression of FSH and ERα in gonadotroph tumours. Acta Neuropathol. Commun. 2022, 10, 18. [Google Scholar] [CrossRef]
- Vajtai, I.; Kappeler, A.; Sahli, R. Folliculo-stellate cells of “true dendritic” type are involved in the inflammatory microenvironment of tumor immunosurveillance of pituitary adenomas. Diagn. Pathol. 2007, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Delfin, L.; Mete, O.; Asa, S.L. Follicular cells in pituitary neuroendocrine tumors. Hum. Pathol. 2021, 114, 1–8. [Google Scholar] [CrossRef]
- Wiesnagrotzki, N.; Bernreuther, C.; Saeger, W.; Flitsch, J.; Glatzel, M.; Hagel, C. Co-expression of intermediate filaments glial fibrillary acidic protein and cytokeratin in pituitary adenoma. Pituitary 2021, 24, 62–67. [Google Scholar] [CrossRef]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130502. [Google Scholar] [CrossRef]
- Herrgott, G.A.; Asmaro, K.P.; Wells, M.; Sabedot, T.S.; Malta, T.M.; Mosella, M.S.; Nelson, K.; Scarpace, L.; Barnholtz-Sloan, J.S.; Sloan, A.E.; et al. Detection of tumor-specific DNA methylation markers in the blood of patients with pituitary neuroendocrine tumors. Neuro Oncol. 2022, 24, 1126–1139. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Ilie, M.-D.; Vasiljevic, A.; Bertolino, P.; Raverot, G. Biological and Therapeutic Implications of the Tumor Microenvironment in Pituitary Adenomas. Endocr. Rev. 2022, 44, 297–311. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Kemeny, H.R.; Elsamadicy, A.A.; Farber, S.H.; Champion, C.D.; Lorrey, S.J.; Chongsathidkiet, P.; Woroniecka, K.I.; Cui, X.; Shen, S.H.; Rhodin, K.E.; et al. Targeting PD-L1 Initiates Effective Antitumor Immunity in a Murine Model of Cushing Disease. Clin. Cancer Res. 2020, 26, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.T.; Vesely, M.D.; Miyagishima, D.F. In silico analysis of the immunological landscape of pituitary adenomas. J. Neuro-Oncol. 2020, 147, 595–598. [Google Scholar] [CrossRef]
- Caccese, M.; Barbot, M.; Ceccato, F.; Padovan, M.; Gardiman, M.P.; Fassan, M.; Denaro, L.; Emanuelli, E.; D’Avella, D.; Scaroni, C.; et al. Rapid disease progression in patient with mismatch-repair deficiency pituitary ACTH-secreting adenoma treated with checkpoint inhibitor pembrolizumab. Anti-Cancer Drugs 2020, 31, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Manzoor, S.; Rothman, Y.; Hagen, M.; Pater, L.; Golnik, K.; Mahammedi, A.; Lin, A.L.; Bhabhra, R.; Forbes, J.A.; et al. Complete Response of a Patient with a Mismatch Repair Deficient Aggressive Pituitary Adenoma to Immune Checkpoint Inhibitor Therapy: A Case Report. Neurosurgery 2022, 91, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, C.; Ilie, M.D.; Salle, H.; Nassouri, A.S.; Gaillard, S.; Deluche, E.; Assaker, R.; Mortier, L.; Cortet, C.; Raverot, G. Immunotherapy in Corticotroph and Lactotroph Aggressive Tumors and Carcinomas: Two Case Reports and a Review of the Literature. J. Pers. Med. 2020, 10, 88. [Google Scholar] [CrossRef]
- Ilie, M.D.; Villa, C.; Cuny, T.; Cortet, C.; Assie, G.; Baussart, B.; Cancel, M.; Chanson, P.; Decoudier, B.; Deluche, E.; et al. Real-life efficacy and predictors of response to immunotherapy in pituitary tumors: A cohort study. Eur. J. Endocrinol. 2022, 187, 685–696. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Meng, X.; Zhou, S.; Zhu, Y.; Xu, J.; Tao, R. Apatinib (YN968D1) and Temozolomide in Recurrent Invasive Pituitary Adenoma: Case Report and Literature Review. World Neurosurg. 2019, 124, 319–322. [Google Scholar] [CrossRef]
- Osterhage, K.; Rotermund, R.; Droste, M.; Dierlamm, J.; Saeger, W.; Petersenn, S.; Aberle, J.; Flitsch, J. Bevacizumab in Aggressive Pituitary Adenomas—Experience with 3 Patients. Exp. Clin. Endocrinol. Diabetes 2021, 129, 178–185. [Google Scholar] [CrossRef]
- Dutta, P.; Reddy, K.S.; Rai, A.; Madugundu, A.K.; Solanki, H.S.; Bhansali, A.; Radotra, B.D.; Kumar, N.; Collier, D.; Iacovazzo, D.; et al. Surgery, Octreotide, Temozolomide, Bevacizumab, Radiotherapy, and Pegvisomant Treatment of an AIP Mutation—Positive Child. J. Clin. Endocrinol. Metab. 2019, 104, 3539–3544. [Google Scholar] [CrossRef]
- Syro, L.V.; Scheithauer, B.W.; Kovacs, K.; Toledo, R.A.; Londoño, F.J.; Ortiz, L.D.; Rotondo, F.; Horvath, E.; Uribe, H. Pituitary tumors in patients with MEN1 syndrome. Clinics 2012, 67 (Suppl. S1), 43–48. [Google Scholar] [CrossRef]
- Korsisaari, N.; Ross, J.; Wu, X.; Kowanetz, M.; Pal, N.; Hall, L.; Eastham-Anderson, J.; Forrest, W.F.; Van Bruggen, N.; Peale, F.V.; et al. Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin level in a mouse model of multiple endocrine neoplasia type 1. Clin. Cancer Res. 2008, 14, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Phase II Trial of Nivolumab Plus Ipilimumab in Patients with Aggressive Pituitary Tumors; National Cancer Institute: Bethesda, MD, USA, 2019.
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Pituitary tumour fibroblast-derived cytokines influence tumour aggressiveness. Endocr.-Relat. Cancer 2019, 26, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’ Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef]
- Marques-Pamies, M.; Gil, J.; Valassi, E.; Pons, L.; Carrato, C.; Jordà, M.; Puig-Domingo, M. New molecular tools for precision medicine in pituitary neuroendocrine tumors. Minerva Endocrinol. 2024, 49, 300–320. [Google Scholar] [CrossRef]
- Grande, G.; Graziani, A.; Toni, L.D.E.; Ferlin, A. Proteomics for the identification of peripheral markers in pituitary disease. Minerva Endocrinol. 2024, 49, 293–299. [Google Scholar] [CrossRef]
- Semenescu, L.E.; Tataranu, L.G.; Dricu, A.; Ciubotaru, G.V.; Radoi, M.P.; Rodriguez, S.M.B.; Kamel, A. A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis. Biomedicines 2023, 11, 2485. [Google Scholar] [CrossRef]
- Aydin, B.; Yildirim, E.; Erdogan, O.; Arga, K.Y.; Yilmaz, B.K.; Bozkurt, S.U.; Bayrakli, F.; Turanli, B. Past, Present, and Future of Therapies for Pituitary Neuroendocrine Tumors: Need for Omics and Drug Repositioning Guidance. Omics 2022, 26, 115–129. [Google Scholar] [CrossRef]
| GENE/PATHWAY | ROLE |
|---|---|
| MEN1 | It has a tumor suppressor function and is involved in cellular proliferation, gene transcription, and genome stability [23]. |
| CDKN1B | It is hypothesized that it is involved in tumorigenesis through unknown mechanisms and cell cycle regulation [24]. |
| PRKAR1A | It is related to the tumorigenesis of somatotrophinomas, lactotrophinomas, mixed PitNETs, and corticotrophinomas, and its loss enhances protein kinase A (PKA) signaling, which is involved in transcriptional regulation, cellular progression and proliferation, and apoptosis [24]. |
| GPR101 | It is associated with the development of somatotroph adenomas and is highly overexpressed in pituitary lesions of X-linked acro gigantism [23,24]. |
| VHL | It is involved in apoptosis and tumorigenesis and is associated with pituitary lesions in Von Hippel–Lindau syndrome [25]. |
| DICER1 | It promotes cellular proliferation. It is involved in tumorigenesis in DICER1 syndrome through incompletely understood mechanisms [26]. |
| MLH1 and MLH2 | They are involved in tumorigenesis through incompletely understood mechanisms in Lynch syndrome [49,50]. |
| AIP | It is involved in the tumorigenesis of all PitNETs through incompletely understood mechanisms [27]. |
| GNAS | The only consistent mutation demonstrated in somatotropinomas is McCune–Albright syndrome [24]. |
| SDHx | Germline mutations were demonstrated in hereditary pituitary lesions with pituitary adenomas, such as phaeochromocytoma/paraganglioma [28]. |
| PTTG1 | Its overexpression is correlated with tumor formation and progression [29,30]. |
| STAT3 | It supports tumorigenesis in somatotropinomas [31,32]. |
| CDH23 | It is involved in tumoral growth, mainly in somatotropinomas [33]. |
| IGSF1 | It increases the secretion of somatotropin hormone and IGF-1 levels and is associated with pituitary hyperplasia [34]. |
| PRDM2 | It is involved in c-Myc regulation [35]. |
| SLC20A | It is associated with the activation of the Wnt–b-catenin signaling pathway [36]. |
| SSTR1-5 and PR/SET Domain 2 | They are involved in the development of somatotroph adenomas. Decreased somatostatin and dopamine receptor expression levels have been associated with the tumorigenesis of somatotropinomas [37,38]. |
| GADD45γ | It is involved in the development of somatotroph and nonfunctioning adenomas through DNA damage and function in the negative regulation of cell growth [38]. |
| RASSF1A | It is involved in tumorigenesis through incompletely understood mechanisms [39]. |
| USP8, USP48, and BRAF | Mutations are associated with dysfunctions in the adrenocorticotropic hormone and can cause the activation of the EGF signaling pathway [42]. |
| SFRP2 | Its overexpression reduces b-catenin and decreases Wnt signaling activity, influencing the development of corticotrophinomas [41]. |
| FGFR2 | It is involved in the tumorigenesis of corticotrophinomas by inducing Rb phosphorylation and the regulation of cell cycle progression via p21 and p27, primarily through deubiquitination [39,42]. |
| USP90, HDAC2, CABLES1, PTAG, TSP-1, and CASP-8 | They are involved in the tumorigenesis of corticotrophinomas through unknown mechanisms [23]. |
| C5orf66-AS1 and ENC1 | Through unelucidated mechanisms, they are involved in the development of null cell adenomas [43,44]. |
| FAM90A1, ING2, ETS2, STAT6, MYT1L, and KCNK1 | They are involved in tumoral regrowth in PitNETs through unknown mechanisms [45]. |
| IL-6R/JAK2/STAT3/MMP9 | They are involved in cell survival, growth, proliferation, and metabolism in nonfunctioning adenomas [46]. |
| MEG and CDKN2A | They are involved in the tumorigenesis of nonfunctioning adenomas and somatotrophinomas [23,47,48]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tataranu, L.G. A Nexus of Biomolecular Complexities in Pituitary Neuroendocrine Tumors: Insights into Key Molecular Drivers. Biomedicines 2025, 13, 968. https://doi.org/10.3390/biomedicines13040968
Tataranu LG. A Nexus of Biomolecular Complexities in Pituitary Neuroendocrine Tumors: Insights into Key Molecular Drivers. Biomedicines. 2025; 13(4):968. https://doi.org/10.3390/biomedicines13040968
Chicago/Turabian StyleTataranu, Ligia Gabriela. 2025. "A Nexus of Biomolecular Complexities in Pituitary Neuroendocrine Tumors: Insights into Key Molecular Drivers" Biomedicines 13, no. 4: 968. https://doi.org/10.3390/biomedicines13040968
APA StyleTataranu, L. G. (2025). A Nexus of Biomolecular Complexities in Pituitary Neuroendocrine Tumors: Insights into Key Molecular Drivers. Biomedicines, 13(4), 968. https://doi.org/10.3390/biomedicines13040968






