Hearts, Data, and Artificial Intelligence Wizardry: From Imitation to Innovation in Cardiovascular Care
Abstract
:1. Introduction
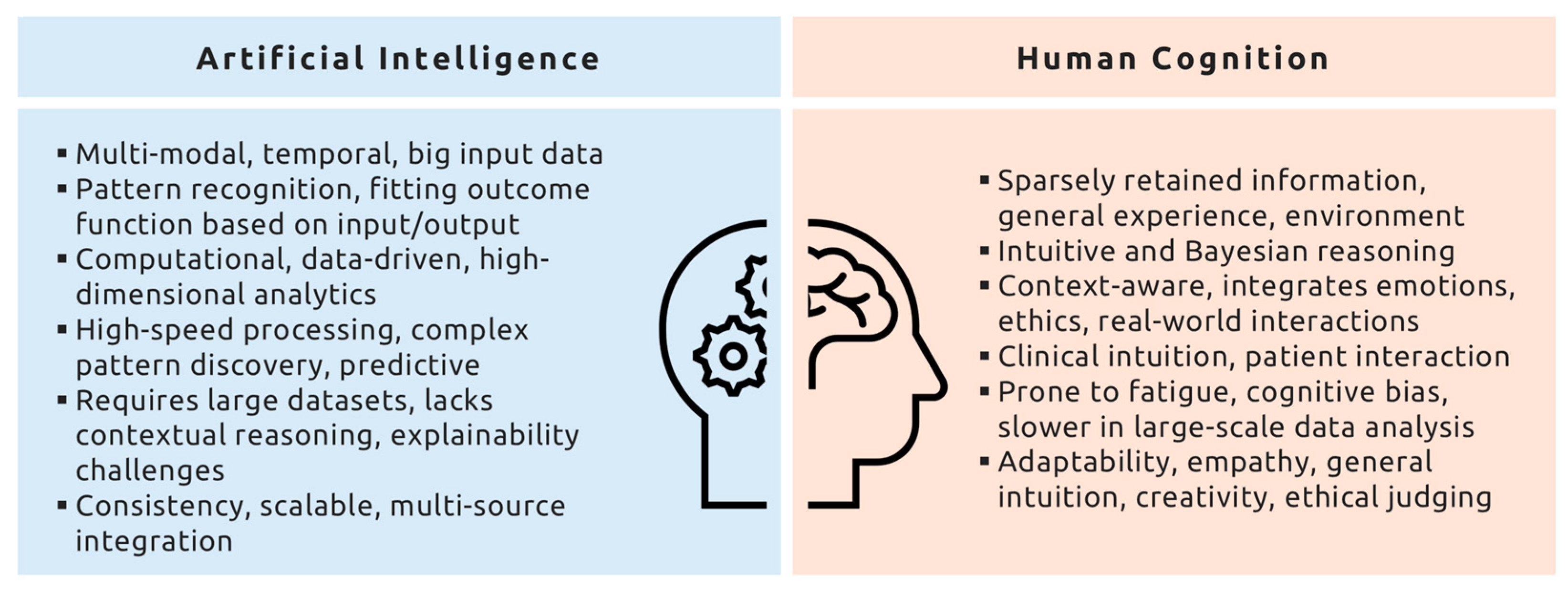
2. Human Cognition vs. Artificial Intelligence
2.1. Literature Search Strategy
2.2. Human Expertise in Context
2.3. AI’s True Strength: Tackling Intractable Problems
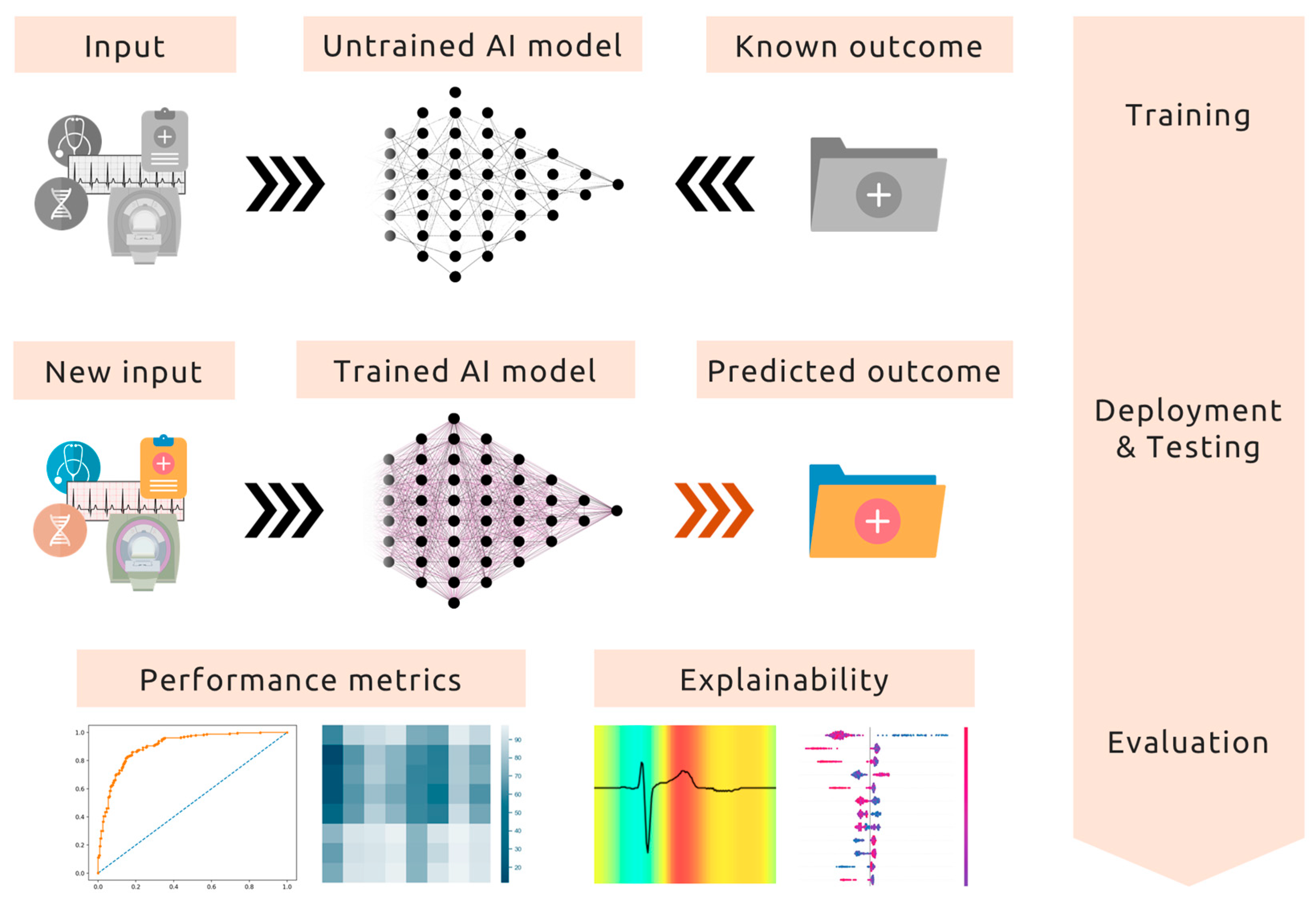
3. From Data to Innovation: AI’s Transformative Applications
3.1. Electrocardiogram
3.2. Cardiovascular Imaging
3.3. Precision Medicine and Omics
3.4. Preventive Cardiology
| Study | Task | AI Approach | Input Modalities | Outcomes and Explainability * |
|---|---|---|---|---|
| Agibetov et al., 2021 [51] | Automatic diagnosis of CA using CMR | Pretrained VGG16 CNN (transfer learning) | CMR imaging | AUC: 0.96, SEN: 94%, SPE: 90%; similar performance to expert radiologists |
| Akita et al., 2024 [29] | Predict myocardial fibrosis from TTE | CNN | TTE, CMR (LGE presence) for ground truth | AUC: 0.86; outperformed reference model based on clinical parameters |
| Attia et al., 2019 [16] | Screen and predict future LVSD using ECG | CNN (6 layers with Relu activation, batch normalization, max pooling, and spatial fusion) | 12-lead ECG | AUC: 0.93, SEN: 86.3%, SPE: 85.7%, ACC: 85.7%; superior AUC compared to traditional screening |
| Attia et al., 2019 [19] | Identify AF from normal sinus rhythm ECGs | CNN | 12-lead ECG | AUC: 0.87, SEN: 79.0%, SPE: 79.5%; outperformed standard risk scores |
| Bos et al., 2021 [52] | Detect LQTS from 12-lead ECGs | CNNs (10 blocks of convolutional, batch normalization, Relu, and max pooling layers) | 12-lead ECG | AUC: 0.90; improved performance over QTc interval measurement-based diagnosis, particularly in concealed cases |
| Chen et al., 2024 [53] | Classification of Fabry Cardiomyopathy vs. HCM | 3D Convolutional Neural Network (3D ResNet18) | CMR imaging | ACC: 91%, AUC: 0.91, F1-score: 0.85; XAI with Grad-CAM |
| Cohen-Shelly et al., 2021 [54] | Detection of moderate-to-severe AVS from ECG | CNN | ECG | AUC: 0.85, SEN: 78%, SPE: 74%; XAI with saliency maps |
| DeGroat et al., 2024 [41] | Novel cardiovascular biomarkers using multi-omics data | XGBoost classifier with Bayesian hyperparameter tuning | Multi-omics (RNA-seq, whole-genome sequencing, clinical demographics) | High prediction accuracy for cardiovascular disease (identified biomarkers showed strong literature-based validation); XAI with SHAP |
| Drouard et al., 2024 [42] | Prediction of cardiovascular risk factors from multi-omic data | Supervised and semi-supervised autoencoders, meta-learners | Blood-derived metabolomics, epigenetics, transcriptomics | AUC improvement of 0.09–0.14 for transcriptomics and 0.07–0.11 for metabolomics with transfer learning; feature importance analysis |
| Economou Lundeberg et al., 2023 [22] | Predicting the risk of VT using 24-h ambulatory ECG data | Elastic Net Regression Model | Ambulatory ECG | AUC: 0.76, NPV of bottom quintile: 98.2% |
| Gavidia et al., 2024 [24] | Prediction of AF onset using wearable device data | CNN (479 layers, EfficientNetV2) | R-to-R interval signals from wearable devices | ACC: 83%, F1-score: 85%, Warning time: 30.8 min before AF onset |
| Goto et al., 2021 [26] | Automated detection of CA using AI-based ECG and TTE models | ECG model: 2D CNN (18 layers); Echo model: 3D-CNN (trained on video sequences of apical 4-chamber views) | ECG and TTE | ECG model: C-statistics of 0.85–0.91 across multiple institutions; Echo model: C-statistics of 0.89–1.00; outperformed expert cardiologists across multiple datasets |
| Gregoire et al., 2024 [25] | Prediction of short-term AF onset using HRV from Holter ECG recordings | XGBoost decision tree trained on HRV parameters | 2-lead Holter ECG data | AUC: 0.92, AUPRC: 0.92, ACC: 84.5%, SEN: 83.0%, SPE: 86.6%, F1-score: 86.2% |
| Grogan et al., 2021 [27] | Detect CA from a standard 12-lead ECG | Deep Neural Network (AI-enhanced ECG model) trained to predict CA presence | 12-lead ECG, single-lead, and 6-lead ECG subsets | AUC: 0.91, SEN: 0.84, SPE: 0.85, PPV: 0.86, NPV: 0.84; predicted CA more than 6 months before clinical diagnosis |
| Jentzer et al., 2021 [55] | Identification of LVSD using ECGs | CNN trained on nearly 100,000 ECGs | 12-lead ECG, TTE for ground truth | AUC: 0.83, SEN: 72.8%, SPE: 77.8%, NPV: 84.9%, ACC: 76.1% |
| Jiang et al., 2024 [21] | Detection of congenital LQTS and differentiation of genotypes based on 12-lead ECGs | CNN (based on ResNetv2, trained using Bayesian optimization) | 12-lead ECG, genetic testing for KCNQ1 and KCNH2 variants | LQTS detection AUC: 0.93, Genotype differentiation AUC: 0.91, SEN: 0.90, F1-score: 0.84; outperformed expert-measured QTc in detecting LQTS, including concealed cases |
| Ko et al., 2020 [18] | Detection of HCM using a 12-lead ECG | CNN | 12-lead ECG | AUC: 0.96, SEN: 87%, SPE: 90%, PPV: 31%, NPV: 99%; outperformed standard ECG interpretation, distinguishing HCM from LVH and normal ECGs |
| Kolk et al., 2023 [34] | Prediction of non-arrhythmic mortality in patients with primary prevention ICD | XGBoost | Multimodal data: 12-lead ECG and clinical variables (demographics, medical history, medications, lab values) | AUC (internal validation): 0.90, AUC (external validation): 0.79, SEN: 70.3%, SPE: 69.6%, F1-score: 74.6%; outperformed traditional risk scores; XAI with SHAP and ECG heatmaps |
| Kolk et al., 2024 [32] | Prediction of malignant ventricular arrhythmias in patients with non-ischemic heart failure | DEEP RISK model: Residual VAE, XGBoost | CMR (LGE), 12-lead ECG, clinical data (demographics, medical history, lab values, medications) | AUC: 0.84, SEN: 0.98, SPE: 0.73, AUPRC: 0.31; XAI with SHAP and latent traversal visualizations |
| Lampert et al., 2023 [56] | Prediction of LVSD in patients with PVCs | Pretrained CNN (ResNet-152) | 12-lead ECG | AUC: 0.79 (internal validation), AUC: 0.85 (external validation); outperformed traditional PVC burden-based risk assessment methods; XAI with Grad-CAM |
| Lee et al., 2016 [57] | Prediction of VT one hour before its occurrence using NNs | NN with one hidden layer (5–13 hidden neurons) | 14 HRV and RRV parameters | AUC: 0.93, SEN: 88.2%, SPE: 82.4%, ACC: 85.3%; outperformed traditional VT prediction methods |
| Lee et al., 2023 [58] | Prediction of significant CAD on coronary CTA in asymptomatic individuals | NN with multi-task learning and feature selection | Coronary CTA, demographics, clinical and laboratory data | AUC: 0.78, ACC: 71.6%; outperformed CAD consortium score (AUC: 0.67), UDF score (AUC: 0.71), and PCE (AUC: 0.72); XAI with SHAP |
| Libiseller-Egger et al., 2022 [59] | Identification of the genetic basis of cardiovascular age and its impact on cardiovascular risk | CNN | 12-lead ECG, GWAS using UK Biobank data | Identified eight genetic loci associated with ECG-derived cardiovascular age, with a correlation between delta age and risk factors; feature importance analysis |
| Liu et al., 2023 [17] | AI TTE for diagnosing four entities: ASD, DCM, HCM, and prior MI | Pre-trained Inception-V3 for feature extraction from single frames, followed by a multiple-layer 1D CNN for video-level classification | TTE (apical 4-chamber videos) | AUCs: 0.99% (ASD), 0.98% (DCM), 0.99% (HCM), 0.98% (prior MI), and 0.98% (Normal); performed comparably to or better than cardiologists; XAI with CAM |
| Melo et al., 2023 [60] | Detection of BrS from an ECG without a sodium channel blocker challenge | Feed-forward NN (optimized with ensemble learning) | 12-lead ECG (9 leads used for classification: I, II, III, V1–V6) | Training cohort: ACC: 84%, SEN: 85.4%, SPE: 82.4%, AUC: 0.91; Validation cohort: ACC: 88.4%, SEN: 79.6%, SPE: 93.6%, AUC: 0.93; outperformed clinicians in BrS diagnosis; XAI with ECG heatmaps |
| Morita et al., 2021 [31] | Prediction of the positive genotype in patients with HCM using TTE images | CNN | TTE images (parasternal short- and long-axis, apical 2-, 3-, 4-, and 5-chamber views) | AUC: 0.86 (CNN & Mayo Score) vs. 0.72 (Mayo Score), AUC: 0.84 (CNN & Toronto Score) vs. 0.75 (Toronto Score); improved SEN, SPE, PPV, and NPV; outperformed reference models; XAI with Grad-CAM |
| O’Driscoll et al., 2023 [61] | Predicting MACE and all-cause mortality from LVEF and GLS | Machine learning-based AI algorithm for automated TTE image processing | AI-contoured left ventricular function from TTE images, GLS, and LVEF | AI-calculated LVEF and GLS were independently associated with MACE |
| Oikonomou et al., 2024 [33] | AI tool for guiding cardiac testing in stable chest pain patients | Machine-learning-derived algorithm (XGBoost) applied to phenomapping-based decision support | Demographic and clinical data (age, sex, BMI, hypertension, diabetes, lipid panel, medications, others) | AI-aligned testing was associated with lower risk of MI or death; improved CAD detection rates in anatomical-first testing; feature importance analysis |
| Oikonomou et al., 2024 [62] | Detection and risk stratification of AVS progression using a video-based AI biomarker | Deep learning-based video AI model (DASSi), saliency maps | TTE, CMR | Faster AVS progression prediction, Hazard Ratios for aortic valve replacement: 4.97 for YNHHS cohort, 4.04 for CSMC cohort, 11.38 for UK Biobank; XAI with saliency maps and feature importance analysis |
| Raghunath et al., 2021 [63] | Predicting new-onset AF from a 12-lead ECG to enable targeted screening | CNN trained on 12-lead ECG signals | 12-lead ECG, patient demographics (age, sex) | AUC: 0.85, AUPRC: 0.22 for predicting AF within 1 year; SEN: 69%, SPE: 81%; Hazard Ratio for high- vs. low-risk groups: 7.2 over 30 years; outperformed CHARGE-AF and an XGBoost model using only age and sex |
| Sahashi et al., 2024 [64] | Deep learning-based estimation of CMR tissue characteristics from TTE | Video-based CNN trained on TTE videos to predict CMR-derived labels | TTE, CMR for ground truth | AUC: 0.56–0.87 for different CMR parameters |
| Schwartz et al., 2022 [38] | Evaluating the feasibility, accuracy, and safety of AI-based left atrium reconstruction | Model-based FAM using an AI algorithm trained on >300 left atrial CTA | Electroanatomic mapping with CARTO 3, ICE, fluoroscopy, and cardiac CTA | Reduced mapping and fluoroscopy time during PVI; no major complications |
| Sun et al., 2024 [65] | Detecting CAD from ECG and PCG signals | Parallel CNN network with autoencoder, SVM classifier | ECG, PCG | ACC: 98.45%, SEN: 98.6%, SPE: 98.6%, F1-score: 98. 9%; outperformed various CAD detection methods |
| Surucu et al., 2021 [66] | Prediction of AF episodes before they occur with HRV | CNN with preprocessing steps | ECG-derived HRV | ACC: 100%, PRE: 100%, SEN: 100%, F1-score: 100% |
| Tokodi et al., 2023 [67] | Prediction of RVEF using 2D TTE videos | Spatiotemporal CNNs, ensemble model of three best-performing networks, saliency maps | 2D TTE videos (apical 4-chamber view) | Mean absolute error for RVEF: 4.57% (internal validation), 5.54% (external validation); ACC for RVEF <45%: 78.4% vs. human expert: 77.0%; performance comparable to human experts, but the model had higher SEN at the cost of SPE; XAI with 2D TTE heatmaps |
| Torres Soto et al., 2022 [68] | Differentiating HCM from HTN LVH using multimodal AI | Multimodal deep learning model (LVH-Fusion; late-average fusion, late-ranked fusion, and pre-trained and random late fusion models) | ECG, TTE | F1-score: 0.73 (HCM), 0.96 (HTN); SEN: 0.73; SPE: 0.96; outperforms single-modal models; false discovery rate reduced from 0.59 to 0.27; outperformed cardiologists in correctly classifying HCM and HTN; XAI with SHAP |
| Venkat et al., 2023 [69] | Identification of genes associated with cardiovascular risk using RNA-seq | RF model, recursive feature elimination, SelectKBest feature selection | RNA-seq (gene expression), demographic data (age, gender, race) | ACC for HF: 90.9%, for AF: 95%, for other cardiovascular diseases: 95.9%; feature importance analysis |
| Yang et al., 2021 [70] | Prediction of ischemia and prognosis from coronary plaque features | Machine learning-based feature selection (Boruta algorithm, hierarchical clustering) | Coronary CTA, FFR and clinical data for ground truth | AUC for low FFR: 0.8, for 5-year vessel-oriented composite outcome: 0.71; improved ischemia prediction; feature importance analysis |
| Zhang et al., 2022 [71] | Identification of CAD-related genes from gene networks | Graph Convolutional Network, three-layer deep learning model | Gene expression, gene interaction networks | AUC: 0.75, AUPR: 0.78, ACC: 66.9%; better performance than other models |
| Zhang et al., 2023 [72] | Identification of CA using TTE and machine learning | Various machine models: LR, RF, SVM, others; texture feature extraction | TTE, myocardial texture analysis | AUC: 0.71–0.81, across models; LR had the best performance (F1-score: 0.34, SEN: 0.21, SPE: 1.0); outperformed traditional TTE |
| Zhao et al., 2022 [73] | Prediction of HF with preserved ejection fraction using DNA methylation and clinical data | Deep learning framework integrating Factorization Machine-based Neural Network, LASSO, and XGBoost-based feature selection | DNA methylation (CpG sites), clinical data | AUC: 0.90, Hosmer–Lemeshow statistic: 6.17 (p: 0.632); outperformed existing risk models |
| Zhou et al., 2023 [30] | Differentiation of ischemic cardiomyopathy from DCM using TTE | Machine learning models: XGBoost (best-performing), logistic regression, RF, NN | TTE, demographic data | XGBoost: AUC: 0.93, ACC: 75%, SEN: 72%, SPE: 78% (internal validation); AUC: 0.80, ACC: 78%, SEN: 64%, SPE: 93% (external validation) |
4. Challenges and Future Opportunities
4.1. The Black-Box Problem: Enhancing AI Transparency and Explainability
4.2. Bias in AI Models: Ensuring Fair and Equitable AI
4.3. Regulatory and Ethical Considerations
4.4. Interdisciplinary Collaboration: Bridging the Gap
4.5. Technological Advances and Open Science
- High-performance computational hardware: advances in graphic processing units (GPU) and tensor processing units (TPU) enable AI models to analyze continuous ECG recordings, high-resolution imaging, and multimodal data integration in real-time, drastically improving efficiency in AI workflows [82].
- Self-supervised learning: This method is an emerging paradigm that enables AI models to learn useful representations from unlabeled data by creating surrogate tasks, such as predicting missing parts of ECG signals or reconstructing corrupted imaging inputs. This is particularly valuable in cardiovascular medicine, where labeled datasets are limited and costly to obtain. Self-supervised learning has already shown promising results in certain areas of cardiovascular research, such as echocardiographic view classification [83,84] and anomaly detection in ECGs [85].
- Federated learning: Federated learning allows multiple institutions to collaboratively train AI models without sharing raw patient data. Instead, model updates are exchanged, preserving patient privacy and complying with data protection regulations [86]. This decentralized approach facilitates access to diverse and representative datasets, improving model generalizability and robustness. Federated learning has already marked some use cases in the field, such as distributed ECG classification [87], cross-center imaging tasks [88], and model development for genomic research [89].
- Foundational models in cardiovascular AI: Foundational models—large-scale, pre-trained neural networks initially developed for broad tasks—are increasingly adapted for cardiovascular medicine [90,91]. These models, such as HeartBEiT for ECG interpretation and EchoCLIP for echo-imaging analysis, are trained on vast and diverse datasets, can be fine-tuned for a variety of downstream clinical applications [92,93,94]. Similar to ChatGPT (OpenAI) in natural language processing, foundational models in medicine can capture universal patterns across different data modalities and patient populations, and offer enhanced performance, adaptability, and transferability to novel cardiovascular tasks, even in low-resource or data-scarce environments.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial Intelligence in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef]
- Faust, O.; Salvi, M.; Barua, P.D.; Chakraborty, S.; Molinari, F.; Acharya, U.R. Issues and Limitations on the Road to Fair and Inclusive AI Solutions for Biomedical Challenges. Sensors 2025, 25, 205. [Google Scholar] [CrossRef] [PubMed]
- Takita, H.; Kabata, D.; Walston, S.L.; Tatekawa, H.; Saito, K.; Tsujimoto, Y.; Miki, Y.; Ueda, D. Diagnostic Performance Comparison between Generative AI and Physicians: A Systematic Review and Meta-Analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- Korteling, J.E.H.; Van De Boer-Visschedijk, G.C.; Blankendaal, R.A.M.; Boonekamp, R.C.; Eikelboom, A.R. Human-versus Artificial Intelligence. Front. Artif. Intell. 2021, 4, 622364. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial Intelligence in Precision Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, London, UK, 13–14 May 2014; pp. 1–10. [Google Scholar]
- Monteith, S.; Glenn, T.; Geddes, J.R.; Achtyes, E.D.; Whybrow, P.C.; Bauer, M. Differences between Human and Artificial/Augmented Intelligence in Medicine. Comput. Hum. Behav. Artif. Hum. 2024, 2, 100084. [Google Scholar] [CrossRef]
- Lussier, M.-T.; Richard, C. Handling Cues from Patients. Can. Fam. Physician Med. Fam. Can. 2009, 55, 1213–1214. [Google Scholar]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key Challenges for Delivering Clinical Impact with Artificial Intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Pantelidis, P.; Bampa, M.; Oikonomou, E.; Papapetrou, P. Machine Learning Models for Automated Interpretation of 12-Lead Electrocardiographic Signals: A Narrative Review of Techniques, Challenges, Achievements and Clinical Relevance. J. Med. Artif. Intell. 2023, 6, 6. [Google Scholar] [CrossRef]
- Moradi, A.; Olanisa, O.O.; Nzeako, T.; Shahrokhi, M.; Esfahani, E.; Fakher, N.; Khazeei Tabari, M.A. Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI. J. Imaging 2024, 10, 193. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Bedoya, A.; Bihorac, A.; Celi, L.; Churpek, M.; Economou-Zavlanos, N.J.; Elbers, P.; Saria, S.; Liu, V.; Lyons, P.G.; et al. Use of Artificial Intelligence in Critical Care: Opportunities and Obstacles. Crit. Care 2024, 28, 113. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.R.; Hampton, J. The ECG Made Easy, 9th ed.; Elsevier: Edinburgh, UK; London, UK; New York, NY, USA; Oxford, UK; Philadelphia, PA, USA; St. Louis, MO, USA; Sydney, Australia, 2019; ISBN 978-0-7020-7457-8. [Google Scholar]
- Kaddoura, S. Echo Made Easy, 3rd ed.; Elsevier: Edinburgh, UK; London, UK; New York, NY, USA; Oxford, UK; Philadelphia, PA, USA; St. Louis, MO, USA; Sydney, Australia; Toronto, ON, Canada, 2016; ISBN 978-0-7020-6656-6. [Google Scholar]
- Pantelidis, P.; Oikonomou, E.; Gialamas, I.; Goliopoulou, A.; Sarantos, S.; Zakynthinos, G.E.; Anastasiou, A.; Gounaridi, M.I.; Kalogeras, K.; Siasos, G. Decoding the Heart: How Artificial Intelligence Is Transforming Cardiology. J. Med. Artif. Intell. 2025, 8, 9. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for Cardiac Contractile Dysfunction Using an Artificial Intelligence–Enabled Electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chang, H.; Yang, D.; Yang, F.; Wang, Q.; Deng, Y.; Li, L.; Lv, W.; Zhang, B.; Yu, L.; et al. A Deep Learning Framework Assisted Echocardiography with Diagnosis, Lesion Localization, Phenogrouping Heterogeneous Disease, and Anomaly Detection. Sci. Rep. 2023, 13, 3. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An Artificial Intelligence-Enabled ECG Algorithm for the Identification of Patients with Atrial Fibrillation during Sinus Rhythm: A Retrospective Analysis of Outcome Prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Ruipérez-Campillo, S.; Alvarez-Florez, L.; Deb, B.; Bekkers, E.J.; Allaart, C.P.; Van Der Lingen, A.-L.C.J.; Clopton, P.; Išgum, I.; Wilde, A.A.M.; et al. Dynamic Prediction of Malignant Ventricular Arrhythmias Using Neural Networks in Patients with an Implantable Cardioverter-Defibrillator. eBioMedicine 2024, 99, 104937. [Google Scholar] [CrossRef]
- Jiang, R.; Cheung, C.C.; Garcia-Montero, M.; Davies, B.; Cao, J.; Redfearn, D.; Laksman, Z.M.; Grondin, S.; Atallah, J.; Escudero, C.A.; et al. Deep Learning–Augmented ECG Analysis for Screening and Genotype Prediction of Congenital Long QT Syndrome. JAMA Cardiol. 2024, 9, 377. [Google Scholar] [CrossRef]
- Economou Lundeberg, J.; Måneheim, A.; Persson, A.; Dziubinski, M.; Sridhar, A.; Healey, J.S.; Slusarczyk, M.; Engström, G.; Johnson, L.S. Ventricular Tachycardia Risk Prediction with an Abbreviated Duration Mobile Cardiac Telemetry. Heart Rhythm O2 2023, 4, 500–505. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Frodi, D.M.; Langford, J.; Jacobsen, P.K.; Risum, N.; Andersen, T.O.; Tan, H.L.; Hastrup Svendsen, J.; Knops, R.E.; Diederichsen, S.Z.; et al. Artificial Intelligence-Enhanced Wearable Technology Enables Ventricular Arrhythmia Prediction. Eur. Heart J. Digit. Health 2024, ztae069. [Google Scholar] [CrossRef]
- Gavidia, M.; Zhu, H.; Montanari, A.N.; Fuentes, J.; Cheng, C.; Dubner, S.; Chames, M.; Maison-Blanche, P.; Rahman, M.M.; Sassi, R.; et al. Early Warning of Atrial Fibrillation Using Deep Learning. Patterns 2024, 5, 100970. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, J.M.; Gilon, C.; Bersini, H.; Groben, L.; Nguyen, T.; Godart, P.; Carlier, S. Heart Rate Variability to Predict 1-Day Atrial Fibrillation Episode Onset Using Machine Learning. Eur. Heart J. 2024, 45, ehae666.339. [Google Scholar] [CrossRef]
- Goto, S.; Mahara, K.; Beussink-Nelson, L.; Ikura, H.; Katsumata, Y.; Endo, J.; Gaggin, H.K.; Shah, S.J.; Itabashi, Y.; MacRae, C.A.; et al. Artificial Intelligence-Enabled Fully Automated Detection of Cardiac Amyloidosis Using Electrocardiograms and Echocardiograms. Nat. Commun. 2021, 12, 2726. [Google Scholar] [CrossRef]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence–Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef]
- Feng, R.; Deb, B.; Ganesan, P.; Tjong, F.V.Y.; Rogers, A.J.; Ruipérez-Campillo, S.; Somani, S.; Clopton, P.; Baykaner, T.; Rodrigo, M.; et al. Segmenting Computed Tomograms for Cardiac Ablation Using Machine Learning Leveraged by Domain Knowledge Encoding. Front. Cardiovasc. Med. 2023, 10, 1189293. [Google Scholar] [CrossRef]
- Akita, K.; Kusunose, K.; Haga, A.; Shimomura, T.; Kosaka, Y.; Ishiyama, K.; Hasegawa, K.; Fifer, M.A.; Maurer, M.S.; Shimada, Y.J. Deep Learning of Echocardiography Distinguishes between Presence and Absence of Late Gadolinium Enhancement on Cardiac Magnetic Resonance in Patients with Hypertrophic Cardiomyopathy. Echo Res. Pract. 2024, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Deng, Y.; Liu, Y.; Su, X.; Zeng, X. Echocardiography-Based Machine Learning Algorithm for Distinguishing Ischemic Cardiomyopathy from Dilated Cardiomyopathy. BMC Cardiovasc. Disord. 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.X.; Kusunose, K.; Haga, A.; Sata, M.; Hasegawa, K.; Raita, Y.; Reilly, M.P.; Fifer, M.A.; Maurer, M.S.; Shimada, Y.J. Deep Learning Analysis of Echocardiographic Images to Predict Positive Genotype in Patients with Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 669860. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Ruipérez-Campillo, S.; Allaart, C.P.; Wilde, A.A.M.; Knops, R.E.; Narayan, S.M.; Tjong, F.V.Y.; DEEP RISK investigators. Multimodal Explainable Artificial Intelligence Identifies Patients with Non-Ischaemic Cardiomyopathy at Risk of Lethal Ventricular Arrhythmias. Sci. Rep. 2024, 14, 14889. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Aminorroaya, A.; Dhingra, L.S.; Partridge, C.; Velazquez, E.J.; Desai, N.R.; Krumholz, H.M.; Miller, E.J.; Khera, R. Real-World Evaluation of an Algorithmic Machine-Learning-Guided Testing Approach in Stable Chest Pain: A Multinational, Multicohort Study. Eur. Heart J. Digit. Health 2024, 5, 303–313. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Ruipérez-Campillo, S.; Deb, B.; Bekkers, E.J.; Allaart, C.P.; Rogers, A.J.; Van Der Lingen, A.-L.C.J.; Alvarez Florez, L.; Isgum, I.; De Vos, B.D.; et al. Optimizing Patient Selection for Primary Prevention Implantable Cardioverter-Defibrillator Implantation: Utilizing Multimodal Machine Learning to Assess Risk of Implantable Cardioverter-Defibrillator Non-Benefit. EP Eur. 2023, 25, euad271. [Google Scholar] [CrossRef] [PubMed]
- Pirruccello, J.P.; Di Achille, P.; Choi, S.H.; Rämö, J.T.; Khurshid, S.; Nekoui, M.; Jurgens, S.J.; Nauffal, V.; Kany, S.; FinnGen; et al. Deep Learning of Left Atrial Structure and Function Provides Link to Atrial Fibrillation Risk. Nat. Commun. 2024, 15, 4304. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Sami, Z.; Cannata, M.; Ciftcikal, Y.; Caron, E.; Thomas, S.V.; Porter, C.R.; Tsioulias, A.; Gujja, M.; Sakai, K.; et al. Artificial Intelligence in Intravascular Imaging for Percutaneous Coronary Interventions: A New Era of Precision. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102506. [Google Scholar] [CrossRef]
- Samant, S.; Panagopoulos, A.N.; Wu, W.; Zhao, S.; Chatzizisis, Y.S. Artificial Intelligence in Coronary Artery Interventions: Preprocedural Planning and Procedural Assistance. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102519. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Chorin, E.; Mann, T.; Levi, M.Y.; Hochstadt, A.; Margolis, G.; Viskin, S.; Banai, S.; Rosso, R. Reconstruction of the Left Atrium for Atrial Fibrillation Ablation Using the Machine Learning CARTO 3 M-FAM Software. J. Interv. Card. Electrophysiol. 2022, 64, 39–47. [Google Scholar] [CrossRef]
- Bahlke, F.; Englert, F.; Popa, M.; Bourier, F.; Reents, T.; Lennerz, C.; Kraft, H.; Martinez, A.T.; Kottmaier, M.; Syväri, J.; et al. First Clinical Data on Artificial Intelligence-guided Catheter Ablation in Long-standing Persistent Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2024, 35, 406–414. [Google Scholar] [CrossRef]
- Martelli, E.; Capoccia, L.; Di Francesco, M.; Cavallo, E.; Pezzulla, M.G.; Giudice, G.; Bauleo, A.; Coppola, G.; Panagrosso, M. Current Applications and Future Perspectives of Artificial and Biomimetic Intelligence in Vascular Surgery and Peripheral Artery Disease. Biomimetics 2024, 9, 465. [Google Scholar] [CrossRef]
- DeGroat, W.; Abdelhalim, H.; Peker, E.; Sheth, N.; Narayanan, R.; Zeeshan, S.; Liang, B.T.; Ahmed, Z. Multimodal AI/ML for Discovering Novel Biomarkers and Predicting Disease Using Multi-Omics Profiles of Patients with Cardiovascular Diseases. Sci. Rep. 2024, 14, 26503. [Google Scholar] [CrossRef]
- Drouard, G.; Mykkänen, J.; Heiskanen, J.; Pohjonen, J.; Ruohonen, S.; Pahkala, K.; Lehtimäki, T.; Wang, X.; Ollikainen, M.; Ripatti, S.; et al. Exploring Machine Learning Strategies for Predicting Cardiovascular Disease Risk Factors from Multi-Omic Data. BMC Med. Inform. Decis. Mak. 2024, 24, 116. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Sytkowski, P.A.; Kannel, W.B.; D’Agostino, R.B. Changes in Risk Factors and the Decline in Mortality from Cardiovascular Disease: The Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can Machine-Learning Improve Cardiovascular Risk Prediction Using Routine Clinical Data? PLoS ONE 2017, 12, e0174944. [Google Scholar] [CrossRef]
- Gerculy, R.; Benedek, I.; Kovács, I.; Rat, N.; Halațiu, V.B.; Rodean, I.; Bordi, L.; Blîndu, E.; Roșca, A.; Mátyás, B.-B.; et al. CT-Assessment of Epicardial Fat Identifies Increased Inflammation at the Level of the Left Coronary Circulation in Patients with Atrial Fibrillation. J. Clin. Med. 2024, 13, 1307. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Inflammatory Related Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Targets. Curr. Pharm. Des. 2020, 26, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Mureddu, S.; Loreni, F.; Ferraraccio, F.; Panarese, I.; Trotta, M.C.; Gatta, G.; et al. Metformin Therapy Effects on the Expression of Sodium-Glucose Cotransporter 2, Leptin, and SIRT6 Levels in Pericoronary Fat Excised from Pre-Diabetic Patients with Acute Myocardial Infarction. Biomedicines 2021, 9, 904. [Google Scholar] [CrossRef]
- Rudnicka, Z.; Proniewska, K.; Perkins, M.; Pregowska, A. Cardiac Healthcare Digital Twins Supported by Artificial Intelligence-Based Algorithms and Extended Reality—A Systematic Review. Electronics 2024, 13, 866. [Google Scholar] [CrossRef]
- Dalakoti, M.; Wong, S.; Lee, W.; Lee, J.; Yang, H.; Loong, S.; Loh, P.H.; Tyebally, S.; Djohan, A.; Ong, J.; et al. Incorporating AI into Cardiovascular Diseases Prevention-Insights from Singapore. Lancet Reg. Health West. Pac. 2024, 48, 101102. [Google Scholar] [CrossRef]
- Agibetov, A.; Kammerlander, A.; Duca, F.; Nitsche, C.; Koschutnik, M.; Donà, C.; Dachs, T.-M.; Rettl, R.; Stria, A.; Schrutka, L.; et al. Convolutional Neural Networks for Fully Automated Diagnosis of Cardiac Amyloidosis by Cardiac Magnetic Resonance Imaging. J. Pers. Med. 2021, 11, 1268. [Google Scholar] [CrossRef]
- Bos, J.M.; Attia, Z.I.; Albert, D.E.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients with Electrocardiographically Concealed Long QT Syndrome from the Surface 12-Lead Electrocardiogram. JAMA Cardiol. 2021, 6, 532. [Google Scholar] [CrossRef]
- Chen, W.-W.; Kuo, L.; Lin, Y.-X.; Yu, W.-C.; Tseng, C.-C.; Lin, Y.-J.; Huang, C.-C.; Chang, S.-L.; Wu, J.C.-H.; Chen, C.-K.; et al. A Deep Learning Approach to Classify Fabry Cardiomyopathy from Hypertrophic Cardiomyopathy Using Cine Imaging on Cardiac Magnetic Resonance. Int. J. Biomed. Imaging 2024, 2024, 6114826. [Google Scholar] [CrossRef]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.-Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram Screening for Aortic Valve Stenosis Using Artificial Intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Kashou, A.H.; Attia, Z.I.; Lopez-Jimenez, F.; Kapa, S.; Friedman, P.A.; Noseworthy, P.A. Left Ventricular Systolic Dysfunction Identification Using Artificial Intelligence-Augmented Electrocardiogram in Cardiac Intensive Care Unit Patients. Int. J. Cardiol. 2021, 326, 114–123. [Google Scholar] [CrossRef]
- Lampert, J.; Vaid, A.; Whang, W.; Koruth, J.; Miller, M.A.; Langan, M.-N.; Musikantow, D.; Turagam, M.; Maan, A.; Kawamura, I.; et al. A Novel ECG-Based Deep Learning Algorithm to Predict Cardiomyopathy in Patients with Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2023, 9, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, S.-Y.; Seo, M.; Nam, G.-B.; Joo, S. Prediction of Ventricular Tachycardia One Hour before Occurrence Using Artificial Neural Networks. Sci. Rep. 2016, 6, 32390. [Google Scholar] [CrossRef]
- Lee, H.; Kang, B.G.; Jo, J.; Park, H.E.; Yoon, S.; Choi, S.-Y.; Kim, M.J. Deep Learning-Based Prediction for Significant Coronary Artery Stenosis on Coronary Computed Tomography Angiography in Asymptomatic Populations. Front. Cardiovasc. Med. 2023, 10, 1167468. [Google Scholar] [CrossRef] [PubMed]
- Libiseller-Egger, J.; Phelan, J.E.; Attia, Z.I.; Benavente, E.D.; Campino, S.; Friedman, P.A.; Lopez-Jimenez, F.; Leon, D.A.; Clark, T.G. Deep Learning-Derived Cardiovascular Age Shares a Genetic Basis with Other Cardiac Phenotypes. Sci. Rep. 2022, 12, 22625. [Google Scholar] [CrossRef]
- Melo, L.; Ciconte, G.; Christy, A.; Vicedomini, G.; Anastasia, L.; Pappone, C.; Grant, E. Deep Learning Unmasks the ECG Signature of Brugada Syndrome. PNAS Nexus 2023, 2, pgad327. [Google Scholar] [CrossRef]
- O’Driscoll, J.M.; Tuttolomondo, D.; Gaibazzi, N. Artificial Intelligence Calculated Global Longitudinal Strain and Left Ventricular Ejection Fraction Predicts Cardiac Events and All-cause Mortality in Patients with Chest Pain. Echocardiography 2023, 40, 1356–1364. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Holste, G.; Yuan, N.; Coppi, A.; McNamara, R.L.; Haynes, N.A.; Vora, A.N.; Velazquez, E.J.; Li, F.; Menon, V.; et al. A Multimodal Video-Based AI Biomarker for Aortic Stenosis Development and Progression. JAMA Cardiol. 2024, 9, 534. [Google Scholar] [CrossRef]
- Raghunath, S.; Pfeifer, J.M.; Ulloa-Cerna, A.E.; Nemani, A.; Carbonati, T.; Jing, L.; vanMaanen, D.P.; Hartzel, D.N.; Ruhl, J.A.; Lagerman, B.F.; et al. Deep Neural Networks Can Predict New-Onset Atrial Fibrillation From the 12-Lead ECG and Help Identify Those at Risk of Atrial Fibrillation–Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef]
- Sahashi, Y.; Ouyang, D.; Kwan, A. Using Deep Learning to Predict Cardiovascular Magnetic Resonance Findings from Echocardiography Videos. Eur. Heart J. 2024, 45, ehae666.3458. [Google Scholar] [CrossRef]
- Sun, C.; Liu, C.; Wang, X.; Liu, Y.; Zhao, S. Coronary Artery Disease Detection Based on a Novel Multi-Modal Deep-Coding Method Using ECG and PCG Signals. Sensors 2024, 24, 6939. [Google Scholar] [CrossRef]
- Surucu, M.; Isler, Y.; Perc, M.; Kara, R. Convolutional Neural Networks Predict the Onset of Paroxysmal Atrial Fibrillation: Theory and Applications. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 113119. [Google Scholar] [CrossRef] [PubMed]
- Tokodi, M.; Magyar, B.; Soós, A.; Takeuchi, M.; Tolvaj, M.; Lakatos, B.K.; Kitano, T.; Nabeshima, Y.; Fábián, A.; Szigeti, M.B.; et al. Deep Learning-Based Prediction of Right Ventricular Ejection Fraction Using 2D Echocardiograms. JACC Cardiovasc. Imaging 2023, 16, 1005–1018. [Google Scholar] [CrossRef]
- Soto, J.T.; Weston Hughes, J.; Sanchez, P.A.; Perez, M.; Ouyang, D.; Ashley, E.A. Multimodal Deep Learning Enhances Diagnostic Precision in Left Ventricular Hypertrophy. Eur. Heart J. Digit. Health 2022, 3, 380–389. [Google Scholar] [CrossRef]
- Venkat, V.; Abdelhalim, H.; DeGroat, W.; Zeeshan, S.; Ahmed, Z. Investigating Genes Associated with Heart Failure, Atrial Fibrillation, and Other Cardiovascular Diseases, and Predicting Disease Using Machine Learning Techniques for Translational Research and Precision Medicine. Genomics 2023, 115, 110584. [Google Scholar] [CrossRef]
- Yang, S.; Koo, B.-K.; Hoshino, M.; Lee, J.M.; Murai, T.; Park, J.; Zhang, J.; Hwang, D.; Shin, E.-S.; Doh, J.-H.; et al. CT Angiographic and Plaque Predictors of Functionally Significant Coronary Disease and Outcome Using Machine Learning. JACC Cardiovasc. Imaging 2021, 14, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, Y.; He, W.; Yuan, F.; Zeng, Y.; Zhang, S. GCN-GENE: A Novel Method for Prediction of Coronary Heart Disease-Related Genes. Comput. Biol. Med. 2022, 150, 105918. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, T.; Su, C.; Qin, S.; Li, J.; Zeng, D.; Cai, Y.; Huang, T.; Wu, J. Deep Learn-Based Computer-Assisted Transthoracic Echocardiography: Approach to the Diagnosis of Cardiac Amyloidosis. Int. J. Cardiovasc. Imaging 2023, 39, 955–965. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, Y.; Ruan, X.; Wang, X.; He, K.; Dong, W.; Qu, H.; Fang, X. A Deep Learning Model for Early Risk Prediction of Heart Failure with Preserved Ejection Fraction by DNA Methylation Profiles Combined with Clinical Features. Clin. Epigenet. 2022, 14, 11. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Arun, N.; Gaw, N.; Singh, P.; Chang, K.; Aggarwal, M.; Chen, B.; Hoebel, K.; Gupta, S.; Patel, J.; Gidwani, M.; et al. Assessing the (Un)Trustworthiness of Saliency Maps for Localizing Abnormalities in Medical Imaging. Radiol. Artif. Intell. 2020, 3, e200267. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tourani, R. Predictive and Causal Implications of Using Shapley Value for Model Interpretation. In Proceedings of the 2020 KDD Workshop on Causal Discovery, San Diego, CA, USA, 24 August 2020. [Google Scholar]
- Svennberg, E.; Han, J.K.; Caiani, E.G.; Engelhardt, S.; Ernst, S.; Friedman, P.; Garcia, R.; Ghanbari, H.; Hindricks, G.; Man, S.H.; et al. State of the Art of Artificial Intelligence in Clinical Electrophysiology in 2025. A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC Working Group in e-Cardiology. EP Eur. 2025, euaf071. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 1 March 2025).
- European Medicines Agency. Artificial Intelligence—EMA. Available online: https://www.ema.europa.eu/en/about-us/how-we-work/big-data/artificial-intelligence (accessed on 1 March 2025).
- Medicines and Healthcare Products Regulatory Agency. MHRA’s AI Regulatory Strategy Ensures Patient Safety and Industry Innovation into 2030. Available online: https://www.gov.uk/government/news/mhras-ai-regulatory-strategy-ensures-patient-safety-and-industry-innovation-into-2030 (accessed on 1 March 2025).
- Nikolić, G.S.; Dimitrijević, B.R.; Nikolić, T.R.; Stojcev, M.K. Survey of Three Types of Processing Units: CPU, GPU and TPU. In Proceedings of the 2022 57th International Scientific Conference on Information, Communication and Energy Systems and Technologies (ICEST), Ohrid, North Macedonia, 16–18 June 2022; pp. 1–6. [Google Scholar]
- Huang, S.-C.; Pareek, A.; Jensen, M.; Lungren, M.P.; Yeung, S.; Chaudhari, A.S. Self-Supervised Learning for Medical Image Classification: A Systematic Review and Implementation Guidelines. npj Digit. Med. 2023, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, L.; Wa Maina, C.; Zühlke, L. Self-Supervised Multi-Task Learning for the Detection and Classification of RHD-Induced Valvular Pathology. J. Imaging 2025, 11, 97. [Google Scholar] [CrossRef]
- Mehari, T.; Strodthoff, N. Self-Supervised Representation Learning from 12-Lead ECG Data. Comput. Biol. Med. 2022, 141, 105114. [Google Scholar] [CrossRef]
- European Union Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data (General Data Protection Regulation). 2016. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng (accessed on 1 March 2025).
- Manocha, A.; Sood, S.K.; Bhatia, M. Federated Learning-Inspired Smart ECG Classification: An Explainable Artificial Intelligence Approach. Multimed. Tools Appl. 2024. [Google Scholar] [CrossRef]
- Goto, S.; Solanki, D.; John, J.E.; Yagi, R.; Homilius, M.; Ichihara, G.; Katsumata, Y.; Gaggin, H.K.; Itabashi, Y.; MacRae, C.A.; et al. Multinational Federated Learning Approach to Train ECG and Echocardiogram Models for Hypertrophic Cardiomyopathy Detection. Circulation 2022, 146, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Calvino, G.; Peconi, C.; Strafella, C.; Trastulli, G.; Megalizzi, D.; Andreucci, S.; Cascella, R.; Caltagirone, C.; Zampatti, S.; Giardina, E. Federated Learning: Breaking Down Barriers in Global Genomic Research. Genes 2024, 15, 1650. [Google Scholar] [CrossRef]
- Mathew, G.; Barbosa, D.; Prince, J.; Venkatraman, S. Foundation Models for Cardiovascular Disease Detection via Biosignals from Digital Stethoscopes. npj Cardiovasc. Health 2024, 1, 25. [Google Scholar] [CrossRef]
- Quer, G.; Topol, E.J. The Potential for Large Language Models to Transform Cardiovascular Medicine. Lancet Digit. Health 2024, 6, e767–e771. [Google Scholar] [CrossRef]
- Vaid, A.; Jiang, J.; Sawant, A.; Lerakis, S.; Argulian, E.; Ahuja, Y.; Lampert, J.; Charney, A.; Greenspan, H.; Narula, J.; et al. A Foundational Vision Transformer Improves Diagnostic Performance for Electrocardiograms. npj Digit. Med. 2023, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Vukadinovic, M.; Yuan, N.; Ouyang, D. Vision–Language Foundation Model for Echocardiogram Interpretation. Nat. Med. 2024, 30, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Brennan, K.A.; Azizi, Z.; Goyal, J.; Deb, B.; Chang, H.J.; Ganesan, P.; Clopton, P.; Pedron, M.; Ruipérez-Campillo, S.; et al. Engineering of Generative Artificial Intelligence and Natural Language Processing Models to Accurately Identify Arrhythmia Recurrence. Circ. Arrhythm. Electrophysiol. 2025, 18, e013023. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Bild, D.E. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am. J. Epidemiol. 2002, 156, 871–881. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantelidis, P.; Dilaveris, P.; Ruipérez-Campillo, S.; Goliopoulou, A.; Giannakodimos, A.; Theofilis, P.; De Lucia, R.; Katsarou, O.; Zisimos, K.; Kalogeras, K.; et al. Hearts, Data, and Artificial Intelligence Wizardry: From Imitation to Innovation in Cardiovascular Care. Biomedicines 2025, 13, 1019. https://doi.org/10.3390/biomedicines13051019
Pantelidis P, Dilaveris P, Ruipérez-Campillo S, Goliopoulou A, Giannakodimos A, Theofilis P, De Lucia R, Katsarou O, Zisimos K, Kalogeras K, et al. Hearts, Data, and Artificial Intelligence Wizardry: From Imitation to Innovation in Cardiovascular Care. Biomedicines. 2025; 13(5):1019. https://doi.org/10.3390/biomedicines13051019
Chicago/Turabian StylePantelidis, Panteleimon, Polychronis Dilaveris, Samuel Ruipérez-Campillo, Athina Goliopoulou, Alexios Giannakodimos, Panagiotis Theofilis, Raffaele De Lucia, Ourania Katsarou, Konstantinos Zisimos, Konstantinos Kalogeras, and et al. 2025. "Hearts, Data, and Artificial Intelligence Wizardry: From Imitation to Innovation in Cardiovascular Care" Biomedicines 13, no. 5: 1019. https://doi.org/10.3390/biomedicines13051019
APA StylePantelidis, P., Dilaveris, P., Ruipérez-Campillo, S., Goliopoulou, A., Giannakodimos, A., Theofilis, P., De Lucia, R., Katsarou, O., Zisimos, K., Kalogeras, K., Oikonomou, E., & Siasos, G. (2025). Hearts, Data, and Artificial Intelligence Wizardry: From Imitation to Innovation in Cardiovascular Care. Biomedicines, 13(5), 1019. https://doi.org/10.3390/biomedicines13051019









