The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
2.2. Study Population and Eligibility Criteria
2.3. Study Groups and Treatment Cohorts
2.4. Propensity Score Matching and Covariates
2.5. Study Outcomes
- H81.0: Ménière’s disease
- H81.1: Benign paroxysmal positional vertigo (BPPV)
- H81.2: Vestibular neuronitis
- H81.3: Other peripheral vertigo
- H81.4: Vertigo of central origin
- H81.8: Other disorders of vestibular function
- H81.9: Unspecified disorder of vestibular function
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Follow-Up Period
3.3. Risk of Vestibular Disease
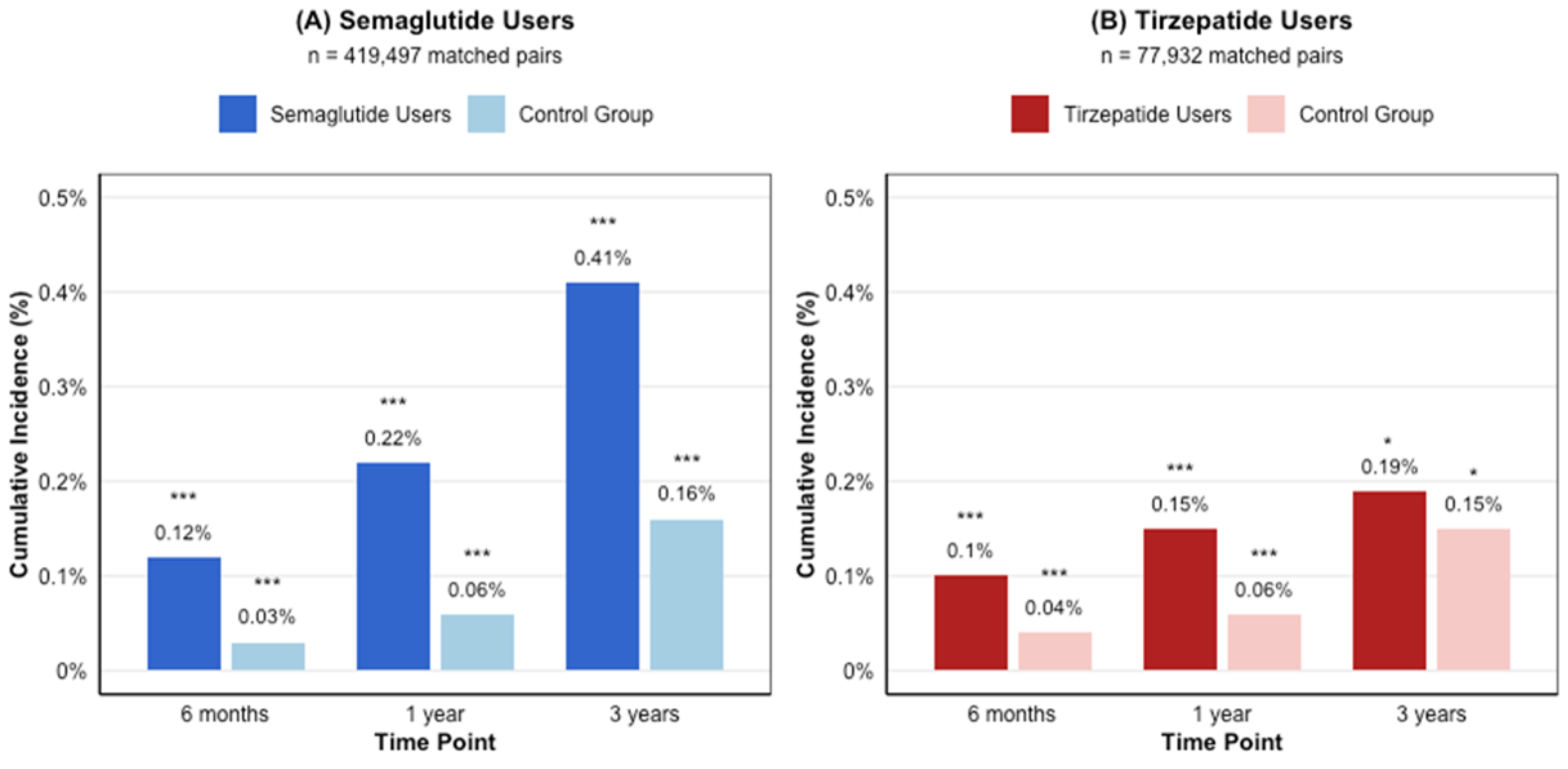
3.3.1. Semaglutide Cohort
3.3.2. Tirzepatide Cohort
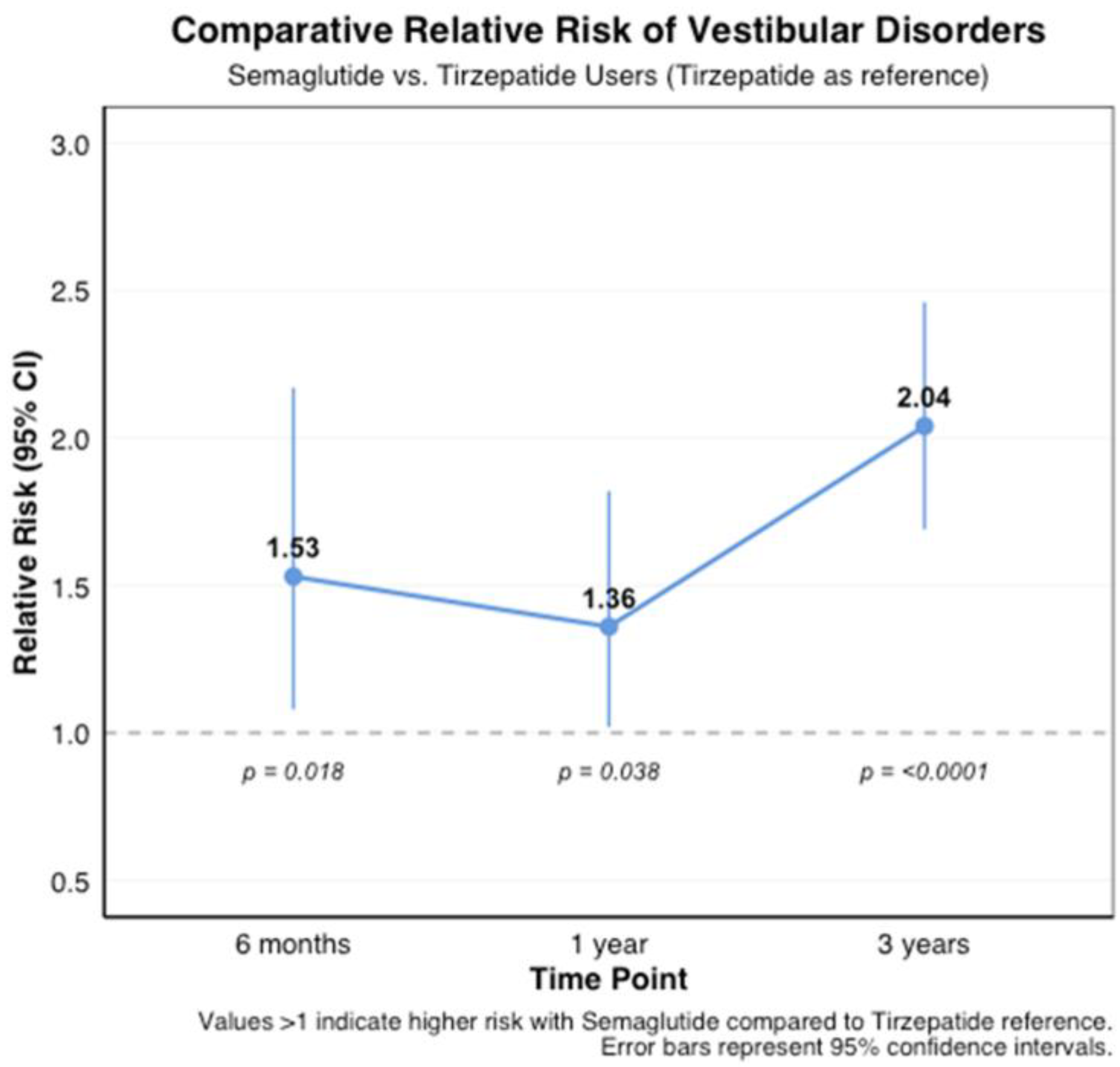
3.3.3. Comparative Risk Between Medications
3.3.4. Distribution of Vestibular Disorder Subtypes
3.3.5. Temporal Patterns of Vestibular Disorder Subtypes
4. Discussion
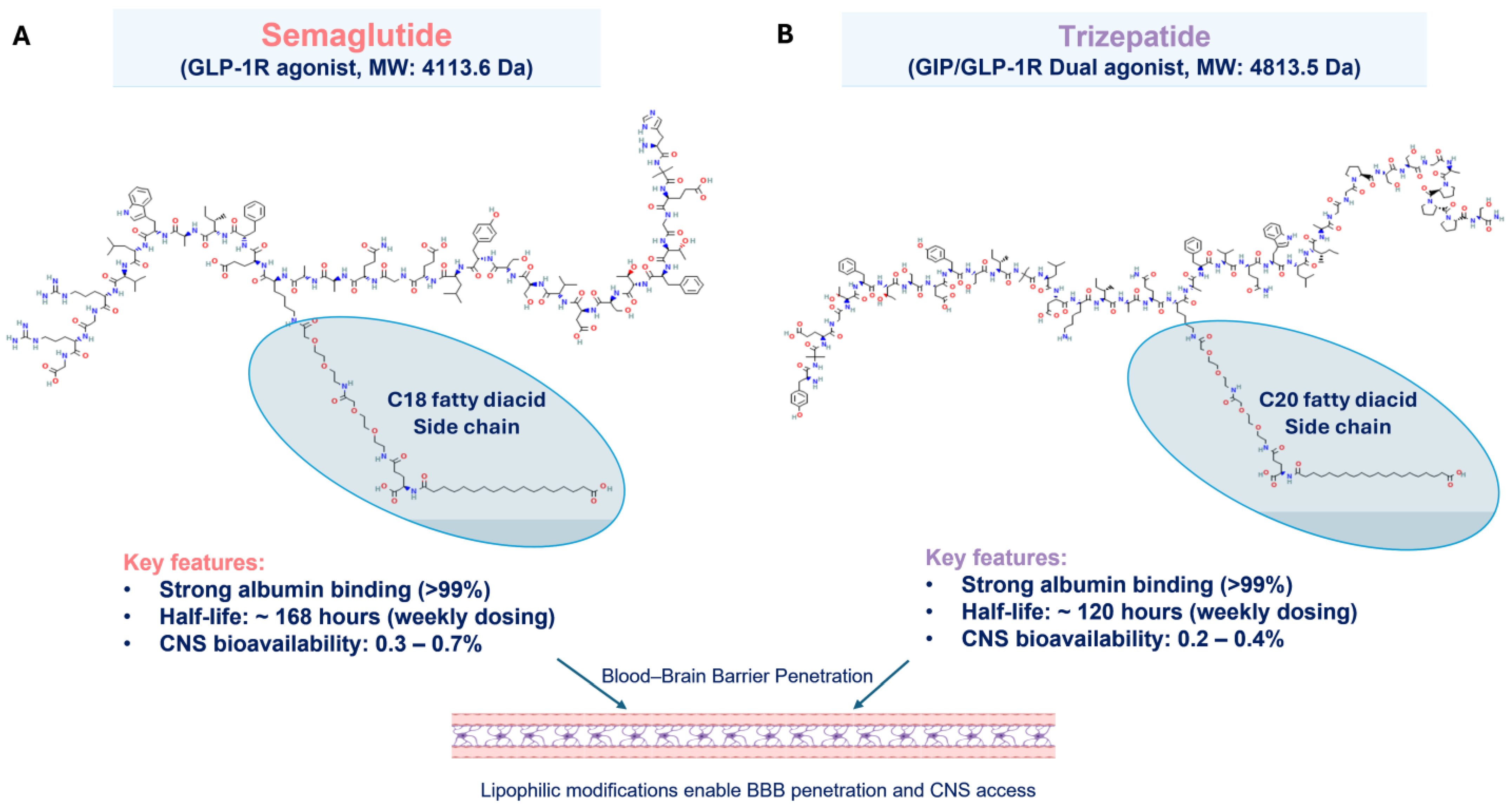
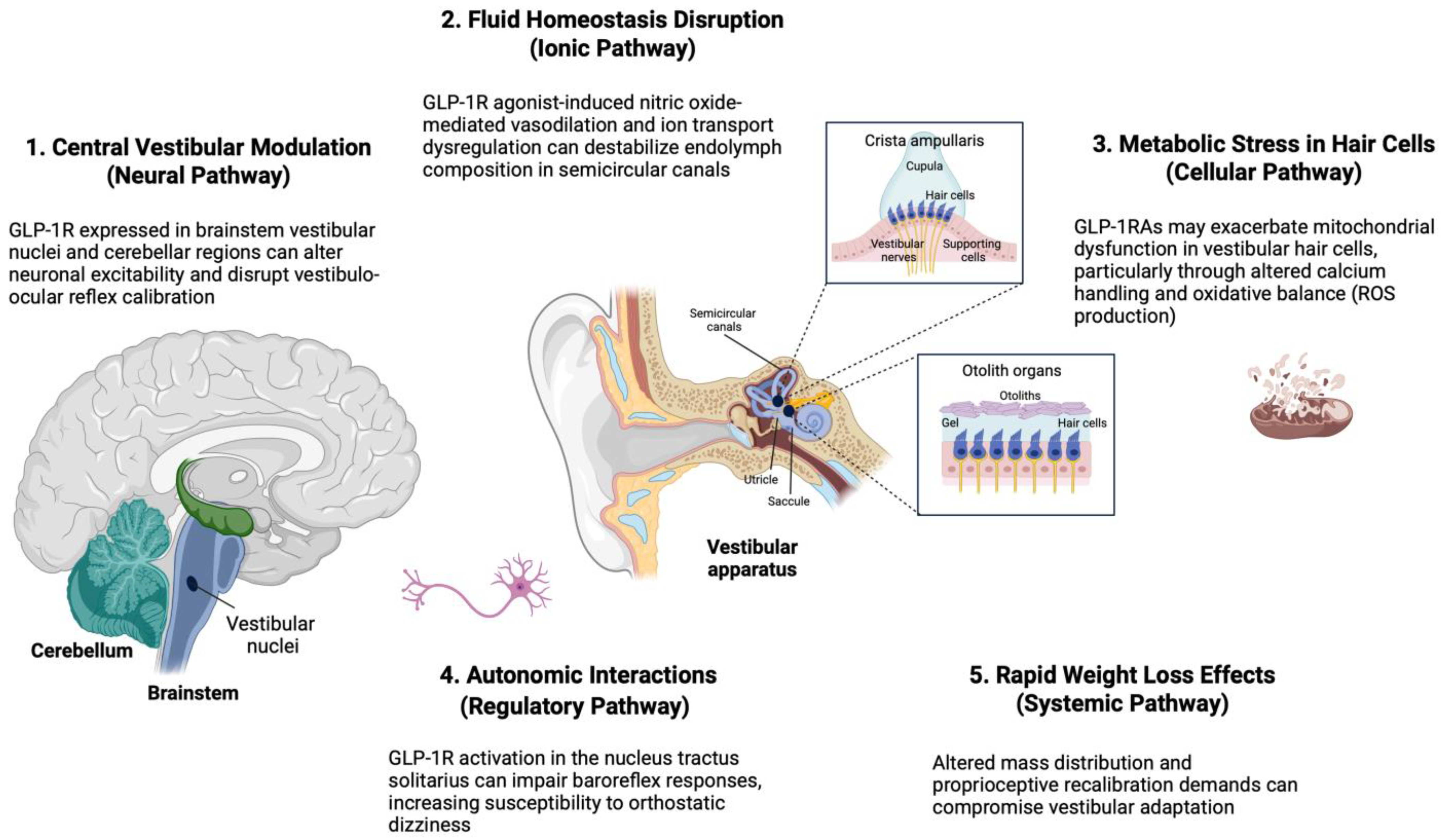
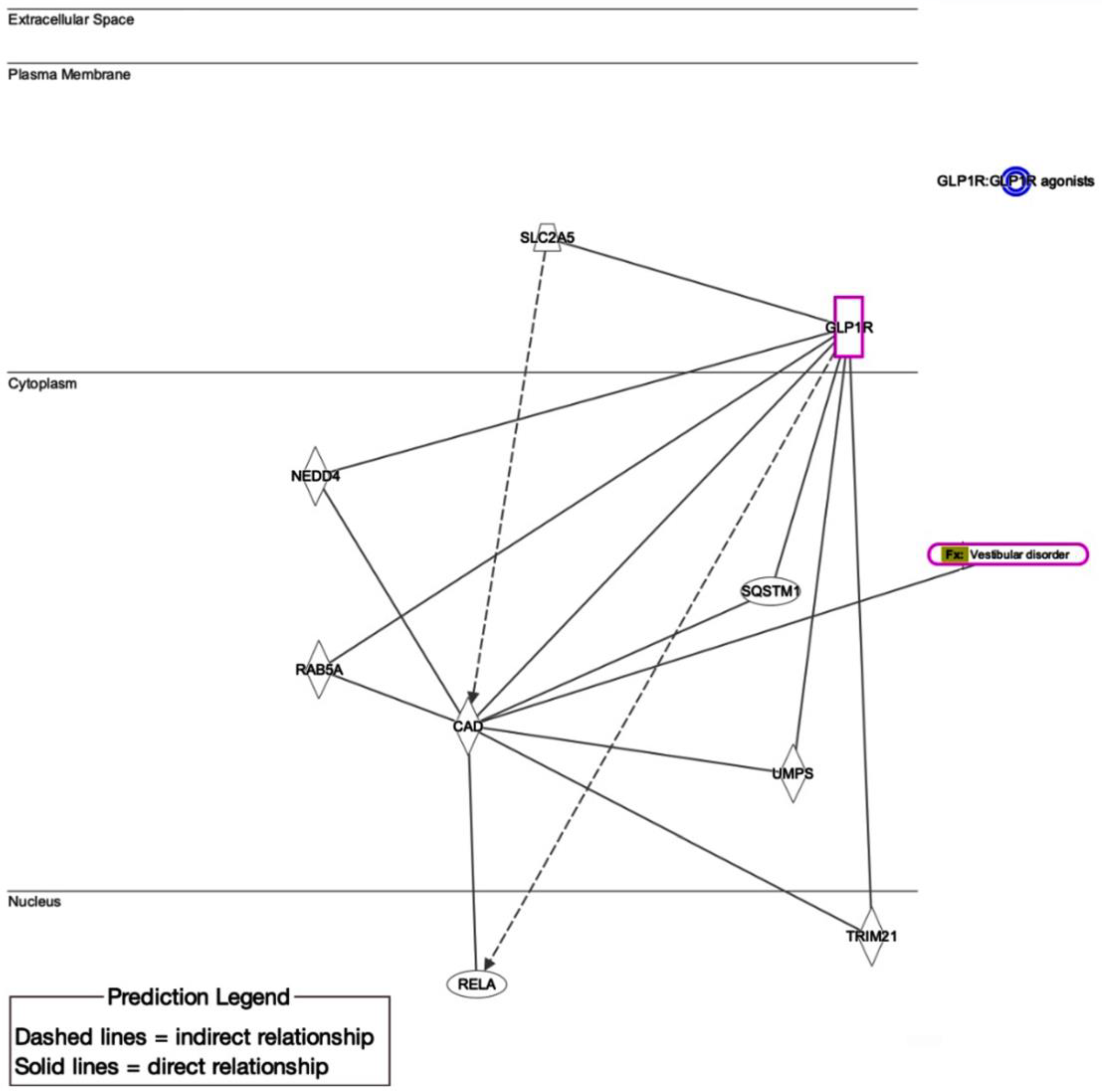
4.1. Putative Biological/Molecular Mechanisms of GLP-1RA-Associated Vestibular Dysfunction
4.2. Differential Medication Effects
4.3. Metabolic Comorbidities and Vestibular Function
4.4. Contrasting Perspectives and Alternative Explanations
4.5. Strengths and Limitations
4.6. Clinical Implications
4.7. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BPPV | Benign paroxysmal positional vertigo |
| CAD | Carbamoyl-phosphate synthetase 2 (CAD) |
| CI | Confidence interval |
| GIP | Gastric inhibitory polypeptide |
| GLP-1RAs | Glucagon-like peptide-1 receptor agonists |
| HR | Hazard ratio |
| NEDD4 | Neural precursor cell-expressed developmentally down-regulated protein 4 |
| RAB8A | Ras-related protein Rab-8A |
| RELA | Rel Avian Reticuloendotheliosis Viral Oncogene Homolog A |
| RR | Risk ratio |
| SLC2A5 | Solute Carrier Family 2 Member 5 |
| SQSTM1 | Sequestosome-1 |
| T2DM | Type 2 diabetes mellitus |
References
- Wen, S.; Nguyen, T.; Gong, M.; Yuan, X.; Wang, C.; Jin, J.; Zhou, L. An Overview of Similarities and Differences in Metabolic Actions and Effects of Central Nervous System Between Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) and Sodium Glucose Co-Transporter-2 Inhibitors (SGLT-2is). Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2955–2972. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.L.; Tsai, M.C.; Tsai, W.H.; Tu, Y.K.; Chien, K.L. Effect of glucagon-like peptide-1 receptor agonists on glycemic control, and weight reduction in adults: A multivariate meta-analysis. PLoS ONE 2023, 18, e0278685. [Google Scholar] [CrossRef] [PubMed]
- Olukorode, J.O.; Orimoloye, D.A.; Nwachukwu, N.O.; Onwuzo, C.N.; Oloyede, P.O.; Fayemi, T.; Odunaike, O.S.; Ayobami-Ojo, P.S.; Divine, N.; Alo, D.J.; et al. Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders. Cureus 2024, 16, e72080. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, A. GLP-1 receptor agonists, are we witnessing the emergence of a paradigm shift for neuro-cardio-metabolic disorders? Pharmacol. Ther. 2025, 269, 108824. [Google Scholar] [CrossRef]
- Kalra, S.; Sahay, R. A Review on Semaglutide: An Oral Glucagon-Like Peptide 1 Receptor Agonist in Management of Type 2 Diabetes Mellitus. Diabetes Ther. 2020, 11, 1965–1982. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, Q.; Cong, Z.; Hang, K.; Zou, X.; Zhang, C.; Chen, Y.; Dai, A.; Liang, A.; Ming, Q.; et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat. Commun. 2022, 13, 1057. [Google Scholar] [CrossRef]
- Myšková, A.; Sýkora, D.; Kuneš, J.; Maletínská, L. Lipidization as a tool toward peptide therapeutics. Drug Deliv. 2023, 30, 2284685. [Google Scholar] [CrossRef]
- Dong, M.; Wen, S.; Zhou, L. The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 2583–2597. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef]
- Kabahizi, A.; Wallace, B.; Lieu, L.; Chau, D.; Dong, Y.; Hwang, E.S.; Williams, K.W. Glucagon-like peptide-1 (GLP-1) signalling in the brain: From neural circuits and metabolism to therapeutics. Br. J. Pharmacol. 2022, 179, 600–624. [Google Scholar] [CrossRef]
- Pak, K.Y.; Cutri, R.M.; Nadeem, W.; Kothari, D.; Wong, Y.T.; Wu, A.W.; Miller, M.E. GLP-1 Receptor Agonist Induced Eustachian Tube Dysfunction: Database and Systematic Review of Otolaryngologic Adverse Events. Otol. Neurotol. 2025, 46, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Disogra, R.M.; Mcelhannon, M. Auditory and Vestibular Side Effects of Pharmaceuticals and Nutraceuticals Used for Diabetes Management: An Overview and Guide for Audiologists and Diabetes Care and Education Specialists. ADCES Pract. 2022, 10, 16–26. [Google Scholar] [CrossRef]
- Back, H.-M.; Choi, S.-A.; Kim, M.G. Adverse events of GLP-1 receptor agonists for weight loss: Twitter and a national pharmacovigilance database. Drug Targets Ther. 2023, 2, 41–48. [Google Scholar] [CrossRef]
- Trapp, S.; Cork, S.C. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am. J. physiol. Regul. Integr. Comp. Physiol. 2015, 309, R795–R804. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Liu, Y.; Cao, L.-X.; Li, Y.-Z.; Wan, P.; Qiu, D.-L. Glucagon-like peptide-1 facilitates cerebellar parallel fiber glutamate release through PKA signaling in mice in vitro. Sci. Rep. 2023, 13, 7948. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Abdalla, M.A.; Sathyapalan, T.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. The Effects of Glucagon-Like Peptide-1 Receptor Agonists and Dipeptydilpeptidase-4 Inhibitors on Blood Pressure and Cardiovascular Complications in Diabetes. J. Diabetes Res. 2021, 2021, 6518221. [Google Scholar] [CrossRef]
- Hosoya, M.; Kitama, T.; Iwabu, K.; Nishiyama, T.; Oishi, N.; Okano, H.; Ozawa, H. Development of the stria vascularis in the common marmoset, a primate model. Sci. Rep. 2022, 12, 19811. [Google Scholar] [CrossRef]
- Oberman, B.S.; Patel, V.A.; Cureoglu, S.; Isildak, H. The aetiopathologies of Ménière’s disease: A contemporary review. Acta Otorhinolaryngol. Ital. 2017, 37, 250–263. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Özgirgin, O.N.; Kingma, H.; Manzari, L.; Lacour, M. Residual dizziness after BPPV management: Exploring pathophysiology and treatment beyond canalith repositioning maneuvers. Front. Neurol. 2024, 15, 1382196. [Google Scholar] [CrossRef]
- Gupta, T.; Kaur, M.; Shekhawat, D.; Aggarwal, R.; Nanda, N.; Sahni, D. Investigating the Glucagon-like Peptide-1 and Its Receptor in Human Brain: Distribution of Expression, Functional Implications, Age-related Changes and Species Specific Characteristics. Basic Clin. Neurosci. 2023, 14, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Silva, J.C.; Tavares, C.A.M.; Girardi, A.C.C. The blood pressure lowering effects of glucagon-like peptide-1 receptor agonists: A mini-review of the potential mechanisms. Curr. Opin. Pharmacol. 2023, 69, 102355. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.R.; Khalsa, S.S. Highway to the danger zone? A cautionary account that GLP-1 receptor agonists may be too effective for unmonitored weight loss. Obes. Rev. 2024, 25, e13709. [Google Scholar] [CrossRef]
- Dzaye, O.; Berning, P.; Razavi, A.C.; Adhikari, R.; Jha, K.; Nasir, K.; Ayers, J.W.; Mortensen, M.B.; Blaha, M.J. Online searches for SGLT-2 inhibitors and GLP-1 receptor agonists correlate with prescription rates in the United States: An infodemiological study. Front. Cardiovasc. Med. 2022, 9, 936651. [Google Scholar] [CrossRef]
- Wan, F. Propensity Score Matching: Should we use it in designing observational studies? BMC Med. Res. Methodol. 2025, 25, 25. [Google Scholar] [CrossRef]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: Crosstalk in Diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef]
- Katsurada, K.; Yada, T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J. Diabetes investig. 2016, 7, 64–69. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.-H. Ménière’s disease. Nat. Rev. Dis. Primers 2016, 2, 16028. [Google Scholar] [CrossRef] [PubMed]
- Luna-Marco, C.; de Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef]
- Greco, C.; Santi, D.; Brigante, G.; Pacchioni, C.; Simoni, M. Effect of the Glucagon-Like Peptide-1 Receptor Agonists on Autonomic Function in Subjects with Diabetes: A Systematic Review and Meta-Analysis. Diabetes Metab. J. 2022, 46, 901–911. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Bastyr, E.J., 3rd; Vignati, L.; Tschöp, M.H.; Schmitt, C.; Owen, K.; Christensen, R.H.; DiMarchi, R.D. The Sustained Effects of a Dual GIP/GLP-1 Receptor Agonist, NNC0090-2746, in Patients with Type 2 Diabetes. Cell Metab. 2017, 26, 343–352.e342. [Google Scholar] [CrossRef]
- Coskun, T.; Sloop, K.W.; Loghin, C.; Alsina-Fernandez, J.; Urva, S.; Bokvist, K.B.; Cui, X.; Briere, D.A.; Cabrera, O.; Roell, W.C.; et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol. Metab. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Secher, A.; Hecksher-Sørensen, J.; Pyke, C. Long-acting glucagon-like peptide-1 receptor agonists have direct access to and effects on pro-opiomelanocortin/cocaine- and amphetamine-stimulated transcript neurons in the mouse hypothalamus. J. Diabetes Investig. 2016, 7, 56–63. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, H.S. An Overview of Existing and Emerging Weight-Loss Drugs to Target Obesity-Related Complications: Insights from Clinical Trials. Biomol. Ther. 2025, 33, 5–17. [Google Scholar] [CrossRef]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef]
- D’Silva, L.J.; Lin, J.; Staecker, H.; Whitney, S.L.; Kluding, P.M. Impact of Diabetic Complications on Balance and Falls: Contribution of the Vestibular System. Phys. Ther. 2016, 96, 400–409. [Google Scholar] [CrossRef]
- Agrawal, Y.; Carey, J.P.; Della Santina, C.C.; Schubert, M.C.; Minor, L.B. Diabetes, vestibular dysfunction, and falls: Analyses from the National Health and Nutrition Examination Survey. Otol. Neurotol. 2010, 31, 1445–1450. [Google Scholar] [CrossRef]
- Holmes, S.; Male, A.J.; Ramdharry, G.; Woodward, C.; James, N.; Skorupinska, I.; Skorupinska, M.; Germain, L.; Kozyra, D.; Bugiardini, E.; et al. Vestibular dysfunction: A frequent problem for adults with mitochondrial disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 838. [Google Scholar] [CrossRef]
- Bettge, K.; Kahle, M.; Abd El Aziz, M.S.; Meier, J.J.; Nauck, M.A. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes. Metab. 2017, 19, 336–347. [Google Scholar] [CrossRef]
- Kawao, N.; Takafuji, Y.; Ishida, M.; Okumoto, K.; Morita, H.; Muratani, M.; Kaji, H. Roles of the vestibular system in obesity and impaired glucose metabolism in high-fat diet-fed mice. PLoS ONE 2020, 15, e0228685. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yu, S.; Cipriani, A.; Wu, S.; Huang, Y.; Zhang, Z.; Yang, J.; Sun, Y.; Yang, Z.; Chai, S.; et al. Neurological Manifestation of Incretin-Based Therapies in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Aging Dis. 2019, 10, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.P.; Menegoni, F.; Vismara, L.; Galli, M.; Romei, M.; Bergamini, E.; Petroni, M.L.; Capodaglio, P. Balance in patients with anorexia and bulimia nervosa. Eur. J. Phys. Rehabil. Med. 2009, 45, 335–340. [Google Scholar] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 6, 275–286. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Rudofsky, G.; Amadid, H.; Braae, U.C.; Catrina, S.B.; Kick, A.; Mandavya, K.; Roslind, K.; Saravanan, P.; van Houtum, W.; Jain, A.B. Oral Semaglutide Use in Type 2 Diabetes: A Pooled Analysis of Clinical and Patient-Reported Outcomes from Seven PIONEER REAL Prospective Real-World Studies. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. disorders 2025, 16, 73–87. [Google Scholar] [CrossRef]
- Hall, C.D.; Heusel-Gillig, L.; Tusa, R.J.; Herdman, S.J. Efficacy of gaze stability exercises in older adults with dizziness. J. Neurol. Phys. Ther JNPT 2010, 34, 64–69. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Before Propensity Matching | After Propensity Matching | ||||
|---|---|---|---|---|---|---|
| Semaglutide (n = 419,497) | Control (n = 8,445,324) | p-Value | Semaglutide (n = 419,497) | Control (n = 419,497) | p-Value | |
| Demographics | ||||||
| Age at index, years | 54.5 ± 14.3 | 56.0 ± 17.6 | <0.001 | 54.5 ± 14.3 | 54.5 ± 14.3 | 0.86 |
| Sex | ||||||
| Female | 246,940 (58.9%) | 4,346,417 (51.5%) | <0.001 | 246,940 (58.9%) | 247,090 (58.9%) | 0.73 |
| Male | 154,139 (36.7%) | 3,609,521 (42.7%) | 154,139 (36.7%) | 155,462 (37.1%) | ||
| Race | ||||||
| White | 270,779 (64.5%) | 5,104,641 (60.4%) | <0.001 | 270,779 (64.5%) | 271,293 (64.7%) | 0.24 |
| Black/African American | 71,040 (16.9%) | 1,398,688 (16.6%) | 71,040 (16.9%) | 71,135 (17%) | ||
| Asian | 13,377 (3.2%) | 287,129 (3.4%) | 13,377 (3.2%) | 12,950 (3.1%) | ||
| Ethnicity | ||||||
| Not Hispanic or Latino | 295,235 (70.4%) | 5,166,505 (61.2%) | <0.001 | 295,235 (70.4%) | 283,110 (67.5%) | 0.96 |
| Hispanic or Latino | 35,948 (8.6%) | 898,451 (10.6%) | 35,948 (8.6%) | 35,961 (8.6%) | ||
| Comorbidities | ||||||
| Diabetes mellitus | 184,117 (43.9%) | 734,247 (8.7%) | <0.001 | 184,117 (43.9%) | 184,106 (43.9%) | 0.98 |
| Thyroid disorders | 59,791 (14.3%) | 504,074 (6%) | <0.001 | 59,791 (14.3%) | 53,016 (12.6%) | 0.78 |
| Obesity | 159,149 (37.9%) | 603,215 (7.1%) | <0.001 | 159,149 (37.9%) | 159,087 (37.9%) | 0.88 |
| Hypertensive diseases | 200,229 (47.7%) | 1,812,957 (21.5%) | <0.001 | 200,229 (47.7%) | 200,747 (47.9%) | 0.25 |
| Ischemic heart diseases | 43,885 (10.5%) | 501,372 (5.9%) | <0.001 | 43,885 (10.5%) | 47,051 (11.2%) | 0.07 |
| Chronic kidney disease | 37,913 (9%) | 436,765 (5.2%) | <0.001 | 37,913 (9%) | 38,074 (9.1%) | 0.54 |
| Neoplasms | 58,833 (14%) | 768,883 (9.1%) | <0.001 | 58,833 (14%) | 58,869 (14%) | 0.91 |
| Characteristics | Before Propensity Matching | After Propensity Matching | ||||
|---|---|---|---|---|---|---|
| Tirzepatide (n = 77,259) | Control (n = 8,445,324) | p-Value | Tirzepatide (n = 77,259) | Control (n = 77,259) | p-Value | |
| Demographics | ||||||
| Age at index, years | 51.9 ± 13.7 | 56.0 ± 17.6 | <0.001 | 51.9 ± 13.7 | 51.9 ± 13.7 | 0.97 |
| Sex | ||||||
| Female | 47,201 (61.1%) | 4,346,417 (51.5%) | <0.001 | 47,201 (61.1%) | 47,199 (61.1%) | 0.99 |
| Male | 25,991 (33.6%) | 3,609,521 (42.7%) | 25,991 (33.6%) | 26,900 (34.8%) | ||
| Race | ||||||
| White | 54,694 (70.8%) | 5,104,641 (60.4%) | <0.001 | 54,694 (70.8%) | 54,725 (70.8%) | 0.86 |
| Black/African American | 10,922 (14.1%) | 1,398,688 (16.6%) | 10,922 (14.1%) | 10,905 (14.1%) | ||
| Asian | 1508 (2%) | 287,129 (3.4%) | 1508 (2%) | 1484 (1.9%) | ||
| Ethnicity | ||||||
| Not Hispanic or Latino | 55,740 (72.1%) | 5,166,505 (61.2%) | <0.001 | 55,740 (72.1%) | 53,832 (69.7%) | 0.75 |
| Hispanic or Latino | 5425 (7%) | 898,451 (10.6%) | 5425 (7%) | 5393 (7%) | ||
| Comorbidities | ||||||
| Diabetes mellitus | 28,125 (36.4%) | 734,247 (8.7%) | <0.001 | 28,125 (36.4%) | 28,116 (36.4%) | 0.96 |
| Thyroid disorders | 11,235 (14.5%) | 504,074 (6%) | <0.001 | 11,235 (14.5%) | 9441 (12.2%) | 0.66 |
| Obesity | 31,395 (40.6%) | 603,215 (7.1%) | <0.001 | 31,395 (40.6%) | 31,375 (40.6%) | 0.92 |
| Hypertensive diseases | 33,624 (43.5%) | 1,812,957 (21.5%) | <0.001 | 33,624 (43.5%) | 33,632 (43.5%) | 0.97 |
| Ischemic heart diseases | 6179 (8%) | 501,372 (5.9%) | <0.001 | 6179 (8%) | 7269 (9.4%) | 0.90 |
| Chronic kidney disease | 5087 (6.6%) | 436,765 (5.2%) | <0.001 | 5087 (6.6%) | 5088 (6.6%) | 0.99 |
| Neoplasms | 10,376 (13.4%) | 768,883 (9.1%) | <0.001 | 10,376 (13.4%) | 10,362 (13.4%) | 0.92 |
| Vestibular Disorder Subtype | Semaglutide (%) | Tirzepatide (%) | p-Value |
|---|---|---|---|
| Benign paroxysmal positional vertigo | 69.8 | 58.4 | <0.001 |
| Other peripheral vertigo | 12.4 | 14.6 | 0.03 |
| Ménière’s disease | 10.8 | 12.2 | 0.17 |
| Vertigo of central origin | 4.2 | 8.6 | <0.001 |
| Vestibular neuronitis | 3.9 | 5.8 | 0.02 |
| Other/unspecified vestibular disorders | 11.2 | 12.8 | 0.13 |
| Vestibular Disorder Subtype | Semaglutide, Months | Tirzepatide, Months | p-Value |
|---|---|---|---|
| Benign paroxysmal positional vertigo | 2.8 (1.6–5.3) | 3.4 (1.9–5.8) | 0.02 |
| Other peripheral vertigo | 3.4 (2.1–6.2) | 3.9 (2.3–6.5) | 0.14 |
| Ménière’s disease | 4.1 (2.5–7.3) | 4.3 (2.7–7.1) | 0.68 |
| Vertigo of central origin | 5.2 (3.4–8.6) | 3.8 (2.5–6.2) | 0.03 |
| Vestibular neuronitis | 2.5 (1.3–4.4) | 4.6 (2.8–6.7) | <0.001 |
| Other/unspecified vestibular disorders | 3.6 (2.1–6.3) | 3.8 (2.2–6.5) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toraih, E.A.; Alenezy, A.; Hussein, M.H.; Hashmat, S.; Mummadi, S.; Alrawili, N.F.; Abdelmaksoud, A.; Fawzy, M.S. The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort. Biomedicines 2025, 13, 1049. https://doi.org/10.3390/biomedicines13051049
Toraih EA, Alenezy A, Hussein MH, Hashmat S, Mummadi S, Alrawili NF, Abdelmaksoud A, Fawzy MS. The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort. Biomedicines. 2025; 13(5):1049. https://doi.org/10.3390/biomedicines13051049
Chicago/Turabian StyleToraih, Eman A., Awwad Alenezy, Mohammad H. Hussein, Shahmeer Hashmat, Saitej Mummadi, Nawaf Farhan Alrawili, Ahmed Abdelmaksoud, and Manal S. Fawzy. 2025. "The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort" Biomedicines 13, no. 5: 1049. https://doi.org/10.3390/biomedicines13051049
APA StyleToraih, E. A., Alenezy, A., Hussein, M. H., Hashmat, S., Mummadi, S., Alrawili, N. F., Abdelmaksoud, A., & Fawzy, M. S. (2025). The Risk of Vestibular Disorders with Semaglutide and Tirzepatide: Findings from a Large Real-World Cohort. Biomedicines, 13(5), 1049. https://doi.org/10.3390/biomedicines13051049








