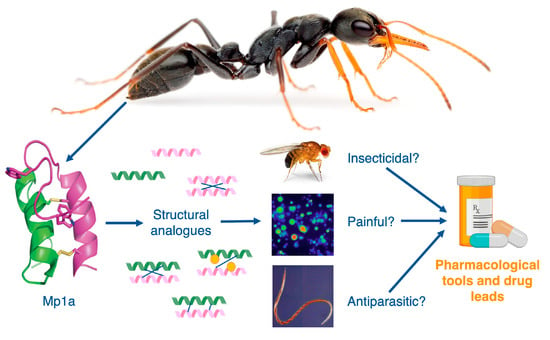
It Takes Two: Dimerization Is Essential for the Broad-Spectrum Predatory and Defensive Activities of the Venom Peptide Mp1a from the Jack Jumper Ant Myrmecia pilosula
Abstract
:1. Introduction
2. Experimental Section
2.1. Peptide Synthesis
2.2. Drosophila Melanogaster Microinjection Assay
2.3. Calcium Imaging of Mammalian Sensory Neurons
2.4. FLIPR Assay
2.5. Haemonchus Contortus Isolation and Larval Development Assay
2.6. Cytotoxicity Assay
2.7. Hemolysis Assay
2.8. Minimum Inhibitory Concentration (MIC) Dilution Assay
2.9. Statistical Analysis
3. Results
3.1. Synthesis of Mp1a and Analogues
3.2. Mp1a Activates Sensory Neurons
3.3. Synthetic Mp1a Shows Insecticidal Activity against D. melanogaster
3.4. Mp1a Is Active against the Gastrointestinal Nematode H. contortus In Vitro
3.5. Cytotoxicity and Antimicrobial Activity
4. Discussion
4.1. Mp1a Shows Insecticidal and Algogenic Activities, Dependent on Dimerization
4.2. Mp1a Shows Antiparasitic Activity against the Veterinary Nematode H. contortus
4.3. Mp1a Analogues Show Some Selectivity Across Bioassasys
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, R.W. Ants with attitude: Australian jack-jumpers of the Myrmecia pilosula species complex, with descriptions of four new species (Hymenoptera: Formicidae: Myrmeciinae). Zootaxa 2015, 3911, 493–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, M.D.; Brown, S.G.; Chataway, T.K.; Davies, N.W.; Milne, R.W.; Aulfrey, S.J.; Heddle, R.J. Myrmecia pilosula (jack jumper) ant venom: Identification of allergens and revised nomenclature. Allergy 2007, 62, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wanandy, T.; Wilson, R.; Gell, D.; Rose, H.E.; Gueven, N.; Davies, N.W.; Brown, S.G.A.; Wiese, M.D. Towards complete identification of allergens in jack jumper (Myrmecia pilosula) ant venom and their clinical relevance: An immunoproteomic approach. Clin. Exp. Allergy 2018, 48, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.W.; Wiese, M.D.; Brown, S.G.A. Characterisation of major peptides in ‘jack jumper’ ant venom by mass spectrometry. Toxicon 2004, 43, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekan, Z.; Headey, S.J.; Scanlon, M.; Baldo, B.A.; Lee, T.-H.; Aguilar, M.-I.; Deuis, J.R.; Vetter, I.; Elliott, A.G.; Amado, M.; et al. Δ-Myrtoxin-Mp1a is a helical heterodimer from the venom of the jack jumper ant that has antimicrobial, membrane-disrupting, and nociceptive activities. Angew. Chem. Int. Ed. 2017, 56, 8495–8499. [Google Scholar] [CrossRef] [Green Version]
- Wiese, M.D.; Chataway, T.K.; Davies, N.W.; Milne, R.W.; Brown, S.G.A.; Gai, W.-P.; Heddle, R.J. Proteomic analysis of Myrmecia pilosula (jack jumper) ant venom. Toxicon 2006, 47, 208–217. [Google Scholar] [CrossRef]
- Touchard, A.; Aili, S.R.; Fox, E.G.P.; Escoubas, P.; Orivel, J.; Nicholson, G.M.; Dejean, A. The biochemical toxin arsenal from ant venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.A.; Robinson, S.D.; Yeates, D.K.; Jin, J.; Baumann, K.; Dobson, J.; Fry, B.G.; King, G.F. Entomo-venomics: The evolution, biology and biochemistry of insect venoms. Toxicon 2018, 154, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Wong, E.S.W.; Morganstern, D.; Mofiz, E.; Gombert, S.; Morris, K.M.; Temple-Smith, P.; Renfree, M.B.; Whittington, C.M.; King, G.F.; Warren, W.C.; et al. Proteomics and deep sequencing comparison of seasonally active venom glands in the platypus reveals novel venom peptides and distinct expression profiles. Mol. Cell. Proteomics 2012, 11, 1354–1364. [Google Scholar] [CrossRef] [Green Version]
- Post, D.C.; Jeanne, R.L. Venom source of a sex pheromone in the social wasp Polistes fuscatus (Hymenoptera: Vespidae). J. Chem. Ecol. 1983, 9, 259–266. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Diebold, P.J.; Orr, M.R.; Gilbert, L.E. Widespread chemical detoxification of alkaloid venom by formicine ants. J. Chem. Ecol. 2015, 41, 884–895. [Google Scholar] [CrossRef]
- Saviola, A.J.; Chiszar, D.; Busch, C.; Mackessy, S.P. Molecular basis for prey relocation in viperid snakes. BMC Biol. 2013, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Tallarovic, S.K.; Melville, J.M.; Brownell, P.H. Courtship and mating in the Giant Hairy Desert Scorpion, Hadrurus arizonensis (Scorpionida, Iuridae). J. Insect Behav. 2000, 13, 827–838. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef] [Green Version]
- Kuhn-Nentwig, L.; Willems, J.; Seebeck, T.; Shalaby, T.; Kaiser, M.; Nentwig, W. Cupiennin 1a exhibits a remarkably broad, non-stereospecific cytolytic activity on bacteria, protozoan parasites, insects, and human cancer cells. Amino Acids 2011, 40, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Kuhn-Nentwig, L.; Fedorova, I.M.; Luscher, B.P.; Kopp, L.S.; Trachsel, C.; Schaller, J.; Vu, X.L.; Seebeck, T.; Streitberger, K.; Nentwig, W.; et al. A venom-derived neurotoxin, CsTx-1, from the spider Cupiennius salei exhibits cytolytic activities. J. Biol. Chem. 2012, 287, 25640–25649. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Xu, J.; Rodriguez Mdel, C.; Lanz-Mendoza, H.; Hernandez-Rivas, R.; Du, W.; Zhu, S. Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 2010, 92, 350–359. [Google Scholar] [CrossRef]
- Dânya Bandeira, L.; Clarissa Perdigão, M.; Izabel Cristina Justino, B.; de Menezes, R.R.P.P.B.; Tiago Lima, S.; Cláudio Borges, F.; Jean-Étienne, R.L.M.; Gandhi, R.-B.; Alice Maria Costa, M. The dinoponeratoxin peptides from the giant ant Dinoponera quadriceps display in vitro antitrypanosomal activity. Biol. Chem. 2018, 399, 187–196. [Google Scholar]
- Aili, S.R.; Touchard, A.; Escoubas, P.; Padula, M.P.; Orivel, J.; Dejean, A.; Nicholson, G.M. Diversity of peptide toxins from stinging ant venoms. Toxicon 2014, 92, 166–178. [Google Scholar] [CrossRef]
- Guo, S.; Herzig, V.; King, G.F. Dipteran toxicity assays for determining the oral insecticidal activity of venoms and toxins. Toxicon 2018, 150, 297–303. [Google Scholar] [CrossRef]
- Herzig, V.; Hodgson, W.C. Neurotoxic and insecticidal properties of venom from the Australian theraphosid spider Selenotholus foelschei. Neurotox 2008, 29, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Mueller, A.; Clayton, D.; Starobova, H.; Hamilton, B.R.; Payne, R.J.; Vetter, I.; King, G.F.; Undheim, E.A.B. A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family. Sci. Adv. 2018, 4, 4640. [Google Scholar] [CrossRef] [Green Version]
- Vetter, I.; Lewis, R.J. Characterization of endogenous calcium responses in neuronal cell lines. Biochem. Pharmacol. 2010, 79, 908–920. [Google Scholar] [CrossRef]
- Albers, G.A.A.; Burgess, S.K. Serial passage of Haemonchus contortus in resistant and susceptible sheep. Vet. Parasitol. 1988, 28, 303–306. [Google Scholar] [CrossRef]
- Kotze, A.C.; O’Grady, J.; Emms, J.; Toovey, A.F.; Hughes, S.; Jessop, P.; Bennell, M.; Vercoe, P.E.; Revell, D.K. Exploring the anthelmintic properties of Australian native shrubs with respect to their potential role in livestock grazing systems. Parasitol 2009, 136, 1065–1080. [Google Scholar] [CrossRef] [Green Version]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for NaV1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef] [Green Version]
- King, J.V.L.; Emrick, J.J.; Kelly, M.J.S.; Herzig, V.; King, G.F.; Medzihradszky, K.F.; Julius, D. A cell-penetrating scorpion toxin enables mode-specific modulation of TRPA1 and pain. Cell 2019, 178, 1362–1374. [Google Scholar] [CrossRef]
- Archer, M.S.; Elgar, M.A. Effects of decomposition on carcass attendance in a guild of carrion-breeding flies. Med. Vet. Entomol. 2003, 17, 263–271. [Google Scholar] [CrossRef]
- Herzig, V.; Cristofori-Armstrong, B.; Israel, M.R.; Nixon, S.A.; Vetter, I.; King, G.F. Animal toxins—Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem. Pharmacol. 2020, in press. [Google Scholar] [CrossRef]
- Nixon, S.A.; Saez, N.J.; Herzig, V.; King, G.F.; Kotze, A.C. The antitrypanosomal diarylamidines, diminazene and pentamidine, show anthelmintic activity against Haemonchus contortus in vitro. Vet. Parasitol. 2019, 270, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kotze, A.C.; Prichard, R.K. Chapter Nine—Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. In Advances in Parasitology; Gasser, R.B., Samson-Himmelstjerna, G.V., Eds.; Academic Press: London, UK, 2016; Volume 93, pp. 397–428. [Google Scholar]
- Lamb, J.; Elliott, T.; Chambers, M.; Chick, B. Broad spectrum anthelmintic resistance of Haemonchus contortus in Northern NSW of Australia. Vet. Parasitol. 2017, 241, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Lamb, J.; Chambers, M.; Hunt, P.W.; Kotze, A.C. Larval development assays reveal the presence of sub-populations showing high- and low-level resistance in a monepantel (Zolvix®)-resistant isolate of Haemonchus contortus. Vet. Parasitol. 2016, 220, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, T.; Henriksen, J.R.; Rasmussen, P.; Clausen, M.H.; Andresen, T.L. Selective acylation enhances membrane charge sensitivity of the antimicrobial peptide mastoparan-x. Biophys. J. 2011, 100, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef]



| Strain | MIC 1a (µM) a (Native Antiparallel Heterodimer) | MIC 5a (µM) (A-Chain Homomer) |
|---|---|---|
| E. coli ATCC 25922 | 0.1–0.2 | 0.42–0.84 |
| K. pneumoniae ATCC 700603 | 0.4–0.8 | 0.84–1.67 |
| K. pneumoniae ATCC BAA-2146 | 0.1–0.2 | 0.84 |
| A. baumannii ATCC 19606 | 0.025 | 0.03–0.1 |
| P. aeruginosa ATCC 27853 | 0.4–0.8 | 0.84 |
| P. aeruginosa FADDI-PA70 | 0.8 | 0.84–3.34 |
| S. aureus ATCC 43300 | 0.8 | 1.67–3.34 |
| S. aureus NRS 1 | 3.2 | 6.7 |
| S. aureus NRS 17 | 0.4–0.8 | 1.67 |
| S. aureus NARSA-VRS1 | 3.2–6.4 | > 6.7 |
| S. aureus NARSA-VRS10 | 0.8 | 1.67 |
| S. pneumoniae ATCC 700677 | 0.4–0.8 | 1.67 |
| Peptide | Description | Neuronal Cell Activation (EC50, µM) | Anthelmintic Activity (IC50, µM) | Insecticidal Activity (LD50, pmol/g) | Cytotoxicity (CC50 µM) b | Hemolysis (HC50 µM) b |
|---|---|---|---|---|---|---|
| 1a | Antiparallel heterodimer (native) | 0.85 ± 0.03 | 6.8 ± 0.5 c | 260.1 ± 16.6 | 0.6 | 2.2 |
| 1c | Antiparallel heterodimer | 1.39 ± 0.34 | 7.2 ± 0.1 | 552.7 ± 18.2 a | 0.7 | > 12 |
| 1d | Antiparallel heterodimer | 3.38 ± 2.8 | 9.5 ± 0.3 | 578 ± 9.7 a | 0.7 | > 12 |
| 2a | Parallel heterodimer | 0.9 ± 0.08 | 35.8 ± 1.9 a | > 600 | > 10 | > 10 |
| 2b | Parallel heterodimer | 38.5 ± 2.3 a | 65.6 ± 14.7 a | > 600 | > 10 | > 10 |
| 3a | A monomer (A16–A23) | inactive | 46.8 ± 0.6 a | > 600 | > 20 | > 20 |
| 3b | A monomer [C16S, C23S] | inactive | 64.9 ± 6.3 a | > 600 | > 20 | > 20 |
| 4a | B monomer (B10–B17) | inactive | 65.5 ± 5.5 a | > 600 | > 15 | > 15 |
| 4b | B monomer [C10S, C17S] | inactive | 61.3 ± 4.4 a | > 600 | > 15 | > 15 |
| 5a | A chain homomer | 4.3 ± 1.3 | 9.2 ± 0.7 | 415.4 ± 29.6 a | 2.4 | > 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nixon, S.A.; Dekan, Z.; Robinson, S.D.; Guo, S.; Vetter, I.; Kotze, A.C.; Alewood, P.F.; King, G.F.; Herzig, V. It Takes Two: Dimerization Is Essential for the Broad-Spectrum Predatory and Defensive Activities of the Venom Peptide Mp1a from the Jack Jumper Ant Myrmecia pilosula. Biomedicines 2020, 8, 185. https://doi.org/10.3390/biomedicines8070185
Nixon SA, Dekan Z, Robinson SD, Guo S, Vetter I, Kotze AC, Alewood PF, King GF, Herzig V. It Takes Two: Dimerization Is Essential for the Broad-Spectrum Predatory and Defensive Activities of the Venom Peptide Mp1a from the Jack Jumper Ant Myrmecia pilosula. Biomedicines. 2020; 8(7):185. https://doi.org/10.3390/biomedicines8070185
Chicago/Turabian StyleNixon, Samantha A., Zoltan Dekan, Samuel D. Robinson, Shaodong Guo, Irina Vetter, Andrew C. Kotze, Paul F. Alewood, Glenn F. King, and Volker Herzig. 2020. "It Takes Two: Dimerization Is Essential for the Broad-Spectrum Predatory and Defensive Activities of the Venom Peptide Mp1a from the Jack Jumper Ant Myrmecia pilosula" Biomedicines 8, no. 7: 185. https://doi.org/10.3390/biomedicines8070185