The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection
Abstract
:1. Introduction
2. Antioxidant and Antinflammatory Properties of n-3 PUFAs
2.1. Mitochondrial Oxidative Stress and n-3 PUFAs
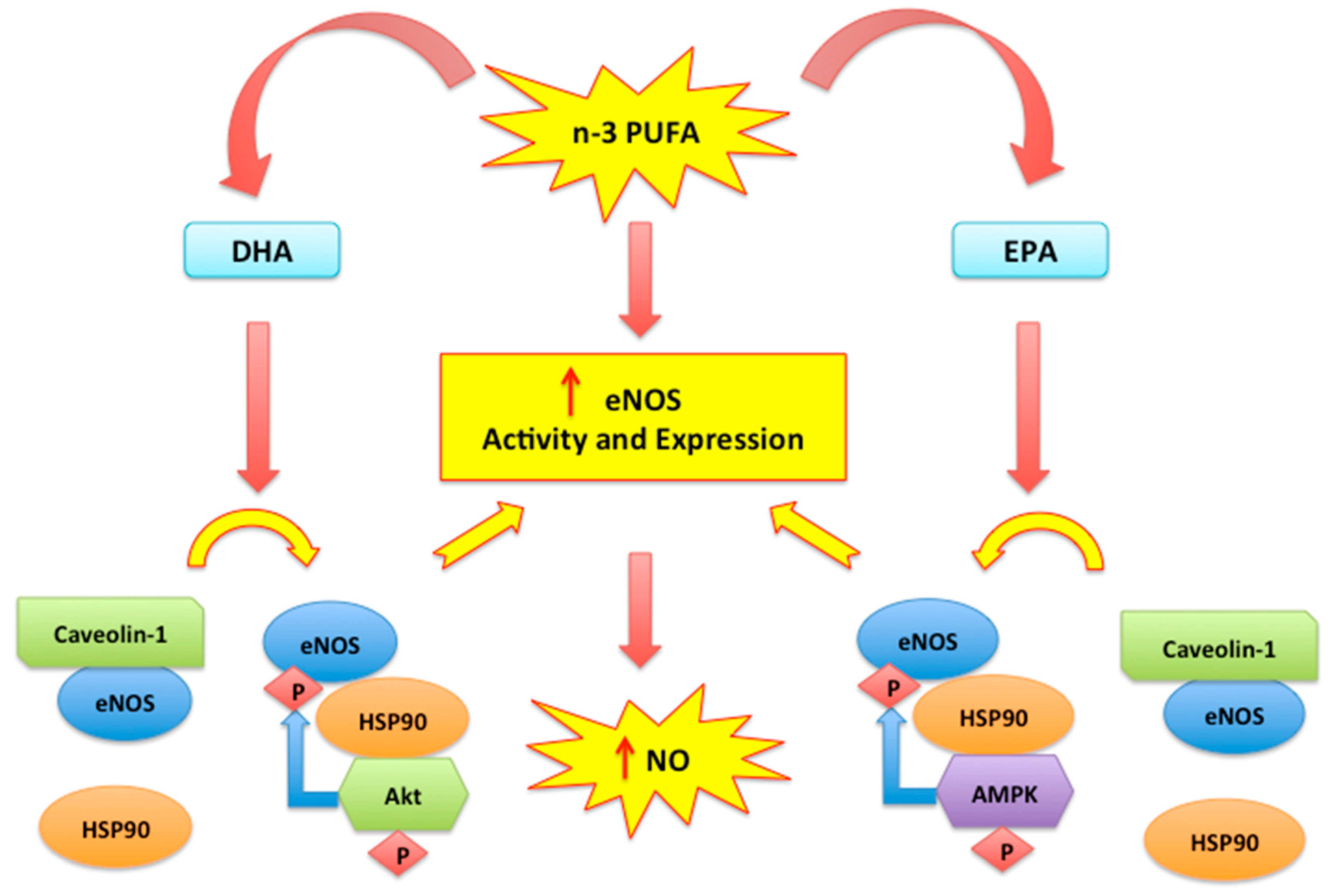
2.2. Nitric Oxide, Endothelial Dysfunction and n-3 PUFAs
2.3. Cell Membranes and Anti-Inflammatory Effects of n-3 PUFAs
2.4. N-3 PUFA-Derived Mediators
3. Vaso-Protective Activities of n-3 PUFAs
3.1. N-3 PUFAs and Atherosclerosis
3.2. The Effect of n-3 PUFAs in Platelet Function
3.3. N-3 PUFAs Index and Coronary Artery Disease (CAD)
4. The Cardio-Protective Response of n-3 PUFAs Supplementation
4.1. The Effects of n-3 PUFAs in Myocardial Ischemia and Reperfusion
4.2. The Potential for n-3 PUFAs Supplementation in Heart Failure
5. Adverse Effects of PUFAs in Cardiovascular Risk Factors
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sosnowska, B.; Penson, P.; Banach, M. The Role of Nutraceuticals in the Prevention of Cardiovascular Disease. Cardiovasc. Diagn. Ther. 2017, 7, S21–S31. [Google Scholar] [CrossRef] [Green Version]
- Rivellese, A.A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The Possible Role of Nutraceuticals in the Prevention of Cardiovascular Disease. High Blood Press. Cardiovasc. Prev. 2019, 26, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Ragusa, S.; Sacco, I.; Muscoli, C.; Sculco, F.; Visalli, V.; Palma, E.; Muscoli, S.; Mondello, L.; Dugo, P.; et al. The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 120–129. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. N-3 PUFAs Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2017, 1, e867–e884. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Gelejinse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of N-3 PUFAs Fatty Acid Supplement Use With Cardiovascular Disease Risks. JAMA Cardiol. 2018, 1, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, R.; Hasanally, D.; Stamenkovic, A.; Maddaford, T.G.; Chaudhary, R.; Pierce, G.N.; Ravandi, A. Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol. Cell. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.M.; Lee, H.; Kang, S.; Park, W.J. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients 2016, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Lu, L.; Liang, J.; Liu, M.; Li, X.; Sun, R.; Zheng, Y.; Zhang, P. N-3 PUFAs Fatty Acids and Primary and Secondary Prevention of Cardiovascular Disease. Cell Biochem. Biophys. 2015, 72, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Chaddha, A.; Eagle, K.A. Cardiology Patient Page. N-3 PUFAs Fatty Acids and Heart Health. Circulation 2015, 132, e350ULATIe352. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Fleming, J.A. Emerging nutrition science on fatty acids and cardiovascular disease: Nutritionists’ perspectives. Adv. Nutr. 2015, 6, 326S–337S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O′Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. ω3-Polyunsaturated fatty acids for heart failure: Effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef] [Green Version]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of n-3 PUFAs polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Tortosa-Caparros, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Pinero, E. Anti-inflammatory Effects of Omega 3 and Omega 6 Polyunsaturated Fatty Acids in Cardiovascular Disease and Metabolic Syndrome. Crit. Rev. Food Sci. Nutr. 2017, 2, 3421–3429. [Google Scholar] [CrossRef]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin synthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [Green Version]
- Mollace, V.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F. Re-assessing the mechanism of action of n-3 PUFAs. Int. J. Cardiol. 2013, 170, S8–S11. [Google Scholar] [CrossRef]
- Awada, M.; Soulage, C.O.; Mevnier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Estienne, M.; Peretti, N.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, H.; Johnson, D.; Gensler, C.; Decker, E.; Zhang, G. Chemistry and Biology of ω-3 PUFA Peroxidation-Derived Compounds. Prostaglandins Other Lipid Mediat. 2017, 132, 84–91. [Google Scholar] [CrossRef]
- Yang, B.; Li, R.; Greenlief, C.M.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling Anti-Oxidative and Anti-Inflammatory Effects of Docosahexaenoic Acid and Its Lipid Peroxidation Product on Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Neuroinflammation 2018, 9, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostowik, M.; Gajos, G.; Zalewski, J.; Nessler, J.; Undas, A. N-3 PUFAs polyunsaturated fatty acids increase plasma adiponectin to leptin ratio in stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, J.; Le Guennec, J.Y. Cardioprotective Effects of Omega 3 Fatty Acids: Origin of the Variability. J. Muscle Res. Cell Motil. 2017, 38, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Metallinos, G.; Georgiopoulos, G.; Mendrinos, D.; Papanikolaou, A.; Magkas, N.; Pitsavos, C.; Vyssoulis, G.; Stefanadis, C.; Tousoulis, D. Short Term n-3 PUFAs Polyunsaturated Fatty Acid Supplementation Induces Favorable Changes in Right Ventricle Function and Diastolic Filling Pressure in Patients With Chronic Heart Failure; A Randomized Clinical Trial. Vasc. Pharmacol. 2016, 79, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Fish, n-3 Fatty Acids, and Cardiovascular Haemodynamics. J. Cardiovasc. Med. 2007, 8, S23–S26. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species (Apex) 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n-3 fatty acids: Molecular mechanisms and potential clinical applications. Clin. Sci. 2013, 124, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.A.; Farías, J.G.; González-Candia, A.; Short, S.E.; Carrasco-Pozo, C.; Castillo, R.L. Ω3 Supplementation and intermittent hypobaric hypoxia induce cardioprotection enhancing antioxidant mechanisms in adult rats. Mar. Drugs 2015, 13, 838–860. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. N-3 PUFAs fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Cabo, J.; Alonso, R.; Mata, P. N-3 PUFAs fatty acids and blood pressure. Br. J. Nutr. 2012, 107, S195–S200. [Google Scholar] [CrossRef] [Green Version]
- Gross, S.S.; Wolin, M.S. Nitric Oxide: Pathophysiological Mechanisms. Annu. Rev. Physiother. 1995, 57, 737–769. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur. J. Clin. Investig. 1991, 21, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelial Dysfunction as a Target for Prevention of Cardiovascular Disease. Diabetes Care 2009, 32, S314–S321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 4, 3294. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie 2007, 89, 169–177. [Google Scholar] [CrossRef]
- Gousset-Dupont, A.; Robert, V.; Grynberg, A.; Lacour, B.; Tardivel, S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 131–139. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Dong, Y.; Wang, S.; Song, P.; Viollet, B.; Zou, M.H. Activation of the AMP-Activated Protein Kinase by Eicosapentaenoic Acid (EPA, 20:5 n-3) Improves Endothelial Function In Vivo. PLoS ONE 2012, 7, e35508. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. N-3 PUFAs Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. BioMed Res. Int. 2015, 2015, 791978. [Google Scholar] [CrossRef] [Green Version]
- Lamoke, F.; Mazzone, V.; Persichini, T.; Maraschi, A.; Harris, M.B.; Venema, R.C.; Colasanti, M.; Gliozzi, M.; Muscoli, C.; Bartoli, M.; et al. Amyloid β peptide-induced inhibition of endothelial nitric oxide production involves oxidative stress-mediated constitutive eNOS/HSP90 interaction and disruption of agonist-mediated Akt activation. J. Neuroinflammation. 2015, 3, 12–84. [Google Scholar] [CrossRef] [Green Version]
- Endo, J.; Arita, M. Cardioprotective mechanism of n-3 PUFAs polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Alzoubi, M.R.; Aldomi Al-Domi, H. Could n-3 PUFAs fatty acids a therapeutic treatment of the immune-metabolic consequence of intermittent hypoxia in obstructive sleep apnea? Diabetes Metab. Syndr. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdukeyum, G.G.; Owen, A.J.; Larkin, T.A.; McLennan, P.L. Up-Regulation of Mitochondrial Antioxidant Superoxide Dismutase Underpins Persistent Cardiac Nutritional-Preconditioning by Long Chain n-3 Polyunsaturated Fatty Acids in the Rat. J. Clin. Med. 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory Activity of Polyunsaturated Fatty Acids in T-Cell Signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakoti, B.B.; Hernandez-Ontiveros, D.G.; Kataki, M.S.; Shah, K.; Pathak, Y.; Panguluri, S.K. Resveratrol and N-3 PUFAs Fatty Acid: Its Implications in Cardiovascular Diseases. Front. Cardiovasc. Med. 2015, 2, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Li, K.; Wang, F.; Yang, B.; Fu, Y.; Zheng, J.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty. PLoS ONE 2016, 25, e0147351. [Google Scholar] [CrossRef]
- Recchiuti, A.; Serhan, C.N. Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating miRNA in Novel Resolution Circuits in Inflammation. Front. Immunol. 2012, 22, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredman, G.; Tabas, I. Boosting Inflammation Resolution in Atherosclerosis. Am. J. Pathol. 2017, 187, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cash, J.L.; Norling, L.V.; Perretti, M. Resolution of inflammation: Targeting GPCRs that interact with lipids and peptides. Drug Discov. Today 2014, 19, 1186–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, B.S.; Hasturk, H.; Kantarci, A.; Freire, M.O.; Nguyen, O.; Kansal, S.; Van Dyke, T.E. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect. Immun. 2015, 83, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H153–H164. [Google Scholar] [CrossRef] [Green Version]
- Kain, V.; Ingle, K.A.; Colas, R.A.; Dalli, J.; Prabhu, S.D.; Serhan, C.N.; Joshi, M.; Halade, G.V. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J. Mol. Cell. Cardiol. 2015, 84, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006, 177, 5902–5911. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Konkel, A.; Mehling, H.; Blossey, K.; Gapelyuk, A.; Wessel, N.; von Schacky, C.; Dechend, R.; Muller, D.N.; Rothe, M.; et al. Dietary n-3 PUFAs fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014, 55, 1150–1164. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, K.; Malick, M.; Madingou, N.; Touchette, C.; Bourque-Riel, V.; Tomaro, L.; Rousseau, G. Metabolites derived from n-3 PUFAs polyunsaturated fatty acids are important for cardioprotection. Eur. J. Pharmacol. 2015, 769, 147–153. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and Prevention of Atherosclerosis: Focus on ω-3 Polyunsaturated Fatty Acids and Mediterranean Diet Polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [Green Version]
- Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Mollace, V. The effect of bergamot polyphenolic fraction in patients with non alcoholic liver steato-hepatitis and metabolic syndrome. PharmaNutrition 2016, 4S, S27–S31. [Google Scholar] [CrossRef]
- Chuchun, L.; Chang, T.S.; Matsuzaki, M.; Worgall, T.S.; Deckelbaum, R.J. n-3 Fatty Acids Reduce Arterial LDL-Cholesterol Delivery and Arterial Lipoprotein Lipase Levels and Lipase Distribution. Arter. Thromb. Vasc. Biol. 2009, 29, 555–561. [Google Scholar]
- Chang, C.L.; Seo, T.; Du, C.B.; Accili, D.; Deckelbaum, R.J. n-3 Fatty acids decrease arterial low-density lipoprotein cholesterol delivery and lipoprotein lipase levels in insulin-resistant mice. Arter. Thromb. Vasc. Biol. 2010, 30, 2510–2517. [Google Scholar] [CrossRef] [Green Version]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovas. 2017, 27, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Wani, O.; May, H.T.; Budoff, M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul. Pharmacol. 2017, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Limbu, R.; Cottrell, G.S.; McNeish, A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE 2018, 13, e0192484. [Google Scholar] [CrossRef]
- Liddle, D.M.; Hutchinson, A.L.; Wellings, H.R.; Power, K.A.; Robinson, L.E.; Monk, J.M. Integrated Immunomodulatory Mechanisms through which Long-Chain n-3 Polyunsaturated Fatty Acids Attenuate Obese Adipose Tissue Dysfunction. Nutrients 2017, 9, 1289. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.H.; Mutch, D.M. Mechanisms underlying N3-PUFA regulation of white adipose tissue endocrine function. Curr. Opin. Pharmacol. 2020, 52, 40–46. [Google Scholar] [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Salic, K.; Morrison, M.C.; Verschuren, L.; Wielinga, P.Y.; Wu, L.; Kleemann, R.; Gjorstrup, P.; Kooistra, T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 2016, 250, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Hung, T.M.; Wei, J.; Chiang, A. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc. Res. 2004, 61, 169–176. [Google Scholar] [CrossRef]
- Casós, K.; Zaragozá, M.C.; Zarkovic, N.; Zarkovic, K.; Andrisic, L.; Portero-Otín, M.; Cacabelos, D.; Mitjavila, M.T. A fish-oil-rich diet reduces vascular oxidative stress in apoE(-/-) mice. Free Radic. Res. 2010, 44, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [Green Version]
- Konishi, T.; Sunaga, D.; Funayama, N.; Yamamoto, T.; Murakami, H.; Hotta, D.; Nojima, M.; Tanaka, S. Eicosapentaenoic acid therapy is associated with decreased coronary plaque instability assessed using optical frequency domain imaging. Clin. Cardiol. 2019, 42, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.K.; Tormoen, G.W.; Weaver, L.J.; Luepke, K.J.; Patel, I.A.; Hjelmen, C.E.; Ensz, N.M.; McComas, L.S.; McCarty, O.J.T. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am. J. Physiol. Cell Physiol. 2013, 304, C273–C279. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.K.; Shearer, G.C.; Ashmore, J.H.; Anderson-Daniels, J.M.; Graslie, E.L.; Tholen, J.T.; Vogelaar, J.L.; Korth, A.J.; Nareddy, V.; Sprehe, M.; et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot Essent Fatty Acids. 2011, 84, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Croset, M.; Lagarde, M. In vitro incorporation and metabolism of icosapentaenoic and docosahexaenoic acids in human platelets--effect on aggregation. Thromb Haemost 1986, 56, 57–62. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstata, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- Sheikh, O.; Vande Hei, A.G.; Battisha, A.; Hammad, T.; Pham, S.; Chilton, R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: As it pertains to the recently published REDUCE-IT trial. Cardiovasc. Diabetol. 2019, 18, 84. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, H.; Hsu-Hage, B.H.H.; Wahlqvist, M.L.; Sinclair, A.J. Original Communication The influence of fish, meat and polyunsaturated fat intakes on platelet phospholipid polyunsaturated fatty acids in male Melbourne Chinese and Caucasian. Eur. J. Clin. Nutr. 2001, 55, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Cao, J.; Mao, Q.; Lu, X.; Zhou, X.; Fan, L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2013, 226, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, V.; Catani, M.V.; Savini, I. Platelet Responses in Cardiovascular Disease: Sex-Related Differences in Nutritional and Pharmacological Interventions. Cardiovasc. Ther. 2020, 2020, 2342837. [Google Scholar] [CrossRef] [PubMed]
- Von Schacky, C. N-3 PUFAs Index and Cardiovascular Health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; An, W.S. Cardioprotective effects of ω-3 PUFAs in chronic kidney disease. BioMed Res. Int. 2013, 2013, 712949. [Google Scholar] [CrossRef] [Green Version]
- Tani, S.; Matsuo, R.; Yagi, T.; Matsumoto, N. Administration of eicosapentaenoic acid may alter high-density lipoprotein heterogeneity in statin-treated patients with stable coronary artery disease: A 6-month randomized trial. J. Cardiol. 2020, 75, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Sugimura, H.; Watanabe, S.; Murakami, Y.; Ebisawa, K.; Ioka, T.; Takahashi, T.; Ando, T.; Kono, K.; Inoue, T. Eicosapantaenoic acid treatment based on the EPA/AA ratio in patients with coronary artery disease: Follow-up data from the Tochigi Ryomo EPA/AA Trial in Coronary Artery Disease (TREAT-CAD) study. Hypertens. Res. 2018. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Liu, M.; Zhang, J.; Liang, W.; Chen, W. Serum ω-3 Polyunsaturated Fatty Acids and Potential Influence Factors in Elderly Patients with Multiple Cardiovascular Risk Factors. Sci. Rep. 2018, 8, 1102. [Google Scholar] [CrossRef]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Franzosi, M.G.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell′Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef] [Green Version]
- Yagi, S.; Fukuda, D.; Aihara, K.I.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Shantakumari, N.; Eldeeb, R.A.; Ibrahim, S.A.M.; Sreedharan, J.; Otoum, S. Effect of PUFA on patients with hypertension: A hospital based study. Indian Heart J. 2014, 66, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.S.; Siegel, D.; Vemuri, M.; Mackey, B.E. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am. J. Clin. Nutr. 2007, 86, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [Green Version]
- Gonzàlez_Montero, J.; Brito, R.; Gajardo, A.J.; Rodrigo, R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018, 26, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, J.K.; Calder, P.C. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Wong, L.L.; Liew, O.W.; Richards, A.M. Heart Failure with Reduced Ejection Fraction (HFrEF) and Preserved Ejection Fraction (HFpEF): The Diagnostic Value of Circulating MicroRNAs. Cells 2019, 8, 1651. [Google Scholar] [CrossRef] [Green Version]
- Bishu, K.; Deswal, A.; Chen, H.H.; LeWinter, M.M.; Lewis, G.D.; Semigran, M.J.; Borlaug, B.A.; McNulty, S.; Hernandez, A.F.; Braunwald, E.; et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am. Heart J. 2012, 164, 763–770.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmann, E.; Brunner-La Rocca, H.P.; Maeder, M.T.; Kaufmann, B.A.; Pfisterer, M.; Rickenbacher, P. Is the clinical presentation of chronic heart failure different in elderly versus younger patients and those with preserved versus reduced ejection fraction? Eur. J. Intern. Med. 2018, 57, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liebelt, J.J.; Madan, N.; Shan, J.; Taub, C.C. Comparison of Predictors of Heart Failure with Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Preclinical Left Ventricular Diastolic Dysfunction. Am. J. Cardiol. 2017, 119, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, B.; Huang, J. The Role of Omega-3 Polyunsaturated Fatty Acids in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 18. [Google Scholar] [CrossRef]
- Eclov, J.A.; Qian, Q.; Redetzke, R.; Chen, Q.; Wu, S.C.; Healy, C.L.; Ortmeier, S.B.; Harmon, E.; Shearer, G.C.; O’Connell, T.D. EPA, not DHA, prevents fibrosis in pressure overload-induced heart failure: Potential role of free fatty acid receptor 4. J. Lipid Res. 2015, 56, 2297–2308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.Y. Role of ω-3 Fatty Acids in Cardiovascular Disease. Cell Biochem. Biophys. 2015, 72, 869–875. [Google Scholar] [CrossRef]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 Long-Chain Polyunsaturated Fatty Acid Content and Oxidation State of Fish Oil Supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef] [Green Version]
- García-Hernández, V.M.; Gallar, M.; Sánchez-Soriano, J.; Micol, V.; Roche, E.; García-García, E. Effect of omega-3 dietary supplements with different oxidation levels in the lipidic profile of women: A randomized controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 993–1000. [Google Scholar] [CrossRef]
- Berliner, A.R.; Fine, D.M. There’s something fishy about this bleeding. NDT Plus 2011, 4, 270–272. [Google Scholar] [CrossRef]
- Stupin, M.; Kibel, A.; Stupin, A.; Selthofer-Relatić, K.; Matić, A.; Mihalj, M.; Mihaljević, Z.; Jukić, I.; Drenjančević, I. The Physiological Effect of n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) Intake and Exercise on Hemorheology, Microvascular Function, and Physical Performance in Health and Cardiovascular Diseases; Is There an Interaction of Exercise and Dietary n-3 PUFA Intake? Front. Physiol. 2019, 10, 112. [Google Scholar]
- Hansen, J.B.; Lyngmo, V.; Svensson, B.; Nordøy, A. Inhibition of exercise-induced shortening of bleeding time by fish oil in familial hypercholesterolemia (type IIa). Arterioscler. Thromb. 1993, 13, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, T.A.; Roshanai, F. The influence of different types of omega 3 polyunsaturated fatty acids on blood lipids and platelet function in healthy volunteers. Clin. Sci. 1983, 64, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Holub, B.J. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. CMAJ 2002, 166, 608–615. [Google Scholar] [PubMed]
- Munk Begtrup, K.M.; Krag, A.E.; Hvas, A.M. No impact of fish oil supplements on bleeding risk: A systematic review. Dan. Med. J. 2017, 64, A5366. [Google Scholar]
- Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: New Recommendations from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akrami, A.; Makiabadi, E.; Askarpour, M.; Zamani, K.; Hadi, A.; Mokari-Yamchi, A.; Babajafari, S.; Faghih, S.; Hojhabrimanesh, A. A Comparative Study of the Effect of Flaxseed Oil and Sunflower Oil on the Coagulation Score, Selected Oxidative and Inflammatory Parameters in Metabolic Syndrome Patients. Clin. Nutr. Res. 2020, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jeansen, S.; Renger, F.W.; Garthoff, J.A.; van Helvoort, A.; Calder, P.C. Fish oil LC-PUFAs do not affect blood coagulation parameters and bleeding manifestations: Analysis of 8 clinical studies with selected patient groups on omega-3-enriched medical nutrition. Clin. Nutr. 2018, 37, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonhardt, M.; Langhans, W. Lipid metabolism: Its role in energy regulation and obesity. In Novel Food Ingredients for Weight Control; Woodhead Publishing Series in Food Science, Technology and Nutrition: ETH Zürich, Switzerland, 2007; pp. 3–27. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Gaundal, L.; Myhrstad, M.C.W. Polyunsaturated Fatty Acids and Glycemic Control in Type 2 Diabetes. Nutrients 2019, 11, 1067. [Google Scholar] [CrossRef] [Green Version]
- Landmark, K.; Aursnes, I. Mercury, fish, fish oil and the risk of cardiovascular disease. Tidsskr Nor Laegeforen. 2004, 124, 198–200. [Google Scholar] [CrossRef]
- Hu, X.F.; Laird, B.D.; Chan, H. Mercury diminishes the cardiovascular protective effect of omega-3 polyunsaturated fatty acids in the modern diet of Inuit in Canada. Environ. Res. 2017, 152, 470–477. [Google Scholar] [CrossRef]
- Guallar, E.; Sanz-Gallardo, M.I.; Veer, P.V.T.; Bode, P.; Aro, A.; Gómez-Aracena, J.; Kark, J.D.; Riemersma, R.A.; Martín-Moreno, J.M.; Kok, F.J. Mercury, Fish Oils, and the Risk of Myocardial Infarction. N. Engl. J. Med. 2002, 347, 1747–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergkvist, C.; Berglund, M.; Glynn, A.; Julin, B.; Wolk, A.; Åkesson, A. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction in men—A population-based prospective cohort study. Environ. Int. 2016, 88, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Luigi Tavazzi, L.; Aldo, P.; Maggioni, A.P.; Roberto Marchioli, R.; Simona Barlera, S.; Maria Grazia Franzosi, M.G.; Roberto Latini, R.; Donata Lucci, D.; Gian Luigi Nicolosi, G.L.; Porcu, M.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Randomized Control. Trial Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. https://doi.org/10.3390/biomedicines8090306
Oppedisano F, Macrì R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, Bosco F, Nucera S, Caterina Zito M, Guarnieri L, et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines. 2020; 8(9):306. https://doi.org/10.3390/biomedicines8090306
Chicago/Turabian StyleOppedisano, Francesca, Roberta Macrì, Micaela Gliozzi, Vincenzo Musolino, Cristina Carresi, Jessica Maiuolo, Francesca Bosco, Saverio Nucera, Maria Caterina Zito, Lorenza Guarnieri, and et al. 2020. "The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection" Biomedicines 8, no. 9: 306. https://doi.org/10.3390/biomedicines8090306
APA StyleOppedisano, F., Macrì, R., Gliozzi, M., Musolino, V., Carresi, C., Maiuolo, J., Bosco, F., Nucera, S., Caterina Zito, M., Guarnieri, L., Scarano, F., Nicita, C., Coppoletta, A. R., Ruga, S., Scicchitano, M., Mollace, R., Palma, E., & Mollace, V. (2020). The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines, 8(9), 306. https://doi.org/10.3390/biomedicines8090306






