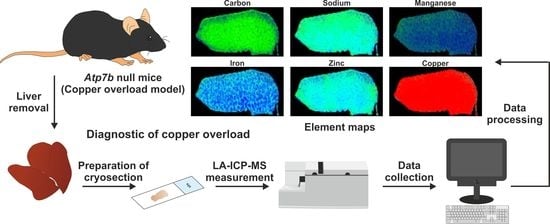
Accurate Measurement of Copper Overload in an Experimental Model of Wilson Disease by Laser Ablation Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Rhodanine Stain for Detection of Cytoplasmic Accumulation of Hepatic Copper
2.3. Scanning Electron Microscopy with Energy-Disperse X-ray (EDX) Spectroscopy Analysis (SEM-EDX)
2.4. Electron Microscopic Analysis
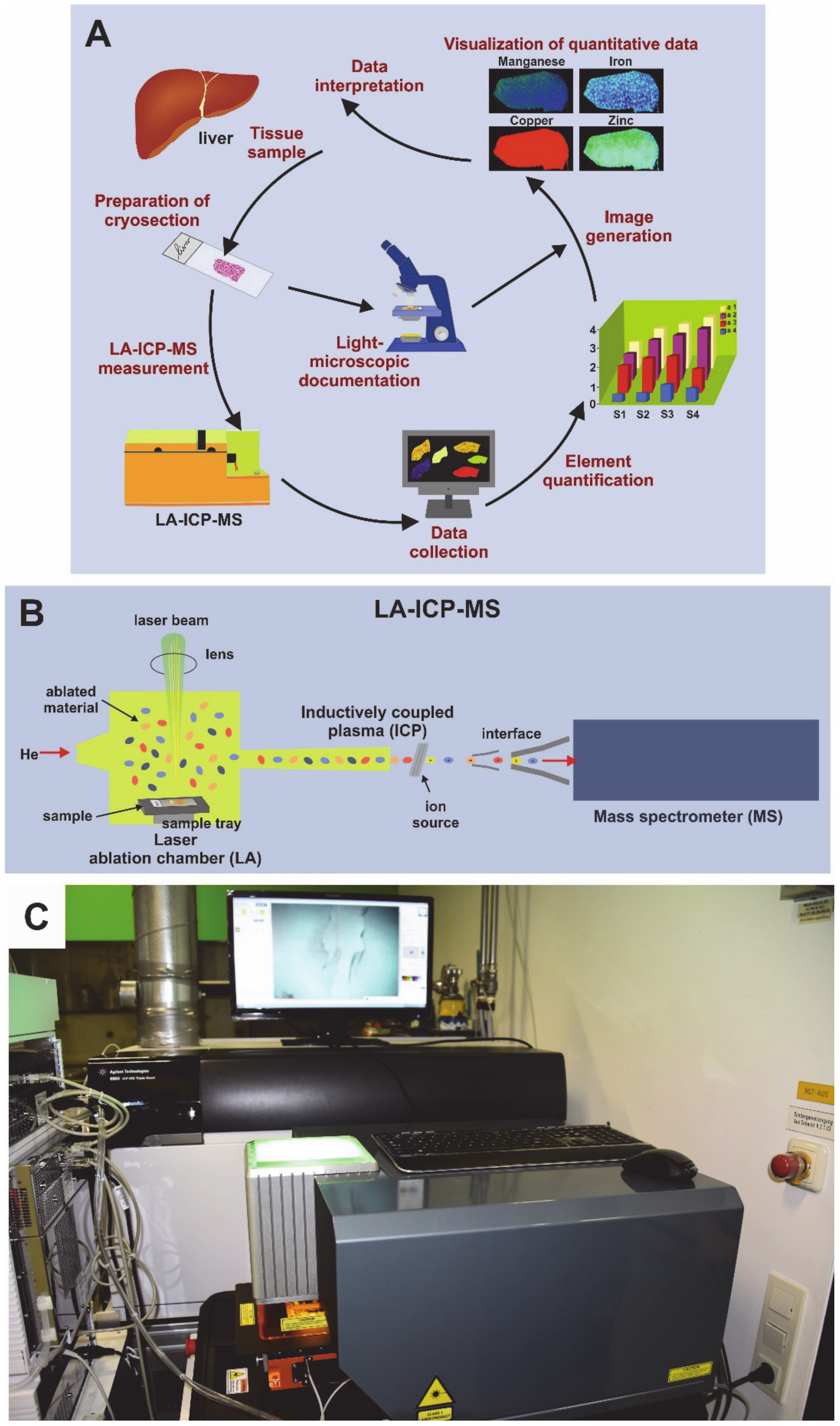
2.5. Sample Preparation for LA-ICP-MS Measurements
2.6. LA-ICP-MS Set Up and Measurements
2.7. Image Generation of Bio-Metal Distribution
3. Results
3.1. Electron Microscopic Analysis of Atp7b–/– Mouse Liver Tissues
3.2. Rhodanine Stain for Detection of Cytoplasmic Accumulation of Hepatic Copper in Atp7b–/– Mice
3.3. Analysis of Liver Tissue of Atp7b–/– Mice by Scanning Electron Microscopy with Energy-Disperse X-Ray Spectroscopy Analysis
3.4. Detection of Hepatic Copper Overload in Atp7b–/– Mice by Laser Ablation Inductively-Coupled Mass Spectrometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7, S61. [Google Scholar] [CrossRef] [PubMed]
- Goldfischer, S.; Sternlieb, I. Changes in the distribution of hepatic copper in relation to the progression of Wilson’s disease (hepatolenticular degeneration). Am. J. Pathol. 1968, 53, 883–901. [Google Scholar] [PubMed]
- Ishak, K.G. Inherited metabolic diseases of the liver. Clin. Liver Dis. 2002, 6, 455–479. [Google Scholar] [CrossRef]
- Pilloni, L.; Lecca, S.; Van Eyken, P.; Flore, C.; Demelia, L.; Pilleri, G.; Nurchi, A.M.; Farci, A.M.G.; Ambu, R.; Callea, F.; et al. Value of histochemical stains for copper in the diagnosis of Wilson’s disease. Histopathology 1998, 33, 28–33. [Google Scholar] [CrossRef]
- Susnea, I.; Weiskirchen, R. Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom. Rev. 2015, 35, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Uerlings, R.; Moreno, D.; Murillo-Sauca, O.; Gázquez, C.; Hernández-Alcoceba, R.; Gonzalez-Aseguinolaza, G.; Weiskirchen, R. Brain copper storage after genetic long-term correction in a mouse model of Wilson disease. Neurol. Genet. 2018, 4, e243. [Google Scholar] [CrossRef] [Green Version]
- Moreno, D.; Murillo-Sauca, O.; Gázquez, C.; Hernandez-Alcoceba, R.; Uerlings, R.; Gonzalez-Aseguinolaza, G.; Weiskirchen, R. Visualization of the therapeutic efficacy of a gene correction approach in Wilson’s disease by laser-ablation inductively coupled mass spectrometry. J. Hepatol. 2018, 68, 1088–1090. [Google Scholar] [CrossRef] [Green Version]
- Buiakova, O.I.; Xu, J.; Lutsenko, S.; Zeitlin, S.; Das, K.; Das, S.; Ross, B.M.; Mekios, C.; Scheinberg, I.H.; Gilliam, T.C. Null Mutation of the Murine ATP7B (Wilson Disease) Gene Results in Intracellular Copper Accumulation and Late-Onset Hepatic Nodular Transformation. Hum. Mol. Genet. 1999, 8, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Sauer, S.; Merle, U.; Opp, S.; Haas, D.; Hoffmann, G.F.; Stremmel, W.; Okun, J.G. Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b−/− mice as a model for Wilson disease. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2011, 1812, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, R.R. Studies on the pathogenesis of hepatolenticular degeneration. II. Cytochemical methods for the localization of copper. Arch. Pathol. 1969, 87, 5766764. [Google Scholar]
- Uerlings, R.; Matusch, A.; Weiskirchen, R. Reconstruction of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) spatial distribution images in Microsoft Excel 2007. Int. J. Mass Spectrom. 2016, 395, 27–35. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Kim, P.; Winkler, R. Software solutions for evaluation and visualization of laser ablation inductively coupled plasma mass spectrometry imaging (LA-ICP-MSI) data: A short overview. J. Cheminform. 2019, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Fanos, V.; Gerosa, C.; Piras, M.; Dessi, A.; Atzei, A.; Van, E.P.; Gibo, Y.; Faa, G. Effects of Iron and Copper Overload on the Human Liver: An Ultrastructural Study. Curr. Med. Chem. 2014, 21, 3768–3774. [Google Scholar] [CrossRef] [PubMed]
- Alt, E.R.; Sternlieb, I.; Goldfischer, S. The Cytopathology of Metal Overload. Int. Rev. Exp. Pathol. 1990, 31, 165–188. [Google Scholar] [CrossRef]
- Iancu, T.C.; Manov, I. Electron microscopy of liver biopsies. In Liver Biopsy; Takahashi, H., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Irons, R.D.; A Schenk, E.; Lee, J.C. Cytochemical methods for copper. Semiquantitative screening procedure for identification of abnormal copper levels in liver. Arch. Pathol. Lab. Med. 1977, 101, 67829. [Google Scholar]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Flechtenmacher, C.; Willheim, C.; Eder, E.; Ferenci, P.; Stremmel, W.; Weiskirchen, R. Laser ablation inductively coupled plasma mass spectrometry imaging of metals in experimental and clinical Wilson’s disease. J. Cell. Mol. Med. 2015, 19, 806–814. [Google Scholar] [CrossRef]
- Jonas, L.; Fulda, G.; Salameh, T.; Schmidt, W.; Kröning, G.; Hopt, U.T.; Nizze, H.; Jonas, G.F.L. Electron Microscopic Detection of Copper in the Liver of Two Patients with Morbus Wilson by EELS and EDX. Ultrastruct. Pathol. 2001, 25, 111–118. [Google Scholar] [CrossRef]
- M-M, P.; Weiskirchen, R.; Gassler, N.; Bosserhoff, A.-K.; Becker, J.S. Novel Bioimaging Techniques of Metals by Laser Ablation Inductively Coupled Plasma Mass Spectrometry for Diagnosis Of Fibrotic and Cirrhotic Liver Disorders. PLoS ONE 2013, 8, e58702. [Google Scholar] [CrossRef]
- Kim, P.; Weiskirchen, S.; Uerlings, R.; Kueppers, A.; Stellmacher, F.; Viveiros, A.; Zoller, H.; Weiskirchen, R. Quantification of liver iron overload disease with laser ablation inductively coupled plasma mass spectrometry. BMC Med. Imaging 2018, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Weiskirchen, S.; Matusch, A.; Stremmel, W.; Weiskirchen, R. Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson’s disease by laser ablation inductively coupled plasma mass spectrometry. BMC Neurosci. 2014, 15, 98. [Google Scholar] [CrossRef] [Green Version]
- Weiskirchen, S.; Kim, P.; Weiskirchen, R. Determination of copper poisoning in Wilson’s disease using laser ablation inductively coupled plasma mass spectrometry. Ann. Transl. Med. 2019, 7, S72. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, S.; Kim, P.; Weiskirchen, R. Laser Ablation Inductively Coupled Plasma Spectrometry: Metal Imaging in Experimental and Clinical Wilson Disease. Inorganics 2019, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Limbeck, A.; Galler, P.; Bonta, M.; Bauer, G.; Nischkauer, W.; Vanhaecke, F. Recent advances in quantitative LA-ICP-MS analysis: challenges and solutions in the life sciences and environmental chemistry. Anal. Bioanal. Chem. 2015, 407, 6593–6617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Roberts, E.A.; Schilsky, M.L. Diagnosis and treatment of Wilson disease: An update. Hepatol. 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Jain, S.; Scheuer, P.J.; Archer, B.; Newman, S.P.; Sherlock, S. Histological demonstration of copper and copper-associated protein in chronic liver diseases. J. Clin. Pathol. 1978, 31, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.J.; Poucell, S.; Patterson, J.; Valencia, P. The Liver. An Atlas and Text of Ultrastructural Pathology; Raven Press: New York, NY, USA, 1987; ISBN 978-0881673029. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Is Scanning Electron Microscopy/Energy Dispersive X-ray Spectrometry (SEM/EDS) Quantitative? Scanning 2012, 35, 141–168. [Google Scholar] [CrossRef]
- Camarata, M.A.; Ala, A.; Schilsky, M.L. Zinc Maintenance Therapy for Wilson Disease: A Comparison Between Zinc Acetate and Alternative Zinc Preparations. Hepatol. Commun. 2019, 3, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Wiernicka, A.; Jańczyk, W.; Dadalski, M.; Avsar, Y.; Schmidt, H.; Socha, P. Gastrointestinal side effects in children with Wilson’s disease treated with zinc sulphate. World J. Gastroenterol. 2013, 19, 4356–4362. [Google Scholar] [CrossRef]
- Shiono, Y.; Hayashi, H.; Wakusawa, S.; Yano, M. Ultrastructural identification of iron and copper accumulation in the liver of a male patient with Wilson disease. Med Mol. Morphol. 2001, 34, 54–60. [Google Scholar] [CrossRef]
- Hayashi, H.; Yano, M.; Fujita, Y.; Wakusawa, S. Compound overload of copper and iron in patients with Wilson’s disease. Med Mol. Morphol. 2006, 39, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y.; Wakusawa, S.; Hayashi, H.; Takikawa, T.; Yano, M.; Okada, T.; Mabuchi, H.; Kono, S.; Miyajima, H. Iron accumulation in the liver of male patients with Wilson’s disease. Am. J. Gastroenterol. 2001, 96, 3147–3151. [Google Scholar] [CrossRef]
- Kaščáková, S.; Kewish, C.; Rouzière, S.; Schmitt, F.; Sobesky, R.; Poupon, J.; Sandt, C.; Francou, B.; Somogyi, A.; Samuel, D.; et al. Rapid and reliable diagnosis of Wilson disease using X-ray fluorescence. J. Pathol. Clin. Res. 2016, 2, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusek, P.; Bahn, E.; Litwin, T.; Jabłonka-Salach, K.; Łuciuk, A.; Huelnhagen, T.; Madai, V.I.; Dieringer, M.A.; Bulska, E.; Knauth, M.; et al. Brain iron accumulation in Wilson disease: Apost mortem7 Tesla MRI—Histopathological study. Neuropathol. Appl. Neurobiol. 2016, 43, 514–532. [Google Scholar] [CrossRef] [Green Version]
- Dusek, P.; Skoloudik, D.; Maskova, J.; Huelnhagen, T.; Bruha, R.; Zahorakova, D.; Niendorf, T.; Ruzicka, E.; Schneider, S.A.; Wuerfel, J. Brain iron accumulation in Wilson’s disease: A longitudinal imaging case study during anticopper treatment using 7.0T MRI and transcranial sonography. J. Magn. Reson. Imaging 2017, 47, 282–285. [Google Scholar] [CrossRef]
- Mai, F.-D.; Chen, B.-J.; Wu, L.-C.; Li, F.-Y.; Chen, W.-K. Imaging of single liver tumor cells intoxicated by heavy metals using ToF-SIMS. Appl. Surf. Sci. 2006, 252, 6809–6812. [Google Scholar] [CrossRef]
- Zou, J.; Talbot, F.; Tata, A.; Ermini, L.; Franjic, K.; Ventura, M.; Zheng, J.; Ginsberg, H.; Post, M.; Ifa, D.R.; et al. Ambient Mass Spectrometry Imaging with Picosecond Infrared Laser Ablation Electrospray Ionization (PIR-LAESI). Anal. Chem. 2015, 87, 12071–12079. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, P.; Zhang, C.C.; Thoröe-Boveleth, S.; Weiskirchen, S.; Gaisa, N.T.; Buhl, E.M.; Stremmel, W.; Merle, U.; Weiskirchen, R. Accurate Measurement of Copper Overload in an Experimental Model of Wilson Disease by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Biomedicines 2020, 8, 356. https://doi.org/10.3390/biomedicines8090356
Kim P, Zhang CC, Thoröe-Boveleth S, Weiskirchen S, Gaisa NT, Buhl EM, Stremmel W, Merle U, Weiskirchen R. Accurate Measurement of Copper Overload in an Experimental Model of Wilson Disease by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Biomedicines. 2020; 8(9):356. https://doi.org/10.3390/biomedicines8090356
Chicago/Turabian StyleKim, Philipp, Chengcheng Christine Zhang, Sven Thoröe-Boveleth, Sabine Weiskirchen, Nadine Therese Gaisa, Eva Miriam Buhl, Wolfgang Stremmel, Uta Merle, and Ralf Weiskirchen. 2020. "Accurate Measurement of Copper Overload in an Experimental Model of Wilson Disease by Laser Ablation Inductively Coupled Plasma Mass Spectrometry" Biomedicines 8, no. 9: 356. https://doi.org/10.3390/biomedicines8090356
APA StyleKim, P., Zhang, C. C., Thoröe-Boveleth, S., Weiskirchen, S., Gaisa, N. T., Buhl, E. M., Stremmel, W., Merle, U., & Weiskirchen, R. (2020). Accurate Measurement of Copper Overload in an Experimental Model of Wilson Disease by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Biomedicines, 8(9), 356. https://doi.org/10.3390/biomedicines8090356