Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development
Abstract
1. Introduction
2. Chronic Obstructive Pulmonary Disease (COPD)
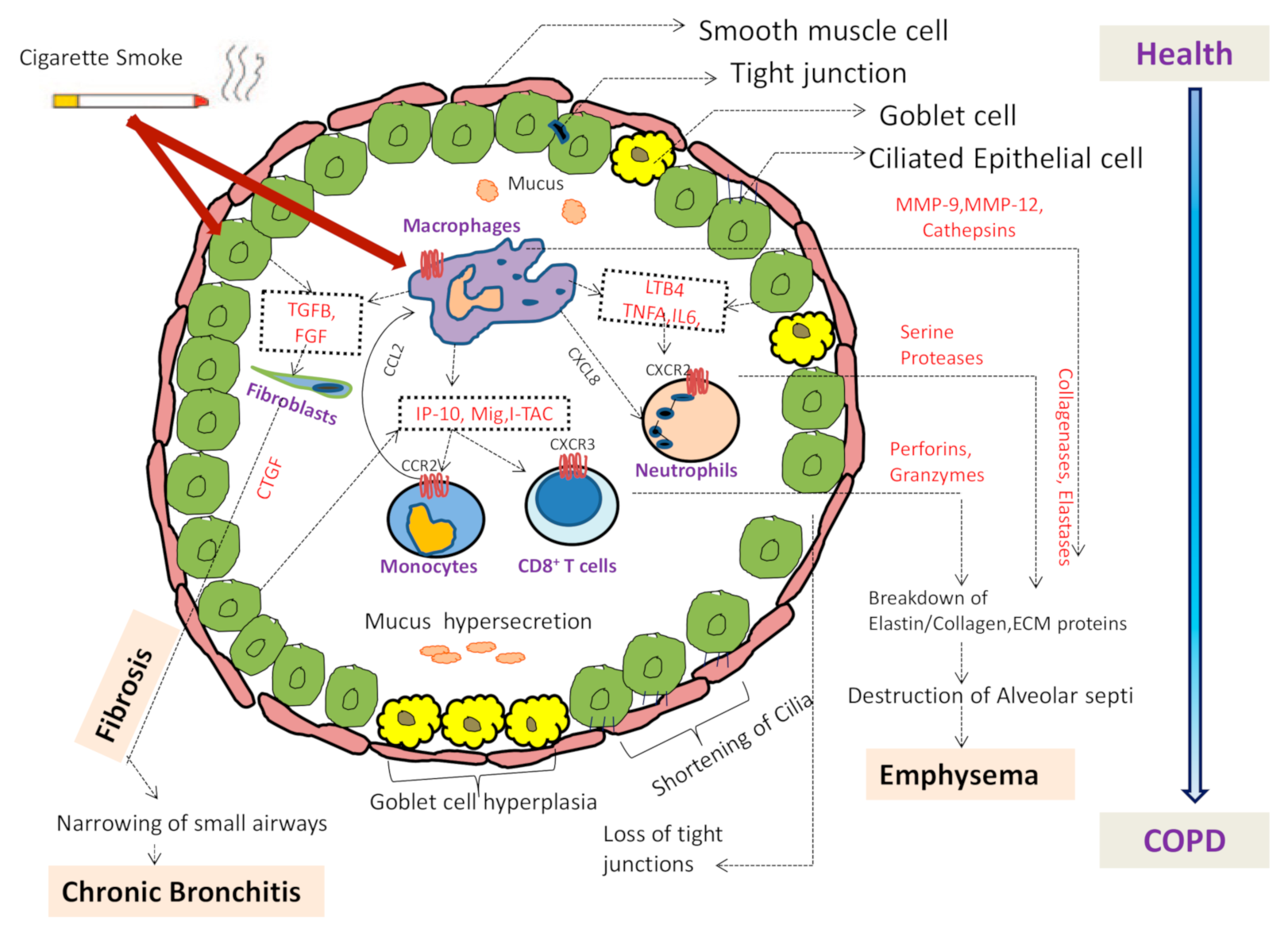
2.1. Pathophysiology of COPD
2.2. Inflammation in COPD
2.3. Adverse Effect of Cigarette Smoke via Neutrophils
3. Innate Immune Cell Neutrophils and COPD
3.1. Neutrophils: Link between Innate and Adaptive Immunity
3.2. Neutrophil Migration
3.3. Functions of Neutrophil and Death Mechanisms
3.3.1. Neutrophil Phagocytosis and Degranulation
3.3.2. Apoptosis of Neutrophils
3.4. Neutrophils Decision to Phagocytosis or Formation of NETs
4. Neutrophil Extracellular Traps (NETs)
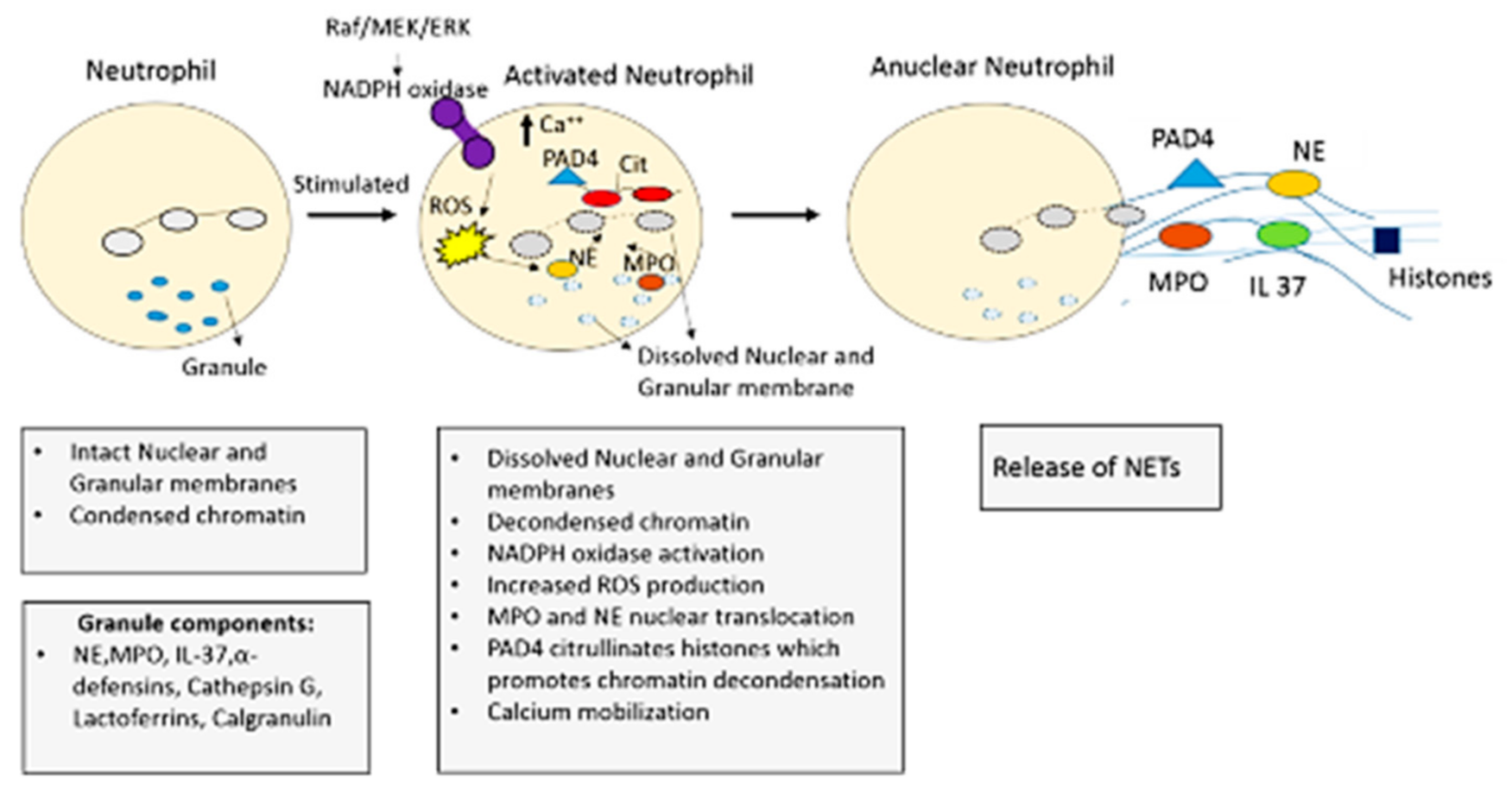
4.1. NETosis: The Formation of NETs
4.2. NETosis Mechanism
4.2.1. NADPH Oxidase (NOX) Dependent NETosis
4.2.2. NADPH Oxidase (NOX) Independent NETosis
5. NETs and COPD
6. Role of CXCR1 and CXCR2 Receptors in Neutrophils and COPD
CXCR2 Inhibition in COPD and NETs
7. Vicious Cycle of the NETs in COPD Inflammation
7.1. Future Prospects: Targeting NETs in COPD
7.2. Potential Anti-Net Therapeutics
- Anti-thrombosis: Heparin is a naturally occurring glycosaminoglycan which is used as an anticoagulant (blood thinner) in the treatment of stable angina, heart attacks and it also antagonizes the effects of histones [194,195,196]. Role of Heparin in reduction/inhibition of NETs is enlisted in Table 3.
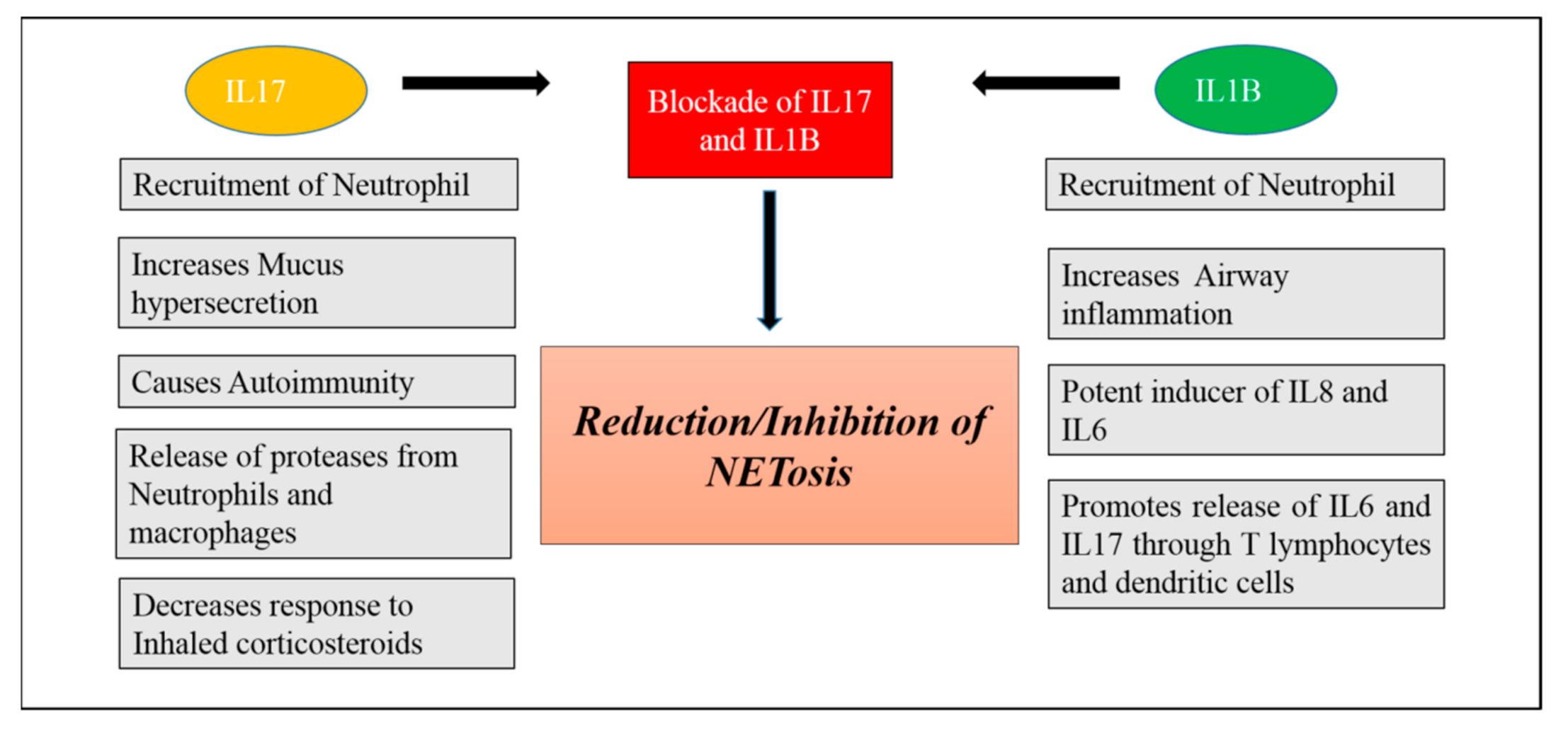
- Blockade of IL-1B and IL17: IL1B and IL-17 are the key mediators of neutrophilic airway inflammation in COPD. Elevated serum levels of these cytokines may be used as a bio-marker for indicating persistent neutrophilic airway inflammation and potential ongoing exacerbation in COPD. Levels of these two inflammatory mediators in serum are associated with important clinical parameters in COPD such as degree of airflow limitation and smoking status. As we know that neutrophils can produce and release NETs in COPD, these NETs in turn can prime macrophages to produce a precursor form of inflammatory cytokine IL-1B (pro- IL1B). NETs can also collaborate with other activation signals such as heat shock proteins and cholesterol signals promoting the release of IL-1B [199]. Thus, serum IL-1B increase in COPD is also found to be associated with neutrophil percentage in COPD. Thus, neutrophils mediate formation of IL-1B that facilitates neutrophil recruitment into airways creating a vicious cycle of neutrophilic airway inflammation and contributing to progression of COPD. IL17 is mainly secreted by IL17 producing T lymphocytes including αβ T cells and ϒ𝛿 T cells and these two kinds of cells can be induced by IL1B in the lung tissue and BALF of COPD patients [200]. Thus, IL1B may be an important factor leading to increased expression of IL17 in COPD. Blockade of IL1B and IL17 could be a valid strategy for prevention and control of COPD [201,202] (Figure 4).
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Singh:, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Quaderi, S.A.; Hurst, J.R. The Unmet Global Burden of COPD. Glob. Health Epidemiol. Genom 2018, 3. [Google Scholar] [CrossRef]
- O’Donnell, R.; Breen, D.; Wilson, S.; Djukanovic, R. Inflammatory Cells in the Airways in COPD. Thorax 2006, 61, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Camicia, G.; Pozner, R.; de Larrañaga, G. Neutrophil Extracellular Traps in Sepsis. Shock 2014, 42, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jasper, A.E.; McIver, W.J.; Sapey, E.; Walton, G.M. Understanding the Role of Neutrophils in Chronic Inflammatory Airway Disease. F1000Research 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory Mechanisms in the Lung. J. Inflamm. Res. 2008, 2, 1–11. [Google Scholar]
- Beeh, K.M.; Kornmann, O.; Buhl, R.; Culpitt, S.V.; Giembycz, M.A.; Barnes, P.J. Neutrophil Chemotactic Activity of Sputum from Patients With COPD. Chest 2003, 123, 1240–1247. [Google Scholar] [CrossRef]
- Uddin, M.; Watz, H.; Malmgren, A.; Pedersen, F. NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma. Front. Immunol. 2019, 10, 47. [Google Scholar] [CrossRef]
- Yousefi, S.; Stojkov, D.; Germic, N.; Simon, D.; Wang, X.; Benarafa, C.; Simon, H.-U. Untangling “NETosis” from NETs. Eur. J. Immunol. 2019, 49, 221–227. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Grinstein, S. Unconventional Roles of the NADPH Oxidase: Signaling, Ion Homeostasis, and Cell Death. Sci. STKE Signal Transduct. Knowl. Environ. 2007, 2007, pe11. [Google Scholar] [CrossRef]
- Mesa, M.A.; Vasquez, G. NETosis. Autoimmune Dis. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.-S.; Ji, A.; Ji, X.-Y.; Li, Y.-Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Kobayashi, S.D.; Quinn, M.T.; DeLeo, F.R. NET Confusion. Front. Immunol. 2016, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pang, Z.; Wang, G.; Guan, X.; Fang, K.; Wang, Z.; Wang, F. Advanced Role of Neutrophils in Common Respiratory Diseases. J. Immunol. Res. 2017, 2017, 1–21. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps (NETs): Double-Edged Swords of Innate Immunity. J. Immunol. Baltim. Md 1950 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Mozzini, C.; Girelli, D. The Role of Neutrophil Extracellular Traps in Covid-19: Only an Hypothesis or a Potential New Field of Research? Thromb. Res. 2020, 191, 26–27. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and Tissue Factor–Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil Extracellular Traps Released by Neutrophils Impair Revascularization and Vascular Remodeling after Stroke. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikari, J.; Anazawa, R.; Tanaka, N.; Katsumata, Y.; Shimada, A.; Suzuki, E.; Tatsumi, K. Neutrophil Extracellular Traps Contribute to Pulmonary Fibrosis Induced by Bleomycin. Eur. Respir. J. 2019, 54 (Suppl. 63). [Google Scholar] [CrossRef]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, H.M.; Lehtonen, S.T.; Harju, T.; Sormunen, R.T.; Lappi-Blanco, E.; Mäkinen, J.M.; Laitakari, K.; Johnson, S.; Kaarteenaho, R.L. Myofibroblast Expression in Airways and Alveoli Is Affected by Smoking and COPD. Respir. Res. 2013, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Kliment, C.R.; Metz, H.E.; Kim, K.-H.; Kargl, J.; Agostini, B.A.; Crum, L.T.; Oczypok, E.A.; Oury, T.A.; Houghton, A.M. Neutrophil Elastase Promotes Myofibroblast Differentiation in Lung Fibrosis. J. Leukoc. Biol. 2015, 98, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ali, Z.S.; Sweezey, N.; Grasemann, H.; Palaniyar, N. Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation. Genes 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil Extracellular Traps Are Associated with Disease Severity and Microbiota Diversity in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils Sense Microbe Size and Selectively Release Neutrophil Extracellular Traps in Response to Large Pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets Induce Neutrophil Extracellular Traps in Transfusion-Related Acute Lung Injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef]
- Lazaar, A.L.; Miller, B.E.; Donald, A.C.; Keeley, T.; Ambery, C.; Russell, J.; Watz, H.; Tal-Singer, R.; Bardin, P.; Bremner, P.; et al. CXCR2 Antagonist for Patients with Chronic Obstructive Pulmonary Disease with Chronic Mucus Hypersecretion: A Phase 2b Trial. Respir. Res. 2020, 21, 149. [Google Scholar] [CrossRef]
- Kim, V.; Criner, G.J. Chronic Bronchitis and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A. Systemic Effects of Chronic Obstructive Pulmonary Disease: What We Know and What We Don’t Know (but Should). Proc. Am. Thorac. Soc. 2007, 4, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1226–1228. [Google Scholar]
- Balmes, J.; Becklake, M.; Blanc, P.; Henneberger, P.; Kreiss, K.; Mapp, C.; Milton, D.; Schwartz, D.; Toren, K.; Viegi, G. Environmental and Occupational Health Assembly, American Thoracic Society. American Thoracic Society Statement: Occupational Contribution to the Burden of Airway Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 787–797. [Google Scholar] [CrossRef]
- Barnes, P.J. Immunology of Asthma and Chronic Obstructive Pulmonary Disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Pathology, Pathogenesis, and Pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative Stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Brashier, B.B.; Kodgule, R. Risk Factors and Pathophysiology of Chronic Obstructive Pulmonary Disease (COPD). J. Assoc. Physicians India 2012, 60, 17–21. [Google Scholar]
- Bourdin, A.; Burgel, P.-R.; Chanez, P.; Garcia, G.; Perez, T.; Roche, N. Recent Advances in COPD: Pathophysiology, Respiratory Physiology and Clinical Aspects, Including Comorbidities. Eur. Respir. Rev. 2009, 18, 198–212. [Google Scholar] [CrossRef]
- Cornwell, W.D.; Kim, V.; Song, C.; Rogers, T.J. Pathogenesis of Inflammation and Repair in Advanced COPD. Semin. Respir. Crit. Care Med. 2010, 31, 257–266. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agustí, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet Lond. Engl. 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Scanlon, S.T.; McKenzie, A.N.J. Type 2 Innate Lymphoid Cells: New Players in Asthma and Allergy. Curr. Opin. Immunol. 2012, 24, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Strange, C. Inhaled Corticosteroids for Chronic Obstructive Pulmonary Disease: What Is Their Role in Therapy? Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette Smoking and Inflammation. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil Elastase, Proteinase 3, and Cathepsin G as Therapeutic Targets in Human Diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An Overview of the Role of Neutrophils in Innate Immunity, Inflammation and Host-Biomaterial Integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Turato, G.; Zuin, R.; Saetta, M. Pathogenesis and Pathology of COPD. Respir. Int. Rev. Thorac. Dis. 2001, 68, 117–128. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic Obstructive Pulmonary Disease: Molecular and Cellular Mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Barnes, P.J.; Cosio, M.G. Characterization of T Lymphocytes in Chronic Obstructive Pulmonary Disease. PLoS Med. 2004, 1. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the Game of T Cells: What Can the CD4/CD8 T-Cell Ratio Tell Us about HIV and Health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of Apoptosis in the Pathogenesis of COPD and Pulmonary Emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef]
- Westwood, J.-P.; Mackay, A.J.; Donaldson, G.; Machin, S.J.; Wedzicha, J.A.; Scully, M. The Role of Complement Activation in COPD Exacerbation Recovery. ERJ Open Res. 2016, 2, 00027-2016. [Google Scholar] [CrossRef]
- Marc, M.M.; Kristan, S.S.; Rozman, A.; Kern, I.; Flezar, M.; Kosnik, M.; Korosec, P. Complement Factor C5a in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Scand. J. Immunol. 2010, 71, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Guzik, K.; Skret, J.; Smagur, J.; Bzowska, M.; Gajkowska, B.; Scott, D.A.; Potempa, J.S. Cigarette Smoke-Exposed Neutrophils Die Unconventionally but Are Rapidly Phagocytosed by Macrophages. Cell Death Dis. 2011, 2, e131. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.A.; Tobias, M.; O’Neill, H.C.; Franklin, C.C. Cigarette Smoke Extract-Induced Suppression of Caspase-3-like Activity Impairs Human Neutrophil Phagocytosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L1572–L1579. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Hirschfeld, J.; Milward, M.R.; Cooper, P.R.; Wright, H.J.; Matthews, J.B.; Chapple, I.L.C. Cigarette Smoke Modifies Neutrophil Chemotaxis, Neutrophil Extracellular Trap Formation and Inflammatory Response-Related Gene Expression. J. Periodontal Res. 2018, 53, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Pouwels, S.D.; Leijendekker, C.; de Bruin, H.G.; Zijlstra, G.J.; van der Vaart, H.; ten Hacken, N.H.T.; van Oosterhout, A.J.M.; Nawijn, M.C.; van der Toorn, M. Cigarette Smoke-Induced Damage-Associated Molecular Pattern Release from Necrotic Neutrophils Triggers Proinflammatory Mediator Release. Am. J. Respir. Cell Mol. Biol. 2015, 52, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Geffen, W.H.; van Jonker, M.R.; Kerstjens, H.A.M.; Nawijn, M.C.; Heijink, I.H. Increased Neutrophil Expression of Pattern Recognition Receptors during COPD Exacerbations. Respirology 2017, 22, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, A.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Mikroulis, D.; Konstantinidis, T.; Sivridis, E.; Koffa, M.; Giatromanolaki, A.; Boumpas, D.T.; et al. Neutrophil Extracellular Traps Promote Differentiation and Function of Fibroblasts. J. Pathol. 2014, 233, 294–307. [Google Scholar] [CrossRef]
- Vij, N.; Chandramani-Shivalingappa, P.; Van Westphal, C.; Hole, R.; Bodas, M. Cigarette Smoke-Induced Autophagy Impairment Accelerates Lung Aging, COPD-Emphysema Exacerbations and Pathogenesis. Am. J. Physiol.-Cell Physiol. 2018, 314, C73–C87. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Lee, S.-J.; Choi, A.M. Autophagy in Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Expert Rev. Respir. Med. 2010, 4, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of Cigarette Smoke-Induced Epithelial Cell Ferroptosis in COPD Pathogenesis. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guillon, A.; Jouan, Y.; Brea, D.; Gueugnon, F.; Dalloneau, E.; Baranek, T.; Henry, C.; Morello, E.; Renauld, J.-C.; Pichavant, M.; et al. Neutrophil Proteases Alter the Interleukin-22-Receptor-Dependent Lung Antimicrobial Defence. Eur. Respir. J. 2015, 46, 771–782. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in Tissue Injury and Repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Meijer, M.; Rijkers, G.T.; van Overveld, F.J. Neutrophils and Emerging Targets for Treatment in Chronic Obstructive Pulmonary Disease. Expert Rev. Clin. Immunol. 2013, 9, 1055–1068. [Google Scholar] [CrossRef]
- Bartoli, M.L.; Costa, F.; Malagrinò, L.; Nieri, D.; Antonelli, S.; Decusatis, G.; Simone, C.D.; Santerini, S.; Cianchetti, S.; Latorre, M.; et al. Sputum Inflammatory Cells in COPD Patients Classified According to GOLD 2011 Guidelines. Eur. Respir. J. 2016, 47, 978–980. [Google Scholar] [CrossRef]
- Guiot, J.; Henket, M.; Corhay, J.L.; Moermans, C.; Louis, R. Sputum Biomarkers in IPF: Evidence for Raised Gene Expression and Protein Level of IGFBP-2, IL-8 and MMP-7. PLoS ONE 2017, 12, e0171344. [Google Scholar] [CrossRef]
- Shanmugam, L.; Ravinder, S.S.; Johnson, P.; Padmavathi, R.; Rajagopalan, B.; Kindo, A.J. Assessment of Phagocytic Activity of Neutrophils in Chronic Obstructive Pulmonary Disease. Lung India Off. Organ Indian Chest Soc. 2015, 32, 437–440. [Google Scholar] [CrossRef]
- Butler, A.; Walton, G.M.; Sapey, E. Neutrophilic Inflammation in the Pathogenesis of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 392–404. [Google Scholar] [CrossRef]
- Venge, P.; Rak, S.; Steinholtz, L.; Håkansson, L.; Lindblad, G. Neutrophil Function in Chronic Bronchitis. Eur. Respir. J. 1991, 4, 536–543. [Google Scholar] [PubMed]
- Müns, G.; Rubinstein, I.; Bergmann, K.C. Phagocytosis and Oxidative Burst of Blood Phagocytes in Chronic Obstructive Airway Disease. Scand. J. Infect. Dis. 1995, 27, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Konrad, F.M.; Braun, S.; Ngamsri, K.-C.; Vollmer, I.; Reutershan, J. Heme Oxygenase-1 Attenuates Acute Pulmonary Inflammation by Decreasing the Release of Segmented Neutrophils from the Bone Marrow. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 307, L707–L717. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C.; Demaurex, N.; Lowell, C.A.; Uribe-Querol, E. Neutrophils: Their Role in Innate and Adaptive Immunity. J. Immunol. Res. 2016, 2016, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Leliefeld, P.H.C.; Koenderman, L.; Pillay, J. How Neutrophils Shape Adaptive Immune Responses. Front. Immunol. 2015, 6, 471. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The Regulatory Roles of Neutrophils in Adaptive Immunity. Cell Commun. Signal. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Hampton, H.R.; Chtanova, T. Lymphatic Migration of Immune Cells. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Monin, L.; Gaffen, S.L. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb. Perspect. Biol. 2018, 10, a028522. [Google Scholar] [CrossRef]
- Malhotra, A.; Shanker, A. NK Cells: Immune Cross-Talk and Therapeutic Implications. Immunotherapy 2011, 3, 1143–1166. [Google Scholar] [CrossRef]
- Stockley, R.A. Neutrophils and the Pathogenesis of COPD. Chest 2002, 121, 151S–155S. [Google Scholar] [CrossRef]
- Cowburn, A.S.; Condliffe, A.M.; Farahi, N.; Summers, C.; Chilvers, E.R. Advances in Neutrophil Biology. Chest 2008, 134, 606–612. [Google Scholar] [CrossRef]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The Balancing Act of Neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, M.; Yan, X.; Cao, L.; Barnes, P.J.; Adcock, I.M.; Huang, M.; Yao, X. Increased Neutrophil Gelatinase-Associated Lipocalin (NGAL) Promotes Airway Remodelling in Chronic Obstructive Pulmonary Disease. Clin. Sci. Lond. Engl. 1979 2017, 131, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U.; van Overveld, F. Role of Elastases in the Pathogenesis of Chronic Obstructive Pulmonary Disease: Implications for Treatment. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Rosales-Mayor, E.; Dale, G.E.; Dembowsky, K.; Torres, A. The Role of Neutrophil Elastase Inhibitors in Lung Diseases. Chest 2017, 152, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Biosynthesis of Human Myeloperoxidase. Arch. Biochem. Biophys. 2018, 642, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ostridge, K.; Williams, N.; Kim, V.; Bennett, M.; Harden, S.; Welch, L.; Bourne, S.; Coombs, N.A.; Elkington, P.T.; Staples, K.J.; et al. Relationship between Pulmonary Matrix Metalloproteinases and Quantitative CT Markers of Small Airways Disease and Emphysema in COPD. Thorax 2016, 71, 126–132. [Google Scholar] [CrossRef]
- Hendrix, A.Y.; Kheradmand, F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog. Mol. Biol. Transl. Sci. 2017, 148, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef]
- Manfredi, A.A.; Ramirez, G.A.; Rovere-Querini, P.; Maugeri, N. The Neutrophil’s Choice: Phagocytose vs Make Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 288. [Google Scholar] [CrossRef]
- Niedźwiedzka-Rystwej, P.; Repka, W.; Tokarz-Deptuła, B.; Deptuła, W. “In Sickness and in Health”—How Neutrophil Extracellular Trap (NET) Works in Infections, Selected Diseases and Pregnancy. J. Inflamm. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by Neutrophils. Microbes Infect. 2003, 5, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. J. Am. Soc. Hematol. 1997, 89, 3503–3521. [Google Scholar]
- Nordenfelt, P.; Tapper, H. The Role of Calcium in Neutrophil Granule-Phagosome Fusion. Commun. Integr. Biol. 2010, 3, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, M.; Catz, S.D. Molecular Mechanisms Regulating Secretory Organelles and Endosomes in Neutrophils and Their Implications for Inflammation. Immunol. Rev. 2016, 273, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Hoenderdos, K.; Condliffe, A. The Neutrophil in Chronic Obstructive Pulmonary Disease. Too Little, Too Late or Too Much, Too Soon? Am. J. Respir. Cell Mol. Biol. 2013, 48, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Koenderman, L.; Kanters, D.; Maesen, B.; Raaijmakers, J.; Lammers, J.W.; de Kruif, J.; Logtenberg, T. Monitoring of Neutrophil Priming in Whole Blood by Antibodies Isolated from a Synthetic Phage Antibody Library. J. Leukoc. Biol. 2000, 68, 58–64. [Google Scholar]
- Oudijk, E.-J.D.; Gerritsen, W.B.M.; Nijhuis, E.H.J.; Kanters, D.; Maesen, B.L.P.; Lammers, J.-W.J.; Koenderman, L. Expression of Priming-Associated Cellular Markers on Neutrophils during an Exacerbation of COPD. Respir. Med. 2006, 100, 1791–1799. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Zucker, R.M.; Hunter, E.S.; Rogers, J.M. Confocal Laser Scanning Microscopy of Morphology and Apoptosis in Organogenesis-Stage Mouse Embryos. Methods Mol. Biol. Clifton NJ 2000, 135, 191–202. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; DeLeo, F.R. Neutrophil Apoptosis and the Resolution of Infection. Immunol. Res. 2009, 43, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Herbert, P.E.; Warrens, A.N. An Introduction to Death Receptors in Apoptosis. Int. J. Surg. 2005, 3, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Mori, Y.; Yamaguchi, M.; Terao, Y.; Hamada, S.; Ooshima, T.; Kawabata, S. α-Enolase of Streptococcus Pneumoniae Induces Formation of Neutrophil Extracellular Traps. J. Biol. Chem. 2012, 287, 10472–10481. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-Containing Complex Regulates Neutrophil Elastase Release and Actin Dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef]
- Capasso, M.; DeCoursey, T.E.; Dyer, M.J.S. PH Regulation and beyond: Unanticipated Functions for the Voltage-Gated Proton Channel, HVCN1. Trends Cell Biol. 2011, 21, 20–28. [Google Scholar] [CrossRef]
- Maueröder, C.; Mahajan, A.; Paulus, S.; Gößwein, S.; Hahn, J.; Kienhöfer, D.; Biermann, M.H.; Tripal, P.; Friedrich, R.P.; Munoz, L.E.; et al. Ménage-à-Trois: The Ratio of Bicarbonate to CO2 and the PH Regulate the Capacity of Neutrophils to Form NETs. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Banerjee, S.; de Freitas, A.; Friggeri, A.; Zmijewski, J.W.; Liu, G.; Abraham, E. Intracellular HMGB1 Negatively Regulates Efferocytosis. J. Immunol. Baltim. Md 1950 2011, 187, 4686–4694. [Google Scholar] [CrossRef]
- Schaper, F.; de Leeuw, K.; Horst, G.; Bootsma, H.; Limburg, P.C.; Heeringa, P.; Bijl, M.; Westra, J. High Mobility Group Box 1 Skews Macrophage Polarization and Negatively Influences Phagocytosis of Apoptotic Cells. Rheumatol. Oxf. Engl. 2016, 55, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- Bilyy, R.O.; Shkandina, T.; Tomin, A.; Muñoz, L.E.; Franz, S.; Antonyuk, V.; Kit, Y.Y.; Zirngibl, M.; Fürnrohr, B.G.; Janko, C.; et al. Macrophages Discriminate Glycosylation Patterns of Apoptotic Cell-Derived Microparticles. J. Biol. Chem. 2012, 287, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.-J.; Kholmukhamedov, A.; Zhou, C.; Jobe, S. Inner Mitochondrial Membrane Disruption Links Apoptotic and Agonist-Initiated Phosphatidylserine Externalization in Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1503–1512. [Google Scholar] [CrossRef]
- Elliott, M.R.; Ravichandran, K.S. The Dynamics of Apoptotic Cell Clearance. Dev. Cell 2016, 38, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, V.A.; Balasubramanian, K.; Winnica, D.; Tyurina, Y.Y.; Vikulina, A.S.; He, R.R.; Kapralov, A.A.; Macphee, C.H.; Kagan, V.E. Oxidatively Modified Phosphatidylserines on the Surface of Apoptotic Cells Are Essential Phagocytic “eat-Me” Signals: Cleavage and Inhibition of Phagocytosis by Lp-PLA2. Cell Death Differ. 2014, 21, 825–835. [Google Scholar] [CrossRef]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid Killing of Human Neutrophils by the Potent Activator Phorbol 12-Myristate 13-Acetate (PMA) Accompanied by Changes Different from Typical Apoptosis or Necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bochenska, O.; Karkowska-Kuleta, J.; Seweryn-Ozog, K.; Aoki, W.; Ueda, M.; Kozik, A.; Rapala-Kozik, M. Aspartic Proteases and Major Cell Wall Components in Candida Albicans Trigger the Release of Neutrophil Extracellular Traps. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- Goldmann, O.; Medina, E. The Expanding World of Extracellular Traps: Not Only Neutrophils but Much More. Front. Immunol. 2012, 3, 420. [Google Scholar] [CrossRef]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New Insights into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.W.R.; Wilton, M.; Poon, K.K.H.; Petri, B.; Lewenza, S. DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, M.; van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as Mediators of Host Defense, Inflammation and Thrombosis. Future Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Beiter, K.; Normark, S.; Henriques-Normark, B. Neutrophil Extracellular Traps: Casting the NET over Pathogenesis. Curr. Opin. Microbiol. 2007, 10, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Niemiec, M.J.; Siler, U.; Urban, C.F.; Reichenbach, J. Restoration of Anti-Aspergillus Defense by Neutrophil Extracellular Traps in Human Chronic Granulomatous Disease after Gene Therapy Is Calprotectin-Dependent. J. Allergy Clin. Immunol. 2011, 127, 1243–1252.e7. [Google Scholar] [CrossRef]
- von Köckritz-Blickwede, M.; Nizet, V. Innate Immunity Turned Inside-out: Antimicrobial Defense by Phagocyte Extracellular Traps. J. Mol. Med. Berl. Ger. 2009, 87, 775–783. [Google Scholar] [CrossRef]
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J. Neutrophil Extracellular Traps Go Viral. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida Albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Santucci, L.; Vaglio, A.; Pratesi, F.; Migliorini, P.; Bertelli, R.; Lavarello, C.; Bartolucci, M.; Candiano, G.; et al. Neutrophil Extracellular Traps Protein Composition Is Specific for Patients with Lupus Nephritis and Includes Methyl-Oxidized Aenolase (Methionine Sulfoxide 93). Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Knight, J.S.; Carmona-Rivera, C.; Kaplan, M.J. Proteins Derived from Neutrophil Extracellular Traps May Serve as Self-Antigens and Mediate Organ Damage in Autoimmune Diseases. Front. Immunol. 2012, 3, 380. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Palaniyar, N. Transcriptional Firing Helps to Drive NETosis. Sci. Rep. 2017, 7, srep41749. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, J.D.; Reinhardt, T.A.; Goff, J.P.; Horst, R.L. Neutrophil Extracellular Trap Formation by Bovine Neutrophils Is Not Inhibited by Milk. Vet. Immunol. Immunopathol. 2006, 113, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil Extracellular Trap Formation as Innate Immune Reactions against the Apicomplexan Parasite Eimeria Bovis. Vet. Immunol. Immunopathol. 2010, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.-P. Neutrophil Extracellular Traps Are Downregulated by Glucocorticosteroids in Lungs in an Equine Model of Asthma. Respir. Res. 2017, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil Extracellular Traps: Is Immunity the Second Function of Chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Hamam, H.J.; Palaniyar, N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules 2019, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Papayannopoulos, V. Molecular Mechanisms Regulating NETosis in Infection and Disease. Semin. Immunopathol. 2013, 35, 513–530. [Google Scholar] [CrossRef]
- Thomas, M.P.; Whangbo, J.; McCrossan, G.; Deutsch, A.; Martinod, K.; Walch, M.; Lieberman, J. Leukocyte Protease Binding to Nucleic Acids Promotes Nuclear Localization and Cleavage of Nucleic Acid Binding Proteins. J. Immunol. Baltim. Md 1950 2014, 192, 5390–5397. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting Neutrophils in Autoimmune Small-Vessel Vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Funchal, G.A.; Jaeger, N.; Czepielewski, R.S.; Machado, M.S.; Muraro, S.P.; Stein, R.T.; Bonorino, C.B.C.; Porto, B.N. Respiratory Syncytial Virus Fusion Protein Promotes TLR-4–Dependent Neutrophil Extracellular Trap Formation by Human Neutrophils. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Farahvash, A.; Douda, D.N.; Licht, J.-C.; Grasemann, H.; Sweezey, N.; Palaniyar, N. JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Sci. Rep. 2017, 7, 3409. [Google Scholar] [CrossRef] [PubMed]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.-D.; Steinbacher, P.; Winterberg, N.; Bathke, A.C.; Klappacher, M.; Studnicka, M. Neutrophil Extracellular Trap (NET) Formation Characterises Stable and Exacerbated COPD and Correlates with Airflow Limitation. Respir. Res. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Stoiber, W.; Krautgartner, W.-D.; Klappacher, M.; Kofler, B.; Steinbacher, P.; Vitkov, L.; Grabcanovic-Musija, F.; Studnicka, M. New Aspects on the Structure of Neutrophil Extracellular Traps from Chronic Obstructive Pulmonary Disease and in Vitro Generation. PLoS ONE 2014, 9, e97784. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Waschki, B.; Marwitz, S.; Goldmann, T.; Kirsten, A.; Malmgren, A.; Rabe, K.F.; Uddin, M.; Watz, H. Neutrophil Extracellular Trap Formation Is Regulated by CXCR2 in COPD Neutrophils. Eur. Respir. J. 2018, 51, 1700970. [Google Scholar] [CrossRef]
- Dworski, R.; Simon, H.-U.; Hoskins, A.; Yousefi, S. Eosinophil and Neutrophil Extracellular DNA Traps in Human Allergic Asthmatic Airways. J. Allergy Clin. Immunol. 2011, 127, 1260–1266. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone Hypercitrullination Mediates Chromatin Decondensation and Neutrophil Extracellular Trap Formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Borregaard, N. Neutrophil Extracellular Traps—The Dark Side of Neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Zabieglo, K.; Majewski, P.; Majchrzak-Gorecka, M.; Wlodarczyk, A.; Grygier, B.; Zegar, A.; Kapinska-Mrowiecka, M.; Naskalska, A.; Pyrc, K.; Dubin, A.; et al. The Inhibitory Effect of Secretory Leukocyte Protease Inhibitor (SLPI) on Formation of Neutrophil Extracellular Traps. J. Leukoc. Biol. 2015, 98, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Herter, J.M.; Van Aken, H.; Napirei, M.; Döring, Y.; Weber, C.; Soehnlein, O.; Zarbock, A. Synchronized Integrin Engagement and Chemokine Activation Is Crucial in Neutrophil Extracellular Trap-Mediated Sterile Inflammation. Blood 2014, 123, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.J.; Thanabalasuriar, A.; Lee, W.-Y.; Sanz, M.-J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular Mechanisms of NET Formation and Degradation Revealed by Intravital Imaging in the Liver Vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Demkow, U. Potent NETosis Inducers Do Not Show Synergistic Effects in Vitro. Cent.-Eur. J. Immunol. 2019, 44, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. Akt Is Essential to Induce NADPH-Dependent NETosis and to Switch the Neutrophil Death to Apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T.L. Oxidative Innate Immune Defenses by Nox/Duox Family NADPH Oxidases. Contrib. Microbiol. 2008, 15, 164–187. [Google Scholar] [CrossRef]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Health and Disease. Biochem. Mosc. 2014, 79, 1286–1296. [Google Scholar] [CrossRef]
- Gupta, A.K.; Giaglis, S.; Hasler, P.; Hahn, S. Efficient Neutrophil Extracellular Trap Induction Requires Mobilization of Both Intracellular and Extracellular Calcium Pools and Is Modulated by Cyclosporine A. PLoS ONE 2014, 9, e97088. [Google Scholar] [CrossRef]
- Neeli, I.; Dwivedi, N.; Khan, S.; Radic, M. Regulation of Extracellular Chromatin Release from Neutrophils. J. Innate Immun. 2009, 1, 194–201. [Google Scholar] [CrossRef]
- Luo, Y.; Arita, K.; Bhatia, M.; Knuckley, B.; Lee, Y.-H.; Stallcup, M.R.; Sato, M.; Thompson, P.R. Inhibitors and Inactivators of Protein Arginine Deiminase 4: Functional and Structural Characterization. Biochemistry 2006, 45, 11727–11736. [Google Scholar] [CrossRef] [PubMed]
- Naffah de Souza, C.; Breda, L.C.D.; Khan, M.A.; Almeida, S.R.D.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline PH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone. Front. Immunol. 2018, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Marwitz, S.; Holz, O.; Kirsten, A.; Bahmer, T.; Waschki, B.; Magnussen, H.; Rabe, K.F.; Goldmann, T.; Uddin, M.; et al. Neutrophil Extracellular Trap Formation and Extracellular DNA in Sputum of Stable COPD Patients. Respir. Med. 2015, 109, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.S. Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer: Epithelial Mesenchymal Transition (EMT), the Missing Link? EBioMedicine 2015, 2, 1578–1579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dubois, A.V.; Gauthier, A.; Bréa, D.; Varaigne, F.; Diot, P.; Gauthier, F.; Attucci, S. Influence of DNA on the Activities and Inhibition of Neutrophil Serine Proteases in Cystic Fibrosis Sputum. Am. J. Respir. Cell Mol. Biol. 2012, 47, 80–86. [Google Scholar] [CrossRef]
- Wright, W.R.; Parzych, K.; Crawford, D.; Mein, C.; Mitchell, J.A.; Paul-Clark, M.J. Inflammatory Transcriptome Profiling of Human Monocytes Exposed Acutely to Cigarette Smoke. PLoS ONE 2012, 7, e30120. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Staab, D.; Zychlinsky, A. Neutrophil Elastase Enhances Sputum Solubilization in Cystic Fibrosis Patients Receiving DNase Therapy. PLoS ONE 2011, 6, e28526. [Google Scholar] [CrossRef]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil Extracellular Traps Are Associated with Inflammation in Chronic Airway Disease. Respirol. Carlton Vic 2016, 21, 467–475. [Google Scholar] [CrossRef]
- Rohrbach, A.S.; Slade, D.J.; Thompson, P.R.; Mowen, K.A. Activation of PAD4 in NET Formation. Front. Immunol. 2012, 3, 360. [Google Scholar] [CrossRef]
- Lugli, E.B.; Correia, R.E.S.M.; Fischer, R.; Lundberg, K.; Bracke, K.R.; Montgomery, A.B.; Kessler, B.M.; Brusselle, G.G.; Venables, P.J. Expression of Citrulline and Homocitrulline Residues in the Lungs of Non-Smokers and Smokers: Implications for Autoimmunity in Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 9. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, I.; Ung, H.K.; Dowling, J.K.; Mansell, A.; D’Andrea, L.; Ellisdon, A.M.; Whisstock, J.C.; Berger, P.J.; Nold-Petry, C.A.; Nold, M.F. Parsing the IL-37-Mediated Suppression of Inflammasome Function. Cells 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. Macrophage Clearance of Neutrophil Extracellular Traps Is a Silent Process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bassler, K.; Aschenbrenner, A.C.; Fujii, W.; Schultze, J.L. Dysregulated Functions of Lung Macrophage Populations in COPD. J. Immunol. Res. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, D.; Shida, H.; Kusunoki, Y.; Miyoshi, A.; Nishio, S.; Tomaru, U.; Atsumi, T.; Ishizu, A. The Responses of Macrophages in Interaction with Neutrophils That Undergo NETosis. J. Autoimmun. 2016, 67, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Thompson, P.R.; Segal, B.H.; Urban, C.F. Nicotine Induces Neutrophil Extracellular Traps. J. Leukoc. Biol. 2016, 100, 1105–1112. [Google Scholar] [CrossRef]
- Gernez, Y.; Tirouvanziam, R.; Chanez, P. Neutrophils in Chronic Inflammatory Airway Diseases: Can We Target Them and How? Eur. Respir. J. 2010, 35, 467–469. [Google Scholar] [CrossRef]
- Chapman, R.W.; Phillips, J.E.; Hipkin, R.W.; Curran, A.K.; Lundell, D.; Fine, J.S. CXCR2 Antagonists for the Treatment of Pulmonary Disease. Pharmacol. Ther. 2009, 121, 55–68. [Google Scholar] [CrossRef]
- Meddows-Taylor, S.; Kuhn, L.; Meyers, T.M.; Sherman, G.; Tiemessen, C.T. Defective Neutrophil Degranulation Induced by Interleukin-8 and Complement 5a and Down-Regulation of Associated Receptors in Children Vertically Infected with Human Immunodeficiency Virus Type 1. Clin. Diagn. Lab. Immunol. 2001, 8, 21–30. [Google Scholar] [CrossRef]
- Stillie, R.; Farooq, S.M.; Gordon, J.R.; Stadnyk, A.W. The Functional Significance behind Expressing Two IL–8 Receptor Types on PMN. J. Leukoc. Biol. 2009, 86, 529–543. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, J.; Bandi, V.; Atmar, R.L.; Hattotuwa, K.; Guntupalli, K.K.; Jeffery, P.K. Biopsy Neutrophilia, Neutrophil Chemokine and Receptor Gene Expression in Severe Exacerbations of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 168, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; De Filippo, K.; Hasenberg, M.; van den Brandt, C.; Nye, E.; Hosking, M.P.; Lane, T.E.; Männ, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-CSF–Mediated Thrombopoietin Release Triggers Neutrophil Motility and Mobilization from Bone Marrow via Induction of Cxcr2 Ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Holz, O.; Khalilieh, S.; Ludwig-Sengpiel, A.; Watz, H.; Stryszak, P.; Soni, P.; Tsai, M.; Sadeh, J.; Magnussen, H. SCH527123, a Novel CXCR2 Antagonist, Inhibits Ozone-Induced Neutrophilia in Healthy Subjects. Eur. Respir. J. 2010, 35, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and Safety of a Monoclonal Antibody Recognizing Interleukin-8 in COPD: A Pilot Study. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef]
- O’Reilly, P.; Jackson, P.L.; Noerager, B.; Parker, S.; Dransfield, M.; Gaggar, A.; Blalock, J.E. N-Alpha-PGP and PGP, Potential Biomarkers and Therapeutic Targets for COPD. Respir. Res. 2009, 10, 38. [Google Scholar] [CrossRef]
- Arumugam, S.; Girish Subbiah, K.; Kemparaju, K.; Thirunavukkarasu, C. Neutrophil Extracellular Traps in Acrolein Promoted Hepatic Ischemia Reperfusion Injury: Therapeutic Potential of NOX2 and P38MAPK Inhibitors. J. Cell. Physiol. 2018, 233, 3244–3261. [Google Scholar] [CrossRef]
- Stevenson, C.S.; Coote, K.; Webster, R.; Johnston, H.; Atherton, H.C.; Nicholls, A.; Giddings, J.; Sugar, R.; Jackson, A.; Press, N.J.; et al. Characterization of Cigarette Smoke-Induced Inflammatory and Mucus Hypersecretory Changes in Rat Lung and the Role of CXCR2 Ligands in Mediating This Effect. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 288, L514–L522. [Google Scholar] [CrossRef]
- Sapey, E.; Stockley, R.A. COPD Exacerbations · 2: Aetiology. Thorax 2006, 61, 250–258. [Google Scholar] [CrossRef]
- Mårdh, C.K.; Root, J.; Uddin, M.; Stenvall, K.; Malmgren, A.; Karabelas, K.; Thomas, M. Targets of Neutrophil Influx and Weaponry: Therapeutic Opportunities for Chronic Obstructive Airway Disease. J. Immunol. Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Radic, M.; Marion, T.N. Neutrophil Extracellular Chromatin Traps Connect Innate Immune Response to Autoimmunity. Semin. Immunopathol. 2013, 35, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Stadtmann, A.; Zarbock, A. CXCR2: From Bench to Bedside. Front. Immunol. 2012, 3, 263. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Bhandari, A.A.; Wagner, D.D. Histones Induce Rapid and Profound Thrombocytopenia in Mice. Blood 2011, 118, 3708–3714. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Opneja, A.; Nayak, L. The Role of Neutrophils in Thrombosis. Thromb. Res. 2018, 170, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Garg, N.; Sharma, A.; Capalash, N.; Singh, R. Nucleases of Bacterial Pathogens as Virulence Factors, Therapeutic Targets and Diagnostic Markers. Int. J. Med. Microbiol. 2019, 309, 151354. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of Action of Hydroxychloroquine and Chloroquine: Implications for Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.; Liu, W.; Wu, K. Expression of Interleukin (IL)-10, IL-17A and IL-22 in Serum and Sputum of Stable Chronic Obstructive Pulmonary Disease Patients. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 459–465. [Google Scholar] [CrossRef]
- Sichelstiel, A.; Yadava, K.; Trompette, A.; Salami, O.; Iwakura, Y.; Nicod, L.P.; Marsland, B.J. Targeting IL-1β and IL-17A Driven Inflammation during Influenza-Induced Exacerbations of Chronic Lung Inflammation. PLoS ONE 2014, 9, e98440. [Google Scholar] [CrossRef]
- Le Rouzic, O.; Pichavant, M.; Frealle, E.; Guillon, A.; Si-Tahar, M.; Gosset, P. Th17 Cytokines: Novel Potential Therapeutic Targets for COPD Pathogenesis and Exacerbations. Eur. Respir. J. 2017, 50, 1602434. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Liu, J.; Zhou, D.B.; Kuang, X.; Xiao, J.; Yu, Q.; Lu, X.; Li, W.; Xie, B.; et al. Serum IL-1β and IL-17 Levels in Patients with COPD: Associations with Clinical Parameters. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1247–1254. [Google Scholar] [CrossRef] [PubMed]

| Determining Factors | Phagocytosis | Formation of NETs | References |
|---|---|---|---|
| Integrity of cytoskeleton | Cytoskeleton integrity is required for phagocytosis | Cytoskeleton disruption is a pre-requisite for NETs formation | [90,107] |
| Size of pathogens | Small size of pathogens favors phagocytosis | NETs released in response to large pathogens | [27,90] |
| Key signals required | MPO is not required | NE and MPO are the key granular proteins which get activated during generation of NETs | [116,117] |
| Immunoglobulin(Ig) opsonization | Ig opsonization required | Ig opsonization is not required | [90,106] |
| Autophagy | PS recognition leads to phagocytosis | Autophagy induced by PMA | [112,113,114,115] |
| Role of platelets: High mobility group box-1 (HMGB1) | HMGB1 is known to be an effective inhibitor of phagocytosis | Neutrophils are instructed by platelets through HMGB1to release NETs via a pathway that involves HMGB1 receptor | [110,111] |
| Role of pH | Phagocytosis of opsonized bulky particulates is ensured by an acute drop in intracellular pH | Acidic environments impair NET formation | [108,109] |
| Role of DEK | DEK is not required for phagocytosis | DEK is necessary for NET generation | [90,105] |
| Compartments | Mediator and Components of NETs | References |
|---|---|---|
| Cytoplasmic | Calprotectin and Catalase PAD4 (mediating citrullination of histone3; CitH3) Kinases; ERK, Akt, JNK, p38, Src etc. | [125,134,135,136] |
| Granular | Primary granules (e.g., MPO, cathepsin G and neutrophil elastase) Secondary granules (e.g., lactoferrin and pentraxin 3) Tertiary granules (e.g., gelatinase and peptidoglycan binding protein) | [124,135,137,138] |
| Nuclear | DNA and Histones (H1, H2A, H2B, H3, and H4) Activation of transcription factors (transcriptional firing) Citrullination of histone by PAD4 (CitH3) Activation of Gasdermin-D to make pores | [26,121,122,137,138] |
| Compartments | Mediator and Components of NETs | References |
|---|---|---|
| Heparin | Interferes with neutrophil autophagy Suppresses Histones Prevents platelets-histone interaction Blocks HMGB1 | [194,195,196] |
| DNAses | Reduces neutrophil infilteration Hydrolyzes DNA Reduces viscosity in lungs | [196,197] |
| Hydroxychloroquine | Targets endosomal NADPH oxidase Inhibits cytokine production Maintains extracellular homeostasis | [196,198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, A.; Khan, M.A.; Bade, G.; Talwar, A. Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines 2021, 9, 53. https://doi.org/10.3390/biomedicines9010053
Trivedi A, Khan MA, Bade G, Talwar A. Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines. 2021; 9(1):53. https://doi.org/10.3390/biomedicines9010053
Chicago/Turabian StyleTrivedi, Anjali, Meraj A. Khan, Geetanjali Bade, and Anjana Talwar. 2021. "Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development" Biomedicines 9, no. 1: 53. https://doi.org/10.3390/biomedicines9010053
APA StyleTrivedi, A., Khan, M. A., Bade, G., & Talwar, A. (2021). Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines, 9(1), 53. https://doi.org/10.3390/biomedicines9010053




