Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs, Chemicals and Solutions Used in This Study
2.2. Cell Preparations
2.3. Electrophysiological Measurements
2.4. Whole-Cell Current Recordings
2.5. Data Analyses
2.6. Curve-Fitting Procedures and Statistical Analyses
3. Results
3.1. Effect of SM-102 on Erg-Mediated K+ Current (IK(erg)) Measured from GH3 Cells
3.2. Concentration-Dependent Analysis of SM-102-Mediated Inhibition of IK(erg) in GH3 Cells
3.3. Inhibitory Effect of SM-102 on Current versus Voltage (I–V) Relationship of IK(erg)
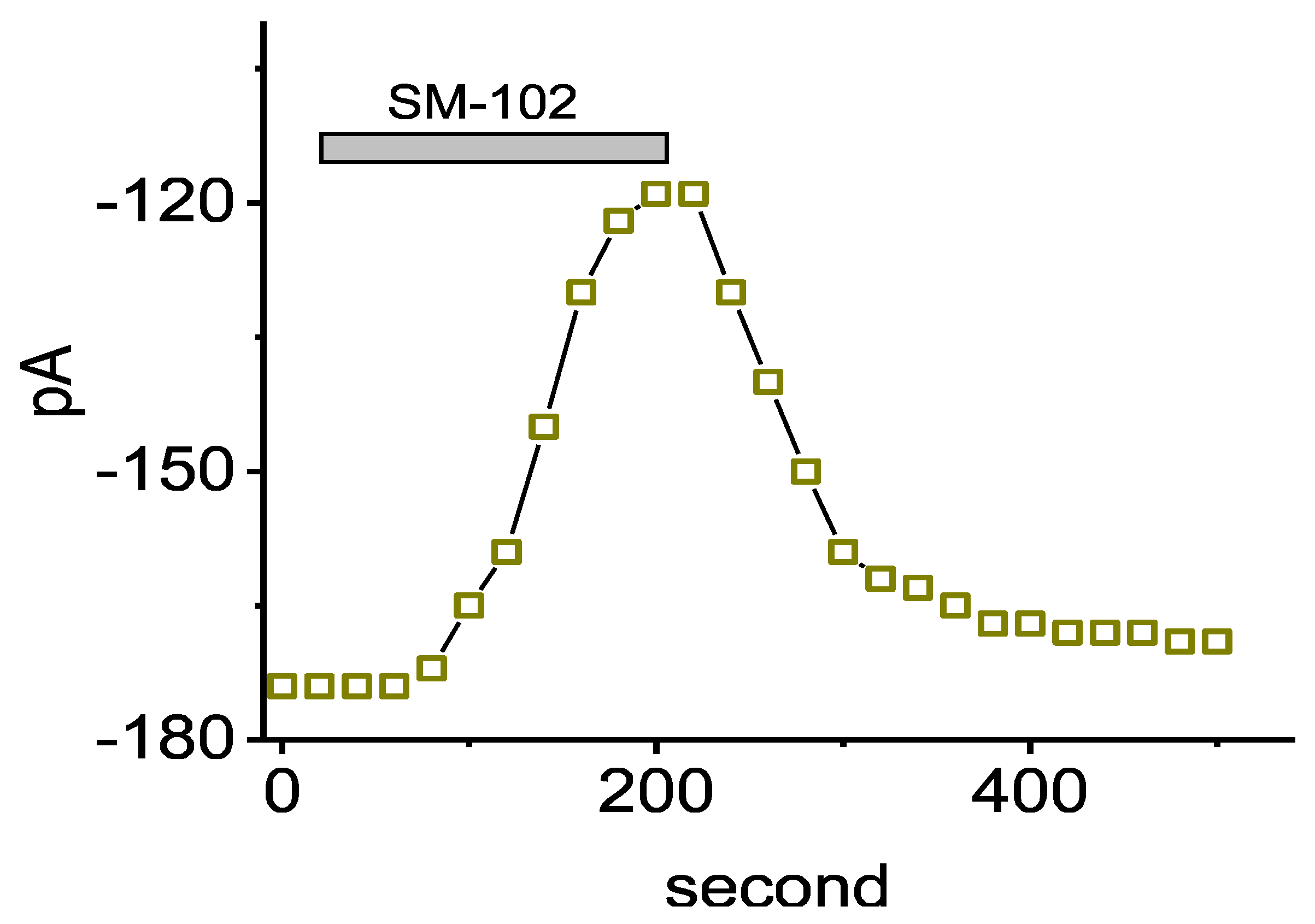
3.4. Effect of SM-102 on Voltage-Dependent Hysteresis of IK(erg)
3.5. Effect of Intracellular Dialysis with SM-102 or Spermine on the Amplitude of IK(erg)
3.6. Effect of SM-102 on IK(erg) Identified in MA-10 Leydig Cells
3.7. Effect of SM-102 on Inwardly Rectifying K+ Current (IK(IR)) in BV2 Microglial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| Erg | ether-à-go-go-related gene |
| GLNVA | glyceryl nonivamide |
| I–V | current versus voltage |
| IC50 | the concentration required for a half-maximal inhibition |
| IK(erg) | erg-mediated K+ current |
| IK(IR) | inwardly rectifying K+ current |
| KD | dissociation constant |
| Kerg channel | erg-mediated K+ channel |
| Kir channel | inwardly rectifying K+ channel |
| τdeact | deactivation time constant |
| TTX | Tetrodotoxin |
References
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther.-Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Davide, J.P.; Cai, M.; Zhang, G.-J.; South, V.J.; Matter, A.; Ng, B.; Zhang, Y.; Sepp-Lorenzino, L. Noninvasive Imaging of Lipid Nanoparticle–Mediated Systemic Delivery of Small-Interfering RNA to the Liver. Mol. Ther. 2010, 18, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P.; Demeneix, B.; Loeffler, J.P.; Perez-Mutul, J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc. Natl. Acad. Sci. USA 1989, 86, 6982–6986. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine—United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1653–1656. [Google Scholar] [CrossRef]
- Mei, R.; Raschi, E.; Forcesi, E.; Diemberger, I.; De Ponti, F.; Poluzzi, E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int. J. Cardiol. 2018, 273, 183–186. [Google Scholar] [CrossRef]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 212828. [Google Scholar] [CrossRef]
- Vidula, M.K.; Ambrose, M.; Glassberg, H.; Chokshi, N.; Chen, T.; Ferrari, V.A.; Han, Y. Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Williams, C.B.; Choi, J.-I.; Hosseini, F.; Roberts, J.; Ramanathan, K.; Ong, K. Acute Myocarditis Following mRNA-1273 SARS-CoV-2 Vaccination. CJC Open 2021, 10. [Google Scholar] [CrossRef]
- Miller, E.R.; Moro, P.L.; Cano, M.; Shimabukuro, T.T. Deaths following vaccination: What does the evidence show? Vaccine 2015, 33, 3288–3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System. JAMA Cardiol. 2020, 5, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschi, E.; Vasina, V.; Poluzzi, E.; De Ponti, F. The hERG K+ channel: Target and antitarget strategies in drug development. Pharmacol. Res. 2008, 57, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Martinson, A.S.; van Rossum, D.; Diatta, F.H.; Layden, M.J.; Rhodes, S.A.; Martindale, M.Q.; Jegla, T. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc. Natl. Acad. Sci. USA 2014, 111, 5712–5717. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-N.; Li, H.-F.; Jan, C.-R.; Chen, I.-J.; Lo, Y.-C. Selective block by glyceryl nonivamide of inwardly rectifying K+ current in rat anterior pituitary GH3 cells. Life Sci. 1998, 63, PL281–PL288. [Google Scholar] [CrossRef]
- Wu, S.-N.; Jan, C.-R.; Li, H.-F.; Chiang, H.-T. Characterization of Inhibition by Risperidone of the Inwardly Rectifying K+ Current in Pituitary GH3 Cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Hardman, R.M.; Forsythe, I. Ether-à-go-go-related gene K+channels contribute to threshold excitability of mouse auditory brainstem neurons. J. Physiol. 2009, 587, 2487–2497. [Google Scholar] [CrossRef]
- Bauer, C.K.; Schwarz, J.R. Ether-à-go-goK+channels: Effective modulators of neuronal excitability. J. Physiol. 2018, 596, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, Y.; Cao, L.; Han, H.; Wang, J.; Yang, B.; Nattel, S.; Wang, Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002, 62, 4843–4848. [Google Scholar] [PubMed]
- Barros, F.; Villalobos, C.; García-Sancho, J.; Del Camino, D.; De La Peña, P.; De-La-Pena-Cortines, P. The role of the inwardly rectifying K+ current in resting potential and thyrotropin-releasing-hormone-induced changes in cell excitability of GH3 rat anterior pituitary cells. Pflügers Archiv 1994, 426, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Jwu-Lai, Y.; Yi-Ching, L.; Yun, W.; Ing-Jun, C. Cardiovascular interactions of nonivamide, glyceryl nonivamide, capsaicin analogues, and substance P antagonist in rats. Brain Res. Bull. 1993, 30, 641–648. [Google Scholar] [CrossRef]
- So, E.C.; Chang, Y.-T.; Hsing, C.-H.; Poon, P.W.-F.; Leu, S.-F.; Huang, B.-M. The effect of midazolam on mouse Leydig cell steroidogenesis and apoptosis. Toxicol. Lett. 2010, 192, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Wu, P.-C.; Shieh, D.-B.; Wu, S.-N. The effects of magnetite (Fe3O4) nanoparticles on electroporation-induced inward currents in pituitary tumor (GH3) cells and in RAW 264.7 macrophages. Int. J. Nanomed. 2012, 7, 1687–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.-L.; Chang, H.-F.; Wu, S.-N. The Inhibition of Inwardly Rectifying K+Channels by Memantine in Macrophages and Microglial Cells. Cell. Physiol. Biochem. 2013, 31, 938–951. [Google Scholar] [CrossRef]
- Chang, W.-T.; Wu, S.-N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Lo, Y.-C.; Wu, S.-N. Characterization of Convergent Suppression by UCL-2077 (3-(Triphenylmethylaminomethyl)pyridine), Known to Inhibit Slow Afterhyperpolarization, of erg-Mediated Potassium Currents and Intermediate-Conductance Calcium-Activated Potassium Channels. Int. J. Mol. Sci. 2020, 21, 1441. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.-Y.; Chang, W.-T.; Wu, S.-N. Characterization of the Synergistic Inhibition of IK(erg) and IK(DR). Int. J. Mol. Sci. 2020, 21, 8078. [Google Scholar] [CrossRef]
- Fürst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef] [Green Version]
- Rappaport, S.M.; Teijido, O.; Hoogerheide, D.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Bezrukov, S.M. Conductance hysteresis in the voltage-dependent anion channel. Eur. Biophys. J. 2015, 44, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Banerjee, K.; Gangopadhyay, G. Entropy hysteresis and nonequilibrium thermodynamic efficiency of ion conduction in a voltage-gated potassium ion channel. Phys. Rev. E 2012, 86, 061915. [Google Scholar] [CrossRef]
- Zhou, J.; Augelli-Szafran, C.E.; Bradley, J.A.; Chen, X.; Koci, B.J.; Volberg, W.A.; Sun, Z.; Cordes, J.S. Novel Potent Human Ether-à-Go-Go-Related Gene (hERG) Potassium Channel Enhancers and Their in Vitro Antiarrhythmic Activity. Mol. Pharmacol. 2005, 68, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [Green Version]
- Weiger, T.M.; Hermann, A. Polyamines block Ca2+-activated K+ channels in pituitary tumor cells (GH3). J. Membr. Biol. 1994, 140, 133–142. [Google Scholar] [CrossRef]
- Laskey, J.; Phelps, P. Effect of cadmium and other metal cations on in vitro Leydig cell testosterone production. Toxicol. Appl. Pharmacol. 1991, 108, 296–306. [Google Scholar] [CrossRef]
- Tenorio, B.; Da Silva, R.P.; Tenorio, F.C.A.M.; Rosales, R.R.C.; Junior, V.A.D.S.; Nogueira, R.D.A. Effect of heat stress and Hsp90 inhibition on T-type calcium currents and voltage-dependent potassium currents in leydig cells. J. Therm. Biol. 2019, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Chen, Y.-W.; Tsai, S.-F.; Wu, S.-N.; Shih, Y.-H.; Jiang-Shieh, Y.-F.; Yang, T.-T.; Kuo, Y.-M. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K+ channel. Sci. Rep. 2016, 6, 22864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, R.J.; Wu, S.-N.; Wu, J.-S.; Chang, H.-D.; Luo, C.-H. Electrophysiological mechanisms of ventricular arrhythmias in relation to Andersen-Tawil syndrome under conditions of reduced IK1: A simulation study. Am. J. Physiol. Circ. Physiol. 2006, 291, H2597–H2605. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-Y.; Chuang, T.-H.; Wu, S.-N. Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines 2021, 9, 1367. https://doi.org/10.3390/biomedicines9101367
Cho H-Y, Chuang T-H, Wu S-N. Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines. 2021; 9(10):1367. https://doi.org/10.3390/biomedicines9101367
Chicago/Turabian StyleCho, Hsin-Yen, Tzu-Hsien Chuang, and Sheng-Nan Wu. 2021. "Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid" Biomedicines 9, no. 10: 1367. https://doi.org/10.3390/biomedicines9101367
APA StyleCho, H.-Y., Chuang, T.-H., & Wu, S.-N. (2021). Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid. Biomedicines, 9(10), 1367. https://doi.org/10.3390/biomedicines9101367




