Abstract
Background: The aim of this systematic review was to pool evidence from studies testing if pentagalloyl glucose (PGG) limited aortic expansion in animal models of abdominal aortic aneurysm (AAA). Methods: The review was conducted according to the PRISMA guidelines and registered with PROSPERO. The primary outcome was aortic expansion assessed by direct measurement. Secondary outcomes included aortic expansion measured by ultrasound and aortic diameter at study completion. Sub analyses examined the effect of PGG delivery in specific forms (nanoparticles, periadventitial or intraluminal), and at different times (from the start of AAA induction or when AAA was established), and tested in different animals (pigs, rats and mice) and AAA models (calcium chloride, periadventitial, intraluminal elastase or angiotensin II). Meta-analyses were performed using Mantel-Haenszel’s methods with random effect models and reported as mean difference (MD) and 95% confidence intervals (CIs). Risk of bias was assessed with a customized tool. Results: Eleven studies reported in eight publications involving 214 animals were included. PGG significantly reduced aortic expansion measured by direct observation (MD: −66.35%; 95% CI: −108.44, −24.27; p = 0.002) but not ultrasound (MD: −32.91%; 95% CI: −75.16, 9.33; p = 0.127). PGG delivered intravenously within nanoparticles significantly reduced aortic expansion, measured by both direct observation (MD: −116.41%; 95% CI: −132.20, −100.62; p < 0.001) and ultrasound (MD: −98.40%; 95% CI: −113.99, −82.81; p < 0.001). In studies measuring aortic expansion by direct observation, PGG administered topically to the adventitia of the aorta (MD: −28.41%; 95% CI −46.57, −10.25; p = 0.002), studied in rats (MD: −56.61%; 95% CI: −101.76, −11.46; p = 0.014), within the calcium chloride model (MD: −56.61%; 95% CI: −101.76, −11.46; p = 0.014) and tested in established AAAs (MD: −90.36; 95% CI: −135.82, −44.89; p < 0.001), significantly reduced aortic expansion. The findings of other analyses were not significant. The risk of bias of all studies was high. Conclusion: There is inconsistent low-quality evidence that PGG inhibits aortic expansion in animal models.
1. Introduction
Abdominal aortic aneurysm (AAA) rupture is estimated to be responsible for approximately 200,000 deaths per year worldwide [1]. The only current treatments for AAA are open or endovascular surgical repair [2,3]. Randomized controlled trials have suggested that the surgical repair of small AAAs (<55 mm) does not reduce mortality [4]. Clinical guidelines recommend that small asymptomatic AAAs are treated conservatively [2,3]; however, up to 70% of non-surgically treated AAAs continue to grow in size, thereby increasing the risk of rupture [5]. A drug therapy for small AAAs would be of great clinical value.
Past preclinical and clinical AAA research has focused on testing drugs that reduce aortic inflammation, inhibit extracellular matrix degradation or lower blood pressure [6,7,8]. Despite hundreds of preclinical studies and multiple clinical trials, none of these drugs have come into routine clinical practice for treating AAA [6,7]. Pentagalloyl glucose (PGG) is a polyphenolic derivate of tannic acid that is currently under investigation as a treatment to stabilize AAA [9]. PGG has been proposed to reduce the turnover of collagen and elastin by cross-linking these key extracellular matrix proteins [9]. A growing number of studies have examined the effect of PGG administration on aortic expansion in animal models of AAA. Many of these studies have reported reduced aortic expansion [10,11,12,13]. However, a recent study reported no effect in two rodent models [14].
Given the conflicting findings of these animal studies and since PGG is now being tested as a treatment for small AAA in patients, a critical review of the past preclinical evidence is needed. The aim of this study was to undertake a systematic review and meta-analysis by pooling data from studies testing the effect of PGG on aortic expansion in animal models of AAA.
2. Methods
2.1. Search Strategy and Eligibility Criteria
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered in the PROSPERO database (Registration number: CRD42021275777) [15]. The PubMed and Web of Science (via ISI Web of Knowledge; 1965) databases were searched from inception to 14 September 2021. The search string ((“Pentagalloyl”[All Fields] AND (“glucose”[MeSH Terms] OR “glucose”[All Fields] OR “glucoses”[All Fields] OR “glucose s”[All Fields])) OR “PGG”[All Fields]) AND (“AAA”[All Fields] OR (“aneurysm”[MeSH Terms] OR “aneurysm”[All Fields] OR “aneurysms”[All Fields] OR “aneurysm s”[All Fields] OR “aneurysmal”[All Fields] OR “aneurysmally”[All Fields] OR “aneurysmic”[All Fields])) was used. No language or date restrictions were used. Reference lists of the studies identified were also searched. Eligibility criteria for inclusion were: an animal study involving any AAA model testing the effect of PGG on aortic diameter increase; aortic diameter reported at a minimum of one time point after PGG administration; and inclusion of a control group not receiving PGG but otherwise receiving similar care. Studies including animals receiving PGG but not reporting aortic diameter, or where this could not be extracted or obtained from the authors, were excluded. In vitro or ex vivo studies were also excluded.
2.2. Data Extraction
The primary outcome was relative increase in the maximum diameter of the aorta after PGG administration, as compared to controls not receiving PGG, reported as percentage. This was required to be measured by direct observation by analysis of the in situ aortas at laparotomy, or the excised aortas using calipers or pictures. Secondary outcomes were aortic expansion measured by ultrasound, final maximum AAA diameter reported in millimeters, and AAA incidence and aortic rupture reported as numbers and percentage in mice allocated to PGG compared to controls. Other data extracted included: the types of AAA models; animal age, sex and strain; sample sizes; method of aortic diameter measurement; definition of AAA incidence; days after AAA induction that PGG or control were first administered; duration over which aortic expansion was studied; PGG form, dose and route of administration; and the findings of histological, biochemical and biomechanical studies. Data were extracted by three authors separately and inconsistencies were resolved through discussion. In studies where aortic diameters were reported only in graphs, they were extracted using ImageJ 64-bit version 1.8.0_172 (National Institute of Health, Bethesda, MD, USA).
2.3. Risk of Bias
A risk of bias tool was developed by combining the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) and a previously developed risk of bias tool for AAA model research [16,17]. This incorporated the first nine questions of the SYRCLE tool and four questions from the AAA model risk of bias tool. These additional questions were focused on: the justification of the dose of PGG used; sample size estimation; whether aortic diameter was reported at first allocation to PGG or control and at study completion; and the reproducibility of aortic diameter measurement. Risk of bias was assessed by three authors and differences were resolved by discussion. The scores of the finally agreed upon risk of bias assessment were summed and reported as a percentage. The studies were rated as high (<50%), medium (51–70%) or low (71–100%) risk of bias.
2.4. Data Analysis
Meta-analyses were planned to be performed for any of the primary and secondary outcomes if data were reported in at least two studies. Sub analyses were also planned, and limited to studies using similar modes of PGG administration (nanoparticle incorporated, aortic periadventitial, or intraluminal); separating treatment starting at the time AAA induction commenced (i.e., testing effect on AAA development) versus starting after AAA had been established for at least one day (i.e., testing effect on AAA growth); performed in the same animals species (e.g., pigs, mice and rats), or AAA model types (calcium chloride, periadventitial, intraluminal elastase or angiotensin II); and excluding studies deemed to be at high risk of bias [18]. A leave-one-out-sensitivity analysis was performed to assess the contribution of each study to the pooled estimates of the primary outcome by excluding individual studies one at a time and recalculating the pooled estimates [19]. All meta-analyses were performed using Mantel-Haenszel’s statistical methods and random effect models anticipating substantial heterogeneity [20]. The results were reported as mean differences (MDs), with 95% confidence intervals (CIs), for aortic diameter increase and relative risk (RR) and 95% CIs for AAA incidence and rupture. All statistical tests were two-sided and p-values < 0.05 were considered significant. Statistical heterogeneity was assessed using the I2 statistic and interpreted as low (0 to 49%), moderate (50 to 74%) or high (75 to 100%) [21]. Publication bias was assessed by funnel plots comparing the summary estimate of each study and its precision (1/standard error) [19]. A minimum of ten studies were required to develop funnel plots to analyze publication bias [19]. Meta-analyses were conducted using ‘meta’ package, and the sensitivity analysis was performed using the ‘dmetar’ package of R program version 4.0.3.
3. Results
3.1. Included Studies
From 139 unique publications identified by the search, eight publications met the inclusion criteria and provided a total of 11 unique studies (Figure 1). Three publications included two different eligible studies [10,13,22], while the other five publications included one eligible study each [11,12,23,24,25]. Six studies used rats, four used mice and one used pigs (see Table 1). Overall, a total of 214 animals were included, with total sample sizes in individual studies varying from 12 to 30 (Table 1). The AAA models used included periadventitial infrarenal aortic calcium chloride application in five studies, intraluminal infrarenal aortic elastase in three studies (including the addition of aortic balloon dilatation and juxta-renal stenosing cuffs in the pig study) [25], periadventitial infrarenal aortic elastase application in two studies and subcutaneous angiotensin II infusion in one study (Table 1). In six studies, PGG and the control interventions were initiated at the time when AAA induction was commenced, whereas in the other five studies, PGG and the control interventions commenced between 10 and 42 days after AAA induction (see Table 2). Animals were monitored for between 14 and 42 days after the PGG and control interventions commenced (Table 2). The routes, forms and doses of the PGG administered varied (see Table 2). Four studies tested the intravenous delivery of PGG incorporated in nanoparticles, another four studies tested PGG applied topically to the adventitia of the aorta and three studies tested PGG infused into the lumen of the aorta (in one case, this was delivered by a drug-eluting balloon). Nine studies included a vehicle control and no intervention was given to the controls in two studies (see Table 2). All eleven studies reported percentage increases in aortic diameter for both the interventional and the control groups. Measurements were performed by direct observation alone in five studies, ultrasound alone in four studies and both measurement methods in two studies (Table 2). Six studies reported the actual aortic diameter at the end of the study. Measurements were performed by direct observation alone in two studies, ultrasound alone in three studies and both measurement methods in one study (Table 2). Only two studies reported AAA incidence [13,24]. Aortic rupture is not a feature of the models used in most studies, with only one study reporting this outcome [10].
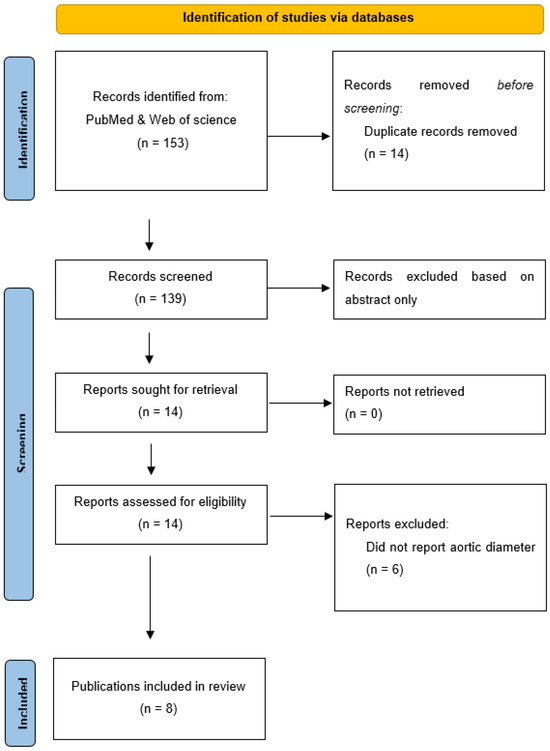
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. A total of 153 publications were screened and, after exclusion of irrelevant studies, 8 publications were included.

Table 1.
Characteristics of included studies and animals.

Table 2.
Pentagalloyl glucose interventions, controls and outcomes.
3.2. Risk of Bias of Included Studies
All 11 studies were considered to have a high risk of bias with overall scores on the 13 item quality assessment tool ranging between 8% and 31% (see Table 3). Common risks of bias identified were failure to randomize animals to the intervention and control group, failure to blind investigators and outcome assessors, failure to justify PGG dose, absence of sample size rationales and not reporting the reproducibility of aortic diameter measurement (Table 3).

Table 3.
Quality assessment of included studies using a modified SYRCLE’s tool for assessing risk of bias.
3.3. Effect of PGG on Aortic Expansion
PGG was reported to significantly reduce the percentage increase in aortic diameter in six of the seven studies where this was measured by direct observation, and three of the six studies that measured aortic diameter percentage increase by ultrasound (see Table 2). A meta-analysis suggested that PGG significantly reduced aortic expansion when measured by direct observation (MD: −66.35%; 95% CI: −108.44, −24.27; p = 0.002), but not ultrasound (MD: −32.91%; 95% CI: −75.16, 9.33; p = 0.127), compared to the controls (Figure 2 and Figure 3). In studies measuring aortic expansion by direct observation, PGG administered intravenously through nanoparticles (MD: −116.41%; 95% CI: −132.20, −100.62; p < 0.001), topically to the adventitia of the aorta (MD: −28.41%; 95% CI: −46.57, −10.25; p = 0.002), studied in rats (MD: −56.61%; 95% CI: −101.76, −11.46; p = 0.014), in the calcium chloride model (MD: −68.17%; 95% CI: −115.12, −21.22; p = 0.004), and where PGG treatment was initiated after model development on days ranging between 10 and 42 (MD: −90.36; 95% CI: −135.82, −44.89; p < 0.001), significantly reduced aortic expansion (Figure 2). A sensitivity analysis of the studies reporting aortic expansion by direct measurement found that the individual removal of any single study did not change the significance of the findings (Supplementary Table S1). In studies measuring aortic expansion by ultrasound measurement, PGG administered intravenously using nanoparticles significantly reduced aortic expansion (MD: −98.40%; 95% CI: −113.99, −82.81; p < 0.001) (Figure 3). The findings of other sub analyses were not significant (Figure 3). Funnel plots were not performed, due to data not being available from a minimum number of 10 studies.
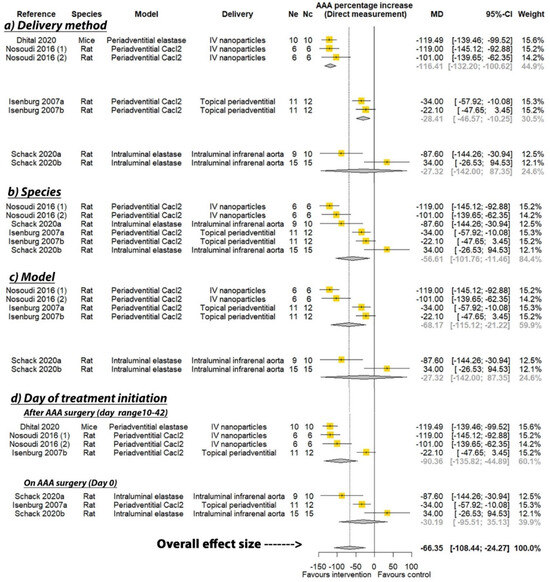
Figure 2.
Meta-analysis of studies testing the effect of pentagalloyl glucose on aortic expansion measured by direct observation. MD = Mean difference; Ne = Number of animals in experimental group; Nc = Number of animals in control group; CI = Confidence interval. a/b: Three of the publications included two separate studies that were considered independently.
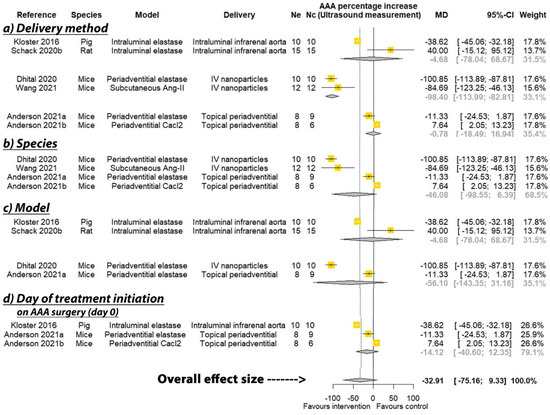
Figure 3.
Meta-analysis of studies testing the effect of pentagalloyl glucose on aortic expansion measured by ultrasound. MD = Mean difference; Ne = Number of animals in experimental group; Nc = Number of animals in control group; CI = Confidence interval. a/b: Three of the publications included two separate studies that were considered independently.
3.4. Effect of PGG on Final AAA Diameter
One of three studies reported that PGG significantly reduced AAA diameter measured by direct observation at study completion (Table 2). One of four studies reported that PGG significantly reduced AAA diameter measured by ultrasound at study completion (Table 2). Meta-analyses suggested that PGG did not significantly reduce aortic diameter assessed by both direct measurement (MD: −0.35 mm; 95% CI −1.82, 1.12; p = 0.642) and ultrasound (MD −0.93 mm; 95% CI −3.00, 1.15; p = 0.381) (Figure 4). The findings of other sub analyses were not significant (Figure 4).
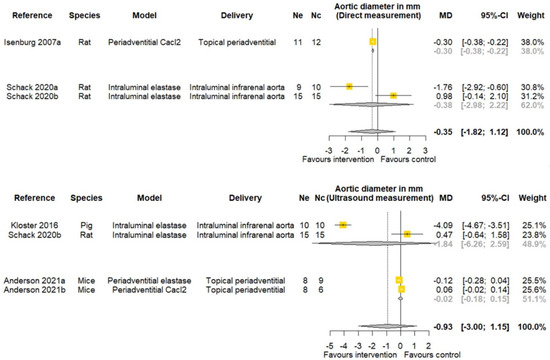
Figure 4.
Meta-analysis of studies testing the effect of pentagalloyl glucose on final aortic diameter in animal models of abdominal aortic aneurysm. MD = Mean difference; Ne = Number of animals in experimental group; Nc = Number of animals in control group; CI = Confidence interval. a/b: Three of the publications included two separate studies that were considered independently.
3.5. Effect of PGG on AAA Incidence
Two studies reported the incidence of AAA (see Table 2), but only one study initiated PGG treatment on the day of AAA induction, with 66.7% of rats in the control group developing AAA, compared to 18.2% of the rats receiving periadventitial aortic PGG at study completion [13]. Another study found that 100% of rats receiving PGG-loaded nanoparticles delivered intravenously 42 days after AAA induction developed AAA similar to the control group [24]. A meta-analysis of the two studies suggested that AAA incidence was not significantly different between rats receiving PGG and the controls, with large CIs (RR: 0.62; 95% CI: 0.00, 1751.32; p = 0.588, Supplementary Figure S1).
3.6. Findings from Histological and Molecular Biology Analyses
Histology findings from some studies found that animals receiving PGG had less aortic media elastic fiber degradation, more desmosine content and decreased macrophage infiltration (See Table 4). PGG was also reported to significantly reduce aortic matrix metalloproteinase (MMP) activity in three studies and increase lysyl oxidase (LOX) activity in two studies (Table 4). One study reported no significant effect of PGG on MMP-2 and MMP-9 [13]. Another two studies reported no significant effect of PGG on LOX or the markers of aortic macrophage infiltration [10].

Table 4.
Reported effects of PGG on aortic histology and molecular biology findings.
4. Discussion
This systematic review of past studies found that the administration of PGG reduced aortic expansion within AAA animal models when measured by direct observation. The findings were not consistent when measured by ultrasound. PGG administered within intravenously injected nanoparticles significantly reduced aortic expansion in studies consistently, whether measured by direct observation or ultrasound. Surprisingly, when PGG treatment was initiated later than when AAA induction commenced (range from 10 to 42 days), it significantly reduced aortic expansion. This was, however, not the case when PGG treatment was started at the time of AAA induction. The findings of other analyses were inconsistent, depending on the method used to measure aortic expansion. A number of important limitations of these prior studies should be noted. Firstly, all studies had a high risk of bias. None of the studies included methods typically thought to be critical in human clinical trials, such as randomization and blinding. Only one study included a sample size calculation [24]. All studies were small and there has been concern that findings from animal models do not translate to AAA patients. This has been particularly reported in relation to doxycycline, but also for fenofibrate, an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor, which have all been reported to limit aortic expansion in animal models but have not been found to limit AAA growth in clinical trials [7,8,26,27,28].
In addition to the animal experiments reported in this study, there have been other experimental studies reporting the beneficial effects of PGG. In vitro studies have suggested that PGG reduces oxidative stress and MMP secretion and improves the elastic properties of a myoblast cell line [22]. Ex vivo studies of the carotid arteries of mice suggest that PGG protected against elastase-induced artery destruction and limited the mechanical failure of the artery by repairing the elastic lamellae and limiting changes in the mechanical properties of the tissue [29]. A similar ex vivo study using pig aortic samples reported that PGG partially protected against elastase- and collagenase-induced biomechanical changes [30].
One of the key challenges to the use of PGG as a clinical treatment is clarity on the most appropriate route of delivery. None of the animal studies used oral administration, which would be the most straightforward way to administer a medical treatment for AAA. The pharmacokinetics of oral PGG administration are poorly understood, as summarized in detail in a recent review [9]. Low and variable bioavailability of PGG has been reported after oral administration [9]. As illustrated in the included animal studies, a wide range of other routes of administration have been proposed, such as nanoparticles and periadventitial routes, but all are not ideal. Given the low risk (approximately 1% per year) of rupture of small AAAs, any treatment needs to have a good safety profile and, ideally, should be minimally invasive [7].
Despite the limitations of the past animal studies, the positive findings of some studies have encouraged the investigation of PGG as an AAA treatment in patients. In a recent presentation at Aortic Asia, it was announced that PGG delivery via an endovascularly placed balloon to the lumen of the infrarenal aorta is being tested as a treatment of small AAA within a clinical trial. Whether this route of administration, given its relatively invasive nature, is appropriate and feasible to use on a wider scale needs further consideration. Most AAAs contain large volumes of intraluminal thrombus that may interfere with PGG delivery to the aortic wall, and also be at risk of embolization during balloon inflation [31]. Further information on the safety and efficacy of intraluminal PGG is thus required. It is possible that, if this initial clinical trial is encouraging, there could be scope to combine PGG treatment with the endovascular repair of large AAA. A recent systematic review reported a long-term reintervention rate of 18% following endovascular aneurysm repair due to the continued expansion of the AAA sac [32]. The combination of an effective drug and surgical treatment could be a valuable addition to the clinical care of patients with large AAAs. This would need widescale testing to ensure that it is an effective and durable treatment.
A number of limitations of this systematic review should be noted. Firstly, the included studies were small and at high risk of bias. There was insufficient investigation or reporting of aortic rupture to assess this outcome. Finally, and most importantly, since all the current evidence is from animal, ex vivo, or in vitro studies, the clinical relevance of these findings remains unclear. The failure to translate past findings from these types of experiments is again emphasized.
In conclusion, this systematic review suggests inconsistent and low-quality evidence from animal studies that PGG may represent a treatment to restore aortic structure in patients with early-stage AAA. Whether this can translate into a clinically useful treatment is currently unclear, but under investigation by at least one company.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines9101442/s1, Figure S1: Meta-analysis of studies testing the effect of pentagalloyl glucose on AAA incidence. RR = Relative risk; Ne = Number of animals in experimental group; Nc = Number of animals in control group; CI = Confidence interval, Table S1: Leave-one-out sensitivity analysis of studies reporting aortic expansion through direct measurement.
Author Contributions
Conceptualization, J.G.; methodology, J.G., S.T. and J.P.; software, S.T.; validation, S.T., J.P. and J.G.; formal analysis, S.T.; investigation, J.G. and S.T.; resources, S.T. and J.P.; data curation, S.T. and J.P.; writing—original draft preparation, J.G.; writing—review and editing, J.G., S.T. and J.P.; visualization, S.T., J.P. and J.G.; supervision, J.G.; project administration, J.G.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Health and Medical Research Council (1180736), Townsville Hospital and Health Service Study, Education and Research Trust Fund, and the Queensland Government. JG holds a Practitioner Fellowship from the National Health and Medical Research Council (1117601), and a Senior Clinical Research Fellowship from the Queensland Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Document Type. This change does not affect the scientific content of the article.
References
- Abubakar, I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M. Editor’s choice–European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Powell, J.T.; Martinez, M.A.M.; Ballard, D.J. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Ballard, D.J.; Jordan, W.D., Jr.; Blebea, J.; Littooy, F.N.; Freischlag, J.A.; Bandyk, D.; Rapp, J.H. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002, 287, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Moxon, J.V.; Singh, T.P.; Bown, M.J.; Mani, K.; Wanhainen, A. Lack of an effective drug therapy for abdominal aortic aneurysm. J. Intern. Med. 2020, 288, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Singh, T.P. Effect of blood pressure lowering drugs and antibiotics on abdominal aortic aneurysm growth: A systematic review and meta-analysis. Heart 2021, 107, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.S.; Simionescu, D.T.; Goergen, C.J.; Hoyt, K.; Sirsi, S.; Finol, E.A. Pentagalloyl Glucose and Its Functional Role in Vascular Health: Biomechanics and Drug-Delivery Characteristics. Ann. Biomed. Eng. 2019, 47, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Schack, A.S.; Stubbe, J.; Steffensen, L.B.; Mahmoud, H.; Laursen, M.S.; Lindholt, J.S. Intraluminal infusion of Penta-Galloyl Glucose reduces abdominal aortic aneurysm development in the elastase rat model. PLoS ONE 2020, 15, e0234409. [Google Scholar] [CrossRef]
- Dhital, S.; Vyavahare, N.R. Nanoparticle-based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PLoS ONE 2020, 15, e0227165. [Google Scholar] [CrossRef] [PubMed]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Parasaram, V.; Karamched, S.; Vyavahare, N. Systemic Delivery of Nanoparticles Loaded with Pentagalloyl Glucose Protects Elastic Lamina and Prevents Abdominal Aortic Aneurysm in Rats. J. Cardiovasc. Transl. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Isenburg, J.C.; Simionescu, D.T.; Starcher, B.C.; Vyavahare, N.R. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation 2007, 115, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Niedert, E.E.; Patnaik, S.S.; Tang, R.; Holloway, R.L.; Osteguin, V.; Finol, E.A.; Goergen, C.J. Animal Model Dependent Response to Pentagalloyl Glucose in Murine Abdominal Aortic Injury. J. Clin. Med. 2021, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Phie, J.; Thanigaimani, S.; Golledge, J. Systematic Review and Meta-Analysis of Interventions to Slow Progression of Abdominal Aortic Aneurysm in Mouse Models. Arter. Thromb. Vasc. Biol. 2021, 41, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Kulinskaya, E.; Morgenthaler, S.; Staudte, R.G. Combining Statistical Evidence. Int. Stat. Rev. 2014, 82, 214–242. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Arnold, F.; Muzzio, N.; Patnaik, S.S.; Finol, E.A.; Romero, G. Pentagalloyl Glucose-Laden Poly(lactide-co-glycolide) Nanoparticles for the Biomechanical Extracellular Matrix Stabilization of an In Vitro Abdominal Aortic Aneurysm Model. ACS Appl. Mater. Interfaces 2021, 13, 25771–25782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parasaram, V.; Dhital, S.; Nosoudi, N.; Hasanain, S.; Lane, B.A.; Lessner, S.M.; Eberth, J.F.; Vyavahare, N.R. Systemic delivery of targeted nanotherapeutic reverses angiotensin II-induced abdominal aortic aneurysms in mice. Sci. Rep. 2021, 11, 8584. [Google Scholar] [CrossRef] [PubMed]
- Nosoudi, N.; Chowdhury, A.; Siclari, S.; Karamched, S.; Parasaram, V.; Parrish, J.; Gerard, P.; Vyavahare, N. Reversal of Vascular Calcification and Aneurysms in a Rat Model Using Dual Targeted Therapy with EDTA- and PGG-Loaded Nanoparticles. Theranostics 2016, 6, 1975–1987. [Google Scholar] [CrossRef]
- Kloster, B.O.; Lund, L.; Lindholt, J.S. Inhibition of early AAA formation by aortic intraluminal pentagalloyl glucose (PGG) infusion in a novel porcine AAA model. Ann. Med. Surg. 2016, 7, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Pinchbeck, J.; Tomee, S.M.; Rowbotham, S.E.; Singh, T.P.; Moxon, J.V.; Jenkins, J.S.; Lindeman, J.H.; Dalman, R.L.; McDonnell, L.; et al. Randomised-controlled trial testing the efficacy of telmisartan to slow growth of small abdominal aortic aneurysms. JAMA Cardiol. 2020, 5, 1374–1381. [Google Scholar] [CrossRef]
- Moxon, J.V.; Rowbotham, S.E.; Pinchbeck, J.L.; Lazzaroni, S.M.; Morton, S.K.; Moran, C.S.; Quigley, F.; Jenkins, J.S.; Reid, C.M.; Cavaye, D.; et al. A Randomised Controlled Trial Assessing the Effects of Peri-operative Fenofibrate Administration on Abdominal Aortic Aneurysm Pathology: Outcomes From the FAME Trial. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 452–460. [Google Scholar] [CrossRef]
- Pinchbeck, J.L.; Moxon, J.V.; Rowbotham, S.E.; Bourke, M.; Lazzaroni, S.; Morton, S.K.; Matthews, E.O.; Hendy, K.; Jones, R.E.; Bourke, B.; et al. Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME-2 Trial. J. Am. Heart Assoc. 2018, 7, e009866. [Google Scholar] [CrossRef]
- Pavey, S.N.; Cocciolone, A.J.; Marty, A.G.; Ismail, H.N.; Hawes, J.Z.; Wagenseil, J.E. Pentagalloyl glucose (PGG) partially prevents arterial mechanical changes due to elastin degradation. Exp. Mech. 2021, 61, 41–51. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Piskin, S.; Pillalamarri, N.R.; Romero, G.; Escobar, G.P.; Sprague, E.; Finol, E.A. Biomechanical Restoration Potential of Pentagalloyl Glucose after Arterial Extracellular Matrix Degeneration. Bioengineering 2019, 6, 58. [Google Scholar] [CrossRef]
- Golledge, J.; Wolanski, P.; Parr, A.; Buttner, P. Measurement and determinants of infrarenal aortic thrombus volume. Eur. Radiol. 2008, 18, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Khan, S.; Salata, K.; Hussain, M.A.; de Mestral, C.; Greco, E.; Aljabri, B.A.; Forbes, T.L.; Verma, S.; Al-Omran, M. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J. Vasc. Surg. 2019, 70, 954–969.e30. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).