Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis
Abstract
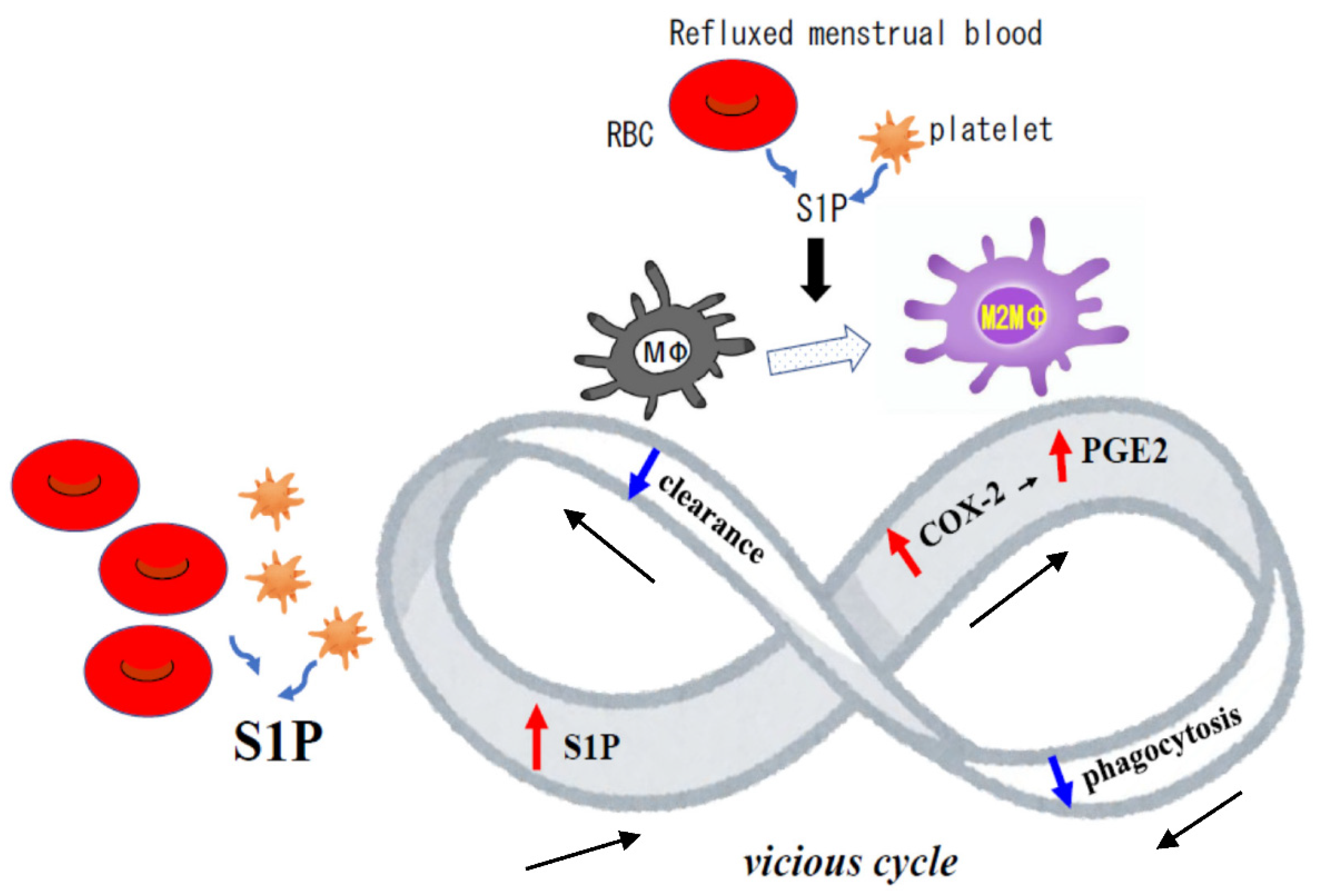
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Collection of Peritoneal Fluid (PF) and Culture of MΦ Derived from the Peritoneal Fluid Mononuclear Cell (PFMC)
2.3. Mass Spectrometry
2.4. In Vivo Study Using a Mouse Model of Endometriosis
2.5. Immunohistochemistry Study
2.6. Reverse Transcription (RT) and Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.7. Cytokine Measurement
2.8. Statistical Analysis
3. Results
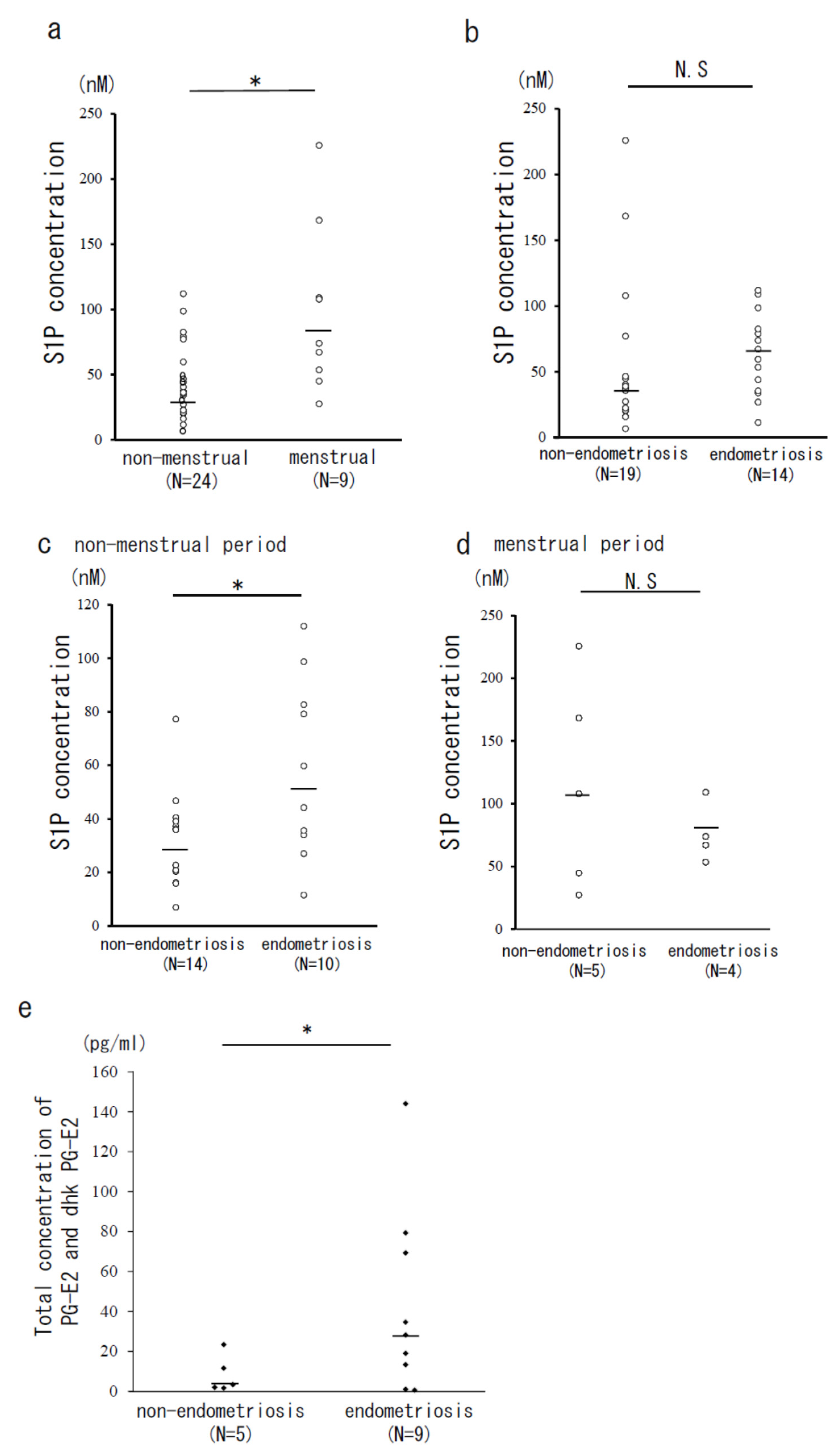
3.1. The Concentrations of S1P, Total PG-E2, and Its Metabolites (dhk PG-E2) in PF
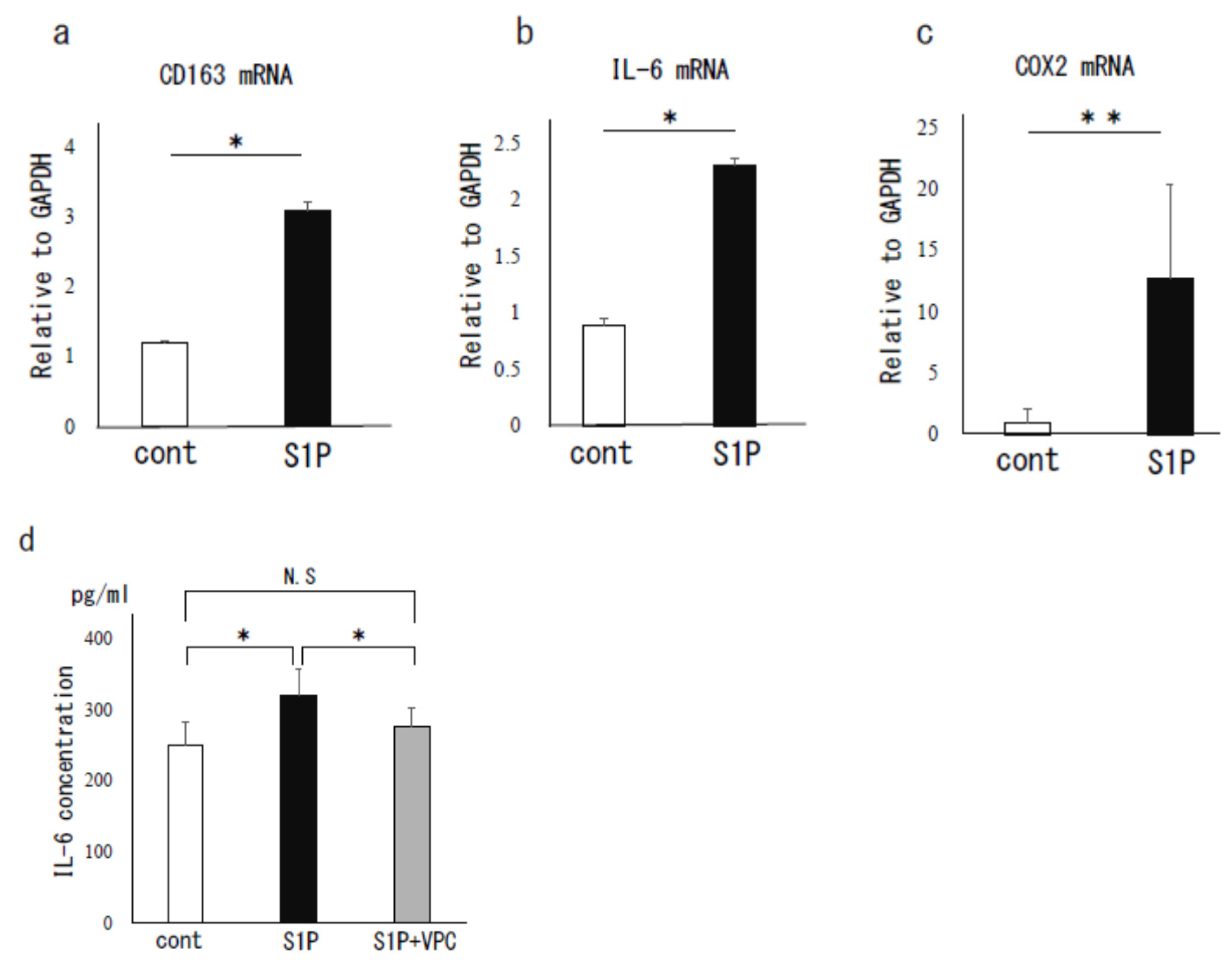
3.2. The Effect of S1P on Human PF MΦ
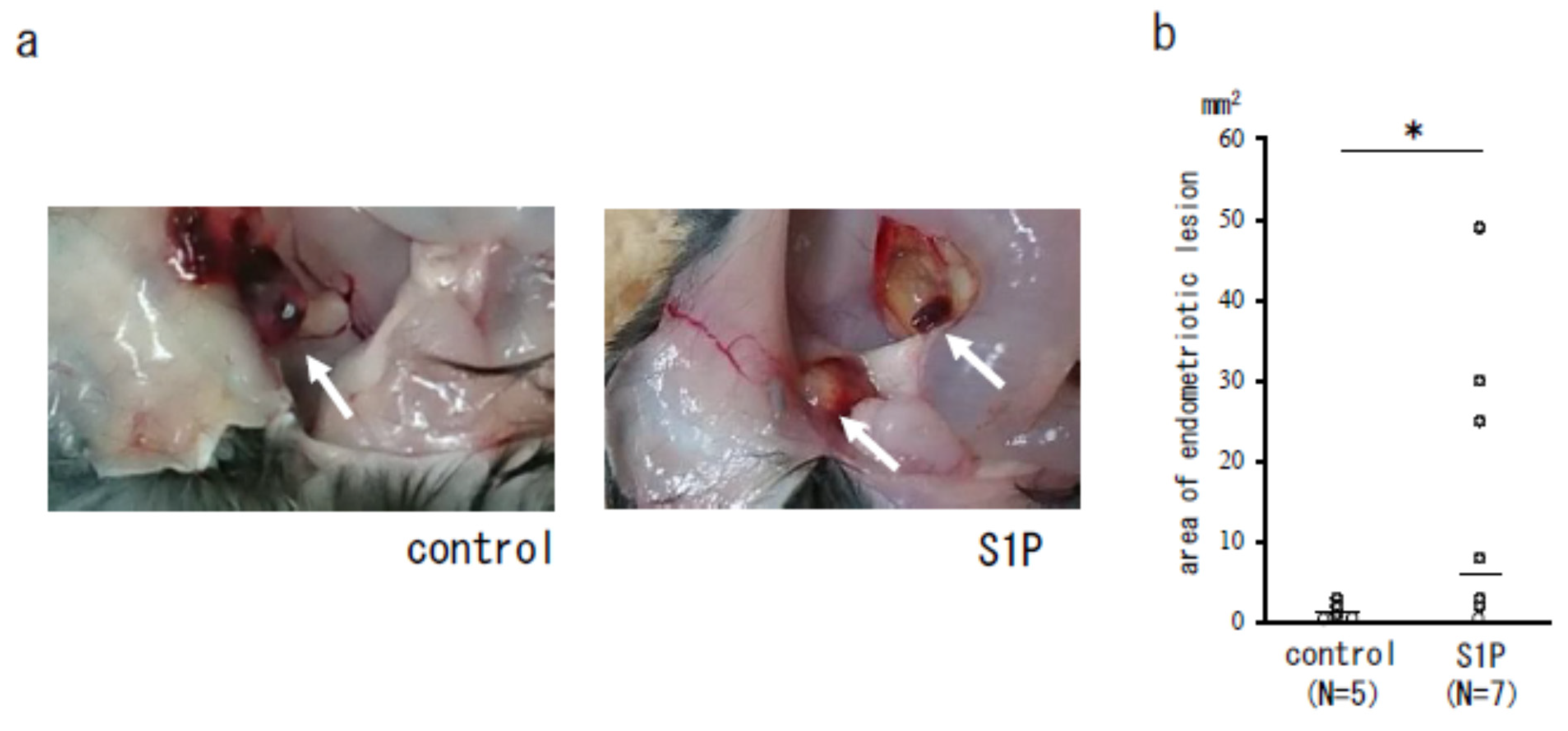
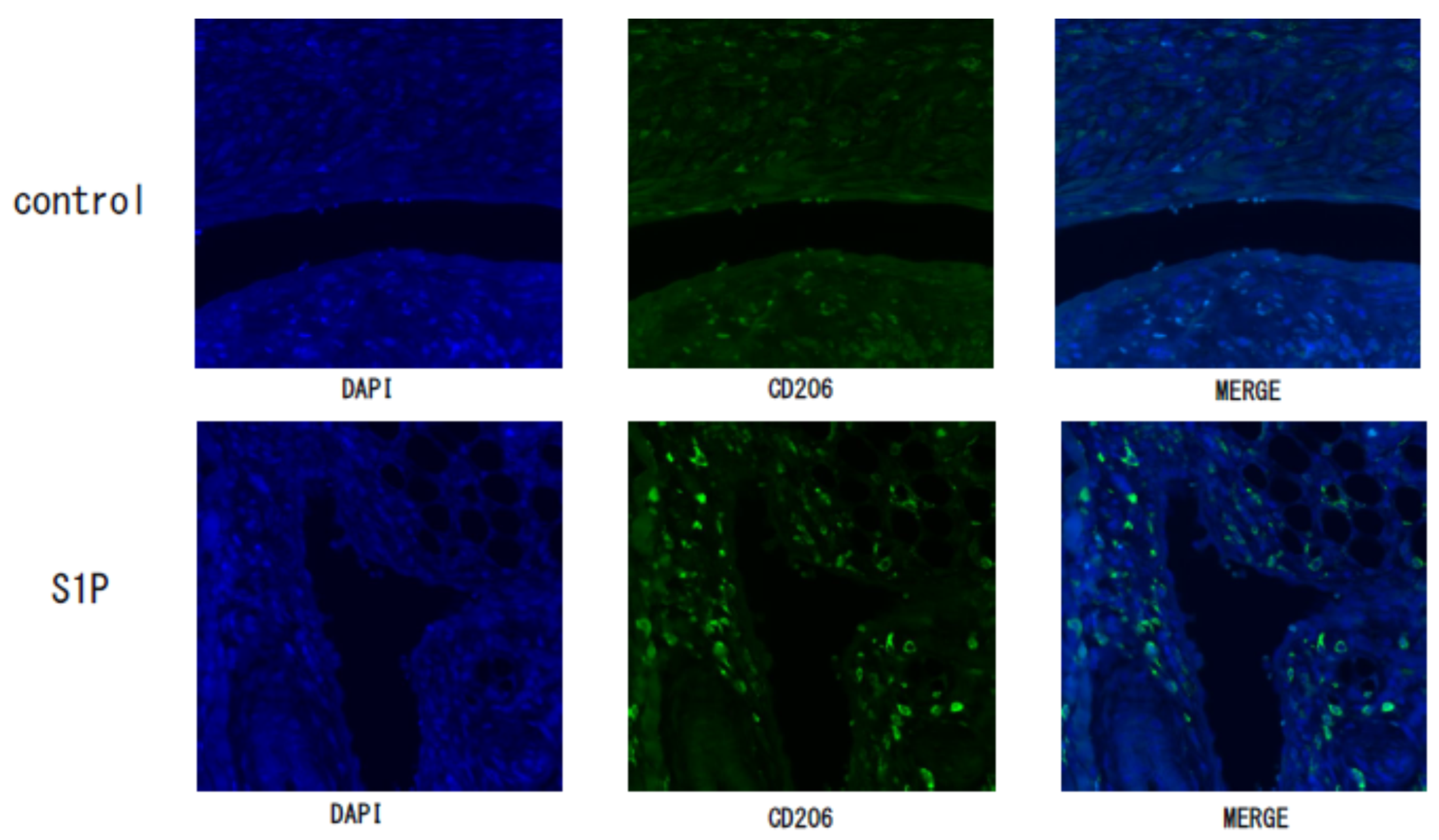
3.3. The Effect of S1P on the Endometriotic-Like Lesion in an Endometriosis Mouse Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, O.; Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Ruimeng, X.; Na, L.; Yano, T.; Tsutsumi, O.; Taketani, Y. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J. Reprod. Immunol. 2006, 72, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Yoshino, O.; Taketani, Y. Lymphocytes in Endometriosis. Am. J. Reprod. Immunol. 2011, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Rovere-querini, P.; Rubartelli, A.; Allavena, P.; Yodoi, J.; University, K.; Tsai, S.-J. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, F.; Komohara, Y.; Takaishi, K.; Honda, R.; Tashiro, H.; Kyo, S.; Katabuchi, H.; Takeya, M. Possible involvement of signal transducer and activator of transcription-3 in cell–cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil. Steril. 2013, 99, 1705–1713.e1. [Google Scholar] [CrossRef]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Sato, E.; Furue, A.; Nawaz, A.; Hatta, H.; Fukushi, Y.; Wada, S.; Tobe, K.; et al. CD206+ macrophage is an accelerator of endometriotic-like lesion via promoting angiogenesis in the endometriosis mouse model. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Yoshino, O.; Yamada-Nomoto, K.; Kano, K.; Ono, Y.; Kobayashi, M.; Ito, M.; Yoneda, S.; Nakashima, A.; Shima, T.; Onda, T.; et al. Sphingosine 1 Phosphate (S1P) Increased IL-6 Expression and Cell Growth in Endometriotic Cells. Reprod. Sci. 2019, 26, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Bernacchioni, C.; Capezzuoli, T.; Vannuzzi, V.; Malentacchi, F.; Castiglione, F.; Cencetti, F.; Ceccaroni, M.; Donati, C.; Bruni, P.; Petraglia, F. Sphingosine 1-phosphate receptors are dysregulated in endometriosis: Possible implication in transforming growth factor β–induced fibrosis. Fertil. Steril. 2021, 115, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Książek, M.; Chacińska, M.; Chabowski, A.; Baranowski, M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid Res. 2015, 56, 1271–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Akiyama, I.; Sato, E.; Ito, M.; Kobayashi, M.; Nakashima, A.; Wada, S.; Onda, T.; et al. IL-33 Exacerbates Endometriotic Lesions via Polarizing Peritoneal Macrophages to M2 Subtype. Reprod. Sci. 2020, 27, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Kato, T.; Kadota, Y.; Erdenebayar, O.; Keyama, K.; Kawakita, T.; Yoshida, K.; Kuwahara, A.; Matsuzaki, T.; Irahara, M. Intraperitoneal administration of activin a promotes development of endometriotic lesions in a mouse model of endometriosis. J. Med. Investig. 2019, 66, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, Y.; Yoshino, O.; Hiraoka, T.; Sato, E.; Fukui, Y.; Ushijima, A.; Nawaz, A.; Hirota, Y.; Wada, S.; Tobe, K.; et al. CD206+ M2-Like Macrophages Are Essential for Successful Implantation. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Darrow, S.L.; Vena, J.E.; Batt, R.E.; Zielezny, M.A.; Michalek, A.M.; Selman, S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology 1993, 4, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Defrère, S.; Lousse, J.C.; González-Ramos, R.; Colette, S.; Donnez, J.; Van Langendonckt, A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.W. Cellular Changes Consistent with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16. [Google Scholar] [CrossRef]
- Chuang, P.C.; Lin, Y.J.; Wu, M.H.; Wing, L.Y.C.; Shoji, Y.; Tsai, S.J. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am. J. Pathol. 2010, 176, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Ebenezer, D.L.; Fu, P.; Natarajan, V. Targeting sphingosine-1-phosphate signaling in lung diseases. Pharmacol. Ther. 2016, 168, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efstathiou, J.A.; Sampson, D.A.; Levine, Z.; Rohan, R.M.; Zurakowski, D.; Folkman, J.; D’Amato, R.J.; Rupnick, M.A. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil. Steril. 2005, 83, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Manabe, I.; Oishi-Tanaka, Y.; Ohsugi, M.; Kono, N.; Ogata, F.; Yagi, N.; Ohto, U.; Kimoto, M.; Miyake, K.; et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012, 15, 518–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, Y.; Kawakita, T.; Yoshino, O.; Sato, E.; Kano, K.; Ohba, M.; Okuno, T.; Ito, M.; Koga, K.; Honda, M.; et al. Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis. Biomedicines 2021, 9, 1519. https://doi.org/10.3390/biomedicines9111519
Ono Y, Kawakita T, Yoshino O, Sato E, Kano K, Ohba M, Okuno T, Ito M, Koga K, Honda M, et al. Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis. Biomedicines. 2021; 9(11):1519. https://doi.org/10.3390/biomedicines9111519
Chicago/Turabian StyleOno, Yosuke, Takako Kawakita, Osamu Yoshino, Erina Sato, Kuniyuki Kano, Mai Ohba, Toshiaki Okuno, Masami Ito, Kaori Koga, Masako Honda, and et al. 2021. "Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis" Biomedicines 9, no. 11: 1519. https://doi.org/10.3390/biomedicines9111519
APA StyleOno, Y., Kawakita, T., Yoshino, O., Sato, E., Kano, K., Ohba, M., Okuno, T., Ito, M., Koga, K., Honda, M., Furue, A., Hiraoka, T., Wada, S., Iwasa, T., Yokomizo, T., Aoki, J., Maeda, N., Unno, N., Osuga, Y., & Hirata, S. (2021). Sphingosine 1-Phosphate (S1P) in the Peritoneal Fluid Skews M2 Macrophage and Contributes to the Development of Endometriosis. Biomedicines, 9(11), 1519. https://doi.org/10.3390/biomedicines9111519






