Truncated Milk Fat Globule-EGF-like Factor 8 Ameliorates Liver Fibrosis via Inhibition of Integrin-TGFβ Receptor Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of NP-011 and Confirmation of the Synthesized Protein
2.2. Rodent Fibrosis Model Induction and Efficacy Tests of NP-011
2.3. Histological Analysis and Immunofluorescence Assay
2.4. Quantitative Reverse-Transcription PCR (RT-qPCR)
2.5. Whole Transcriptome Analysis of Mouse Livers
2.6. Cell Culture and In Vitro Fibrosis Modeling
2.7. TGF-β Luciferase Signaling Reporter Assay
2.8. Western Blot Analysis and Immunoprecipitation (IP) Analysis
2.9. EdU Incorporation Assays
2.10. Proximity Ligation Assay (PLA)
2.11. Radioligand Binding Assay
2.12. Collagenase Activity Assay
2.13. Statistical Analysis
3. Results
3.1. Structural Truncation of MFG-E8 Enhances the Anti-Fibrotic Effect of MFG-E8
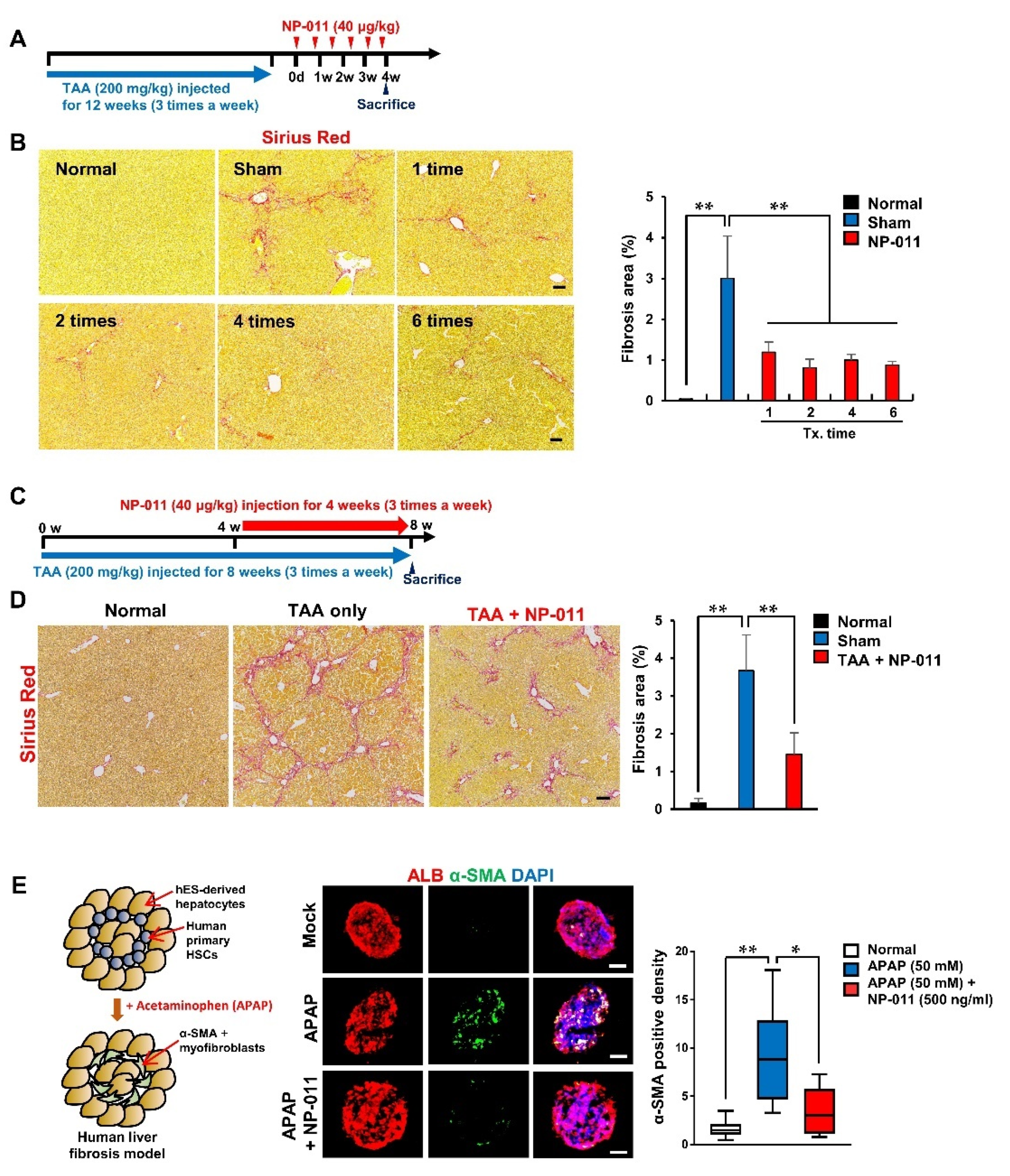
3.2. NP-011 Significantly Reverses Liver Fibrosis at Minimal Dosage
3.3. The Efficacious Dose of NP-011 Shows Therapeutic Efficacy in Different Models Associated with Fibrosis
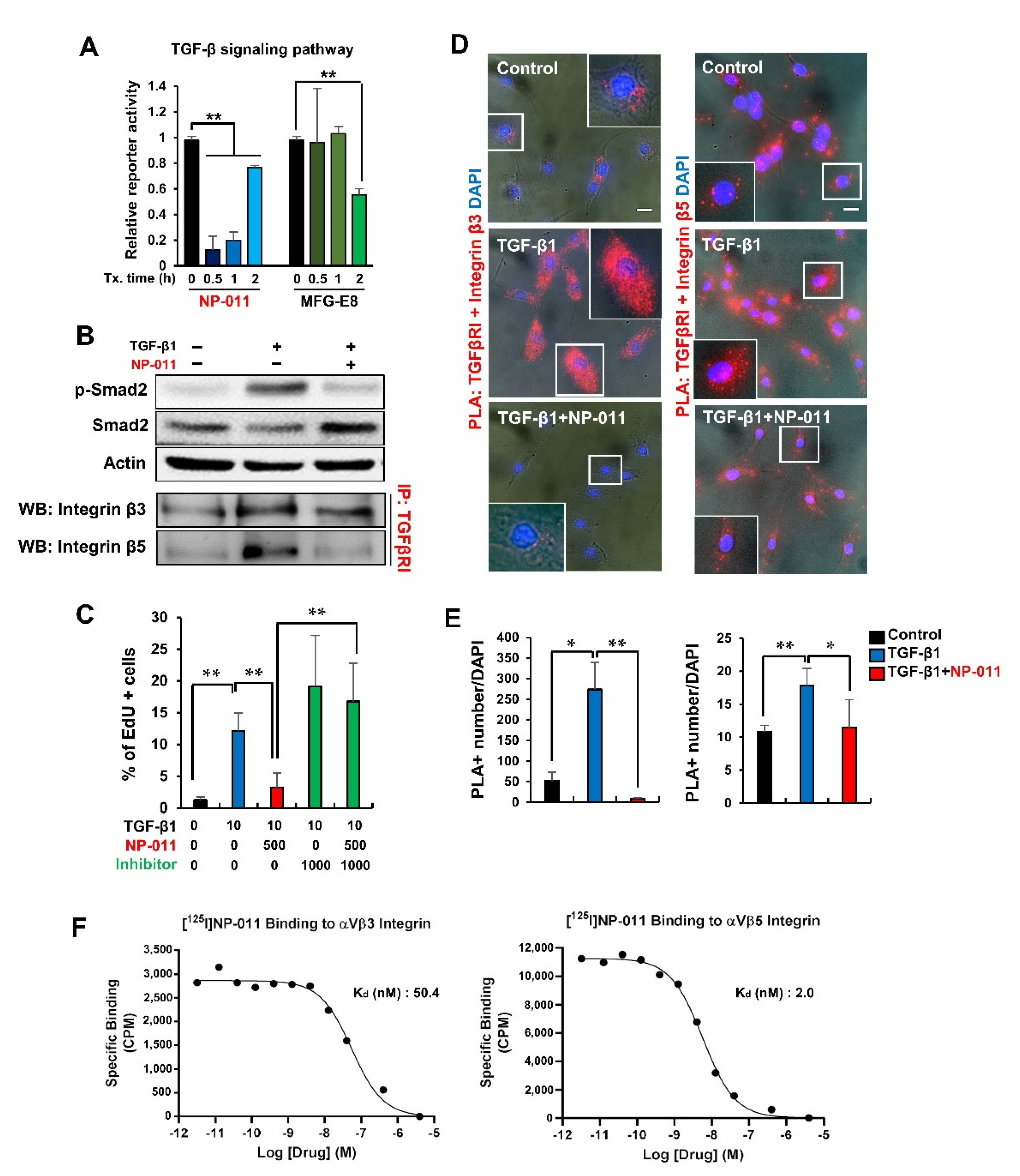
3.4. NP-011 Deactivates HSCs through the Suppression of TGF-β/Smad 2 Signaling and Prevents Fibrogenesis in Human Hepatic Stellate Cells via the Inhibition of Integrin–TGFβ Receptor Interaction
3.5. Bio-Distribution and Safety Profiles of NP-011
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchiyama, A.; Yamada, K.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S. MFG-E8 regulates angiogenesis in cutaneous wound healing. Am. J. Pathol. 2014, 184, 1981–1990. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Kawane, K.; Koike, M.; Mori, Y.; Uchiyama, Y.; Nagata, S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 2005, 437, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.S. Functional Role of Milk Fat Globule-Epidermal Growth Factor VIII in Macrophage-Mediated Inflammatory Responses and Inflammatory/Autoimmune Diseases. Mediators Inflamm. 2016, 2016, 5628486. [Google Scholar] [CrossRef] [Green Version]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Jang, Y.J.; Lim, H.J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.Y.; Kim, J.H.; Do, B.R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atabai, K.; Jame, S.; Azhar, N.; Kuo, A.; Lam, M.; McKleroy, W.; Dehart, G.; Rahman, S.; Xia, D.D.; Melton, A.C.; et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Investig. 2009, 119, 3713–3722. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; An, G.H.; Kim, J.Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.H.; Han, C.; Yang, S.R.; Kim, J.H.; et al. Human pluripotent stem-cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Younossi, Z.M. Epidemiology and natural history of NAFLD and NASH. Clin. Liver Dis. 2007, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty infiltration of liver in hyperlipidemic patients. Dig. Dis. Sci. 2000, 45, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Jo, C.; Chung, W.J.; Kim, D.J. Liver cirrhosis and cancer: Comparison of mortality. Hepatol. Int. 2018, 12, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dooley, S.; ten Dijke, P. TGF-beta in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Troeger, J.S.; Mederacke, I.; Gwak, G.Y.; Dapito, D.H.; Mu, X.; Hsu, C.C.; Pradere, J.P.; Friedman, R.A.; Schwabe, R.F. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012, 143, 1073–1083 e1022. [Google Scholar] [CrossRef] [Green Version]
- Patsenker, E.; Stickel, F. Role of integrins in fibrosing liver diseases. Am. J. Physiol. Gastrointest Liver Physiol. 2011, 301, G425–G434. [Google Scholar] [CrossRef] [PubMed]
- Dorison, A.; Dussaule, J.C.; Chatziantoniou, C. The Role of Discoidin Domain Receptor 1 in Inflammation, Fibrosis and Renal Disease. Nephron 2017, 137, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Borza, C.M.; Pozzi, A. Discoidin domain receptors in disease. Matrix Biol. 2014, 34, 185–192. [Google Scholar] [CrossRef]
- Ye, H.; Li, B.; Subramanian, V.; Choi, B.H.; Liang, Y.; Harikishore, A.; Chakraborty, G.; Baek, K.; Yoon, H.S. NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim. Biophys. Acta. 2013, 1828, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Iredale, J.P.; Benyon, R.C.; Arthur, M.J.; Ferris, W.F.; Alcolado, R.; Winwood, P.J.; Clark, N.; Murphy, G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 1996, 24, 176–184. [Google Scholar] [CrossRef]
- Liu, B.; Shi, D.; Chang, S.; Gong, X.; Yu, Y.; Sun, Z.; Wu, J. Characterization and immunological activity of different forms of recombinant secreted Hc of botulinum neurotoxin serotype B products expressed in yeast. Sci. Rep. 2015, 5, 7678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabregat, I.; Caballero-Diaz, D. Transforming Growth Factor-beta-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-beta in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [Green Version]
- Sarrazy, V.; Koehler, A.; Chow, M.L.; Zimina, E.; Li, C.X.; Kato, H.; Caldarone, C.A.; Hinz, B. Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc. Res. 2014, 102, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theret, N.; Musso, O.; L’Helgoualc’h, A.; Clement, B. Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am. J. Pathol. 1997, 150, 51–58. [Google Scholar] [PubMed]
- Shah, K.G.; Wu, R.; Jacob, A.; Molmenti, E.P.; Nicastro, J.; Coppa, G.F.; Wang, P. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive Care Med. 2012, 38, 128–136. [Google Scholar] [CrossRef]
- Cheyuo, C.; Jacob, A.; Wu, R.; Zhou, M.; Qi, L.; Dong, W.; Ji, Y.; Chaung, W.W.; Wang, H.; Nicastro, J.; et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology 2012, 62, 890–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajakaiye, M.A.; Jacob, A.; Wu, R.; Yang, W.L.; Nicastro, J.; Coppa, G.F.; Wang, P. Recombinant human MFG-E8 attenuates intestinal injury and mortality in severe whole body irradiation in rats. PLoS ONE 2012, 7, e46540. [Google Scholar] [CrossRef]
- Matsuda, A.; Jacob, A.; Wu, R.; Zhou, M.; Aziz, M.; Wang, P. Milk fat globule--EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J. Surg. Res. 2013, 180, e37–e46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Shah, K.G.; Qi, L.; Wu, R.; Barrera, R.; Nicastro, J.; Coppa, G.F.; Wang, P. Milk fat globule epidermal growth factor-factor 8 mitigates inflammation and tissue injury after hemorrhagic shock in experimental animals. J. Trauma Acute Care Surg. 2012, 72, 861–869. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Brenner, M.; Yang, W.L.; Wang, P. Recombinant human MFG-E8 ameliorates colon damage in DSS- and TNBS-induced colitis in mice. Lab. Investig. 2015, 95, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koseki, H.; Imai, K.; Nakayama, F.; Sado, T.; Moriwaki, K.; Taniguchi, M. Pillars article: Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc. Natl. Acad. Sci. USA 1990, 87, 5248–5252. J. Immunol. 2014, 193, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Henderson, N.C.; Arnold, T.D.; Katamura, Y.; Giacomini, M.M.; Rodriguez, J.D.; McCarty, J.H.; Pellicoro, A.; Raschperger, E.; Betsholtz, C.; Ruminski, P.G.; et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 2013, 19, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Haggqvist, B.; Naslund, J.; Sletten, K.; Westermark, G.T.; Mucchiano, G.; Tjernberg, L.O.; Nordstedt, C.; Engstrom, U.; Westermark, P. Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc. Natl. Acad. Sci. USA 1999, 96, 8669–8674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermark, P.; Johnson, K.H.; O’Brien, T.D.; Betsholtz, C. Islet amyloid polypeptide—A novel controversy in diabetes research. Diabetologia 1992, 35, 297–303. [Google Scholar] [CrossRef]
- Westermark, P.; Mucchiano, G.; Marthin, T.; Johnson, K.H.; Sletten, K. Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am. J. Pathol. 1995, 147, 1186–1192. [Google Scholar] [PubMed]
- Peng, S.; Glennert, J.; Westermark, P. Medin-amyloid: A recently characterized age-associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid 2005, 12, 96–102. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef]
- Agarwal, S.K. Integrins and cadherins as therapeutic targets in fibrosis. Front. Pharmacol. 2014, 5, 131. [Google Scholar] [CrossRef] [Green Version]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. alphav integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sola, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, G.H.; Lee, J.; Jin, X.; Chung, J.; Kim, J.-C.; Park, J.-H.; Kim, M.; Han, C.; Kim, J.-H.; Woo, D.-H. Truncated Milk Fat Globule-EGF-like Factor 8 Ameliorates Liver Fibrosis via Inhibition of Integrin-TGFβ Receptor Interaction. Biomedicines 2021, 9, 1529. https://doi.org/10.3390/biomedicines9111529
An GH, Lee J, Jin X, Chung J, Kim J-C, Park J-H, Kim M, Han C, Kim J-H, Woo D-H. Truncated Milk Fat Globule-EGF-like Factor 8 Ameliorates Liver Fibrosis via Inhibition of Integrin-TGFβ Receptor Interaction. Biomedicines. 2021; 9(11):1529. https://doi.org/10.3390/biomedicines9111529
Chicago/Turabian StyleAn, Geun Ho, Jaehun Lee, Xiong Jin, Jinwoo Chung, Joon-Chul Kim, Jung-Hyuck Park, Minkyung Kim, Choongseong Han, Jong-Hoon Kim, and Dong-Hun Woo. 2021. "Truncated Milk Fat Globule-EGF-like Factor 8 Ameliorates Liver Fibrosis via Inhibition of Integrin-TGFβ Receptor Interaction" Biomedicines 9, no. 11: 1529. https://doi.org/10.3390/biomedicines9111529





