The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers
Abstract
:1. Introduction
2. Current Approaches for Detecting Ovarian Cancers
3. Ovarian Cancer Biology and Microenvironment
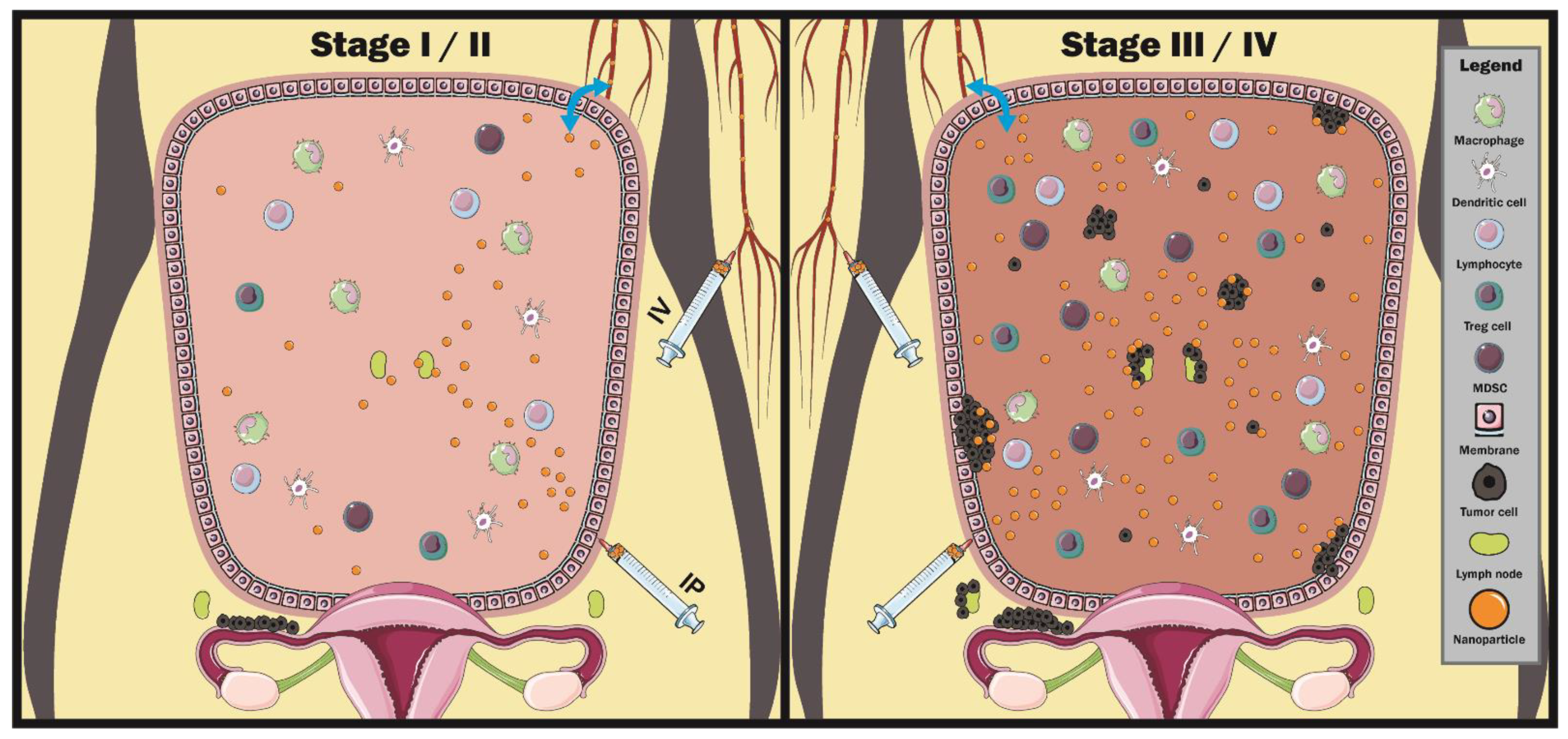
4. Utilizing Nanoparticles in the Ovarian Cancer Microenvironment
5. Recent Developments of Nanoparticles for Ovarian Cancer Diagnostics
5.1. Optical Detection
5.2. Ultrasound
5.3. Photoacoustics
5.4. Magnetic Resonance Imaging
5.5. Positron Emission and Computed Tomography
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Noone, A.M.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. Seer Cancer Statistics Review, 1975–2015; National Cancer Institute: Bethesda, MD, USA, 2018.
- Giornelli, G.H. Management of relapsed ovarian cancer: A review. Springerplus 2016, 5, 1197. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef]
- Chang, S.J.; Hodeib, M.; Chang, J.; Bristow, R.E. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol. Oncol. 2013, 130, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef]
- Tabar, L.; Fagerberg, G.; Chen, H.-H.; Duffy, S.W.; Smart, C.R.; Gad, A.; Smith, R.A. Efficacy of breast cancer screening by age. New results swedish two-county trial. Cancer 1995, 75, 2507–2517. [Google Scholar] [CrossRef]
- Myers, E.R.; Moorman, P.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.J.; Ghate, S.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and harms of breast cancer screening: A systematic review. JAMA 2015, 314, 1615–1634. [Google Scholar] [CrossRef]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of hpv-based screening for prevention of invasive cervical cancer: Follow-up of four european randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- van Nagell, J.R.J.; Miller, R.W.; DeSimone, C.P.; Ueland, F.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef]
- van Nagell, J.R., Jr.; DePriest, P.D.; Ueland, F.R.; DeSimone, C.P.; Cooper, A.L.; McDonald, J.M.; Pavlik, E.J.; Kryscio, R.J. Ovarian cancer screening with annual transvaginal sonography: Findings of 25,000 women screened. Cancer 2007, 109, 1887–1896. [Google Scholar] [CrossRef]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Skates, S.J.; Greene, M.H.; Buys, S.S.; Mai, P.L.; Brown, P.; Piedmonte, M.; Rodriguez, G.; Schorge, J.O.; Sherman, M.; Daly, M.B.; et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent ca125 testing in women at increased familial risk-combined results from two screening trials. Clin. Cancer Res. 2017, 23, 3628–3637. [Google Scholar] [CrossRef] [Green Version]
- Skates, S.J. Ovarian cancer screening: Development of the risk of ovarian cancer algorithm (roca) and roca screening trials. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2012, 22 (Suppl. 1), S24–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging bio–nano science and cancer nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Weiss, A.C.G.; Kelly, H.G.; Faria, M.; Besford, Q.A.; Wheatley, A.K.; Ang, C.-S.; Crampin, E.J.; Caruso, F.; Kent, S.J. Link between low-fouling and stealth: A whole blood biomolecular corona and cellular association analysis on nanoengineered particles. ACS Nano 2019, 13, 4980–4991. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N/A. Cancer Facts & Figures. 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-special-section-ovarian-cancer-2018.pdf (accessed on 25 January 2021).
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the challenge of ascites in ovarian cancer: New avenues for therapy and research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Goff, B.A.; Mandel, L.S.; Melancon, C.H.; Muntz, H.G. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 2004, 291, 2705–2712. [Google Scholar] [CrossRef] [Green Version]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR. Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.; Oram, D.; Fairbanks, J.; Turner, J.; Frost, C.; Grudzinskas, J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol. 1990, 97, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and ovarian cancer: A comprehensive review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci Rep. 2020, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Skates, S.; Allard, W.J.; Verch, T.; Steinhoff, M.; Messerlian, G.; DiSilvestro, P.; Granai, C.O.; et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008, 108, 402–408. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B. Ovarian cancer biomarkers: Moving forward in early detection. In Tumor Microenvironment: The Main Driver of Metabolic Adaptation; Serpa, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–363. [Google Scholar]
- Russo, F.; Lastoria, S.; Svanera, G.; Capobianco, G.; de Chiara, A.; Francia, R.; Squame, E.; de Martinis, F.; Pinto, A. Long-term follow-up study on the role of serum CA-125 as a prognostic factor in 221 newly diagnosed patients with hodgkin’s lymphoma. Leuk. Lymphoma 2007, 48, 723–730. [Google Scholar] [CrossRef]
- Moore, R.G.; Miller, M.C.; Disilvestro, P.; Landrum, L.M.; Gajewski, W.; Ball, J.J.; Skates, S.J. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet. Gynecol. 2011, 118, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2008, 112, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Shih, I.-M. The dualistic model of ovarian carcinogenesis: Revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.H.; Ohman, A.W.; Stepule, C.D.; Kwak, S.; et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef] [Green Version]
- Soslow, R.A. Histologic subtypes of ovarian carcinoma: An overview. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2008, 27, 161–174. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer ii: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Szabova, L.; Yin, C.; Bupp, S.; Guerin, T.M.; Schlomer, J.J.; Householder, D.B.; Baran, M.L.; Yi, M.; Song, Y.; Sun, W.; et al. Perturbation of rb, p53, and brca1 or brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012, 72, 4141–4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Teer, J.K.; Yoder, S.; Gjyshi, A.; Nicosia, S.V.; Zhang, C.; Monteiro, A.N.A. Mutational heterogeneity in non-serous ovarian cancers. Sci. Rep. 2017, 7, 9728. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Uruski, P.; Szubert, S.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Książek, K. Malignant ascites determine the transmesothelial invasion of ovarian cancer cells. Int. J. Biochem. Cell Biol. 2017, 92, 6–13. [Google Scholar] [CrossRef]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [Green Version]
- Mikuła-Pietrasik, J.; Uruski, P.; Szubert, S.; Moszyński, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Książek, K. Biochemical composition of malignant ascites determines high aggressiveness of undifferentiated ovarian tumors. Med. Oncol. 2016, 33, 94. [Google Scholar] [CrossRef]
- Baron, M.A. Structure of the intestinal peritoneum in man. Am. J. Anat. 1941, 69, 439–497. [Google Scholar] [CrossRef]
- Flessner, M.F. The transport barrier in intraperitoneal therapy. Am. J. Physiol. Ren. Physiol. 2005, 288, F433–F442. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.L.; Jin, L.-P. Immune cell population in ovarian tumor microenvironment. J. Cancer 2017, 8, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Glass, J.J.; De Rose, R.; Sperling, C.; Kent, S.J.; Houston, Z.H.; Fletcher, N.L.; Rolfe, B.E.; Thurecht, K.J. Influence of charge on hemocompatibility and immunoreactivity of polymeric nanoparticles. ACS Appl. Bio Mater. 2018, 1, 756–767. [Google Scholar] [CrossRef]
- Ju, Y.; Kelly, H.G.; Dagley, L.F.; Reynaldi, A.; Schlub, T.E.; Spall, S.K.; Bell, C.A.; Cui, J.; Mitchell, A.J.; Lin, Z.; et al. Person-specific biomolecular coronas modulate nanoparticle interactions with immune cells in human blood. ACS Nano 2020, 14, 15723–15737. [Google Scholar] [CrossRef]
- Xu, C.; Shi, S.; Feng, L.; Chen, F.; Graves, S.A.; Ehlerding, E.B.; Goel, S.; Sun, H.; England, C.G.; Nickles, R.J.; et al. Long circulating reduced graphene oxide-iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale 2016, 8, 12683–12692. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Wilson, A.L.; Wilson, K.L.; Bilandzic, M.; Moffitt, L.R.; Makanji, M.; Gorrell, M.D.; Oehler, M.K.; Rainczuk, A.; Stephens, A.N.; Plebanski, M. Non-invasive fluorescent monitoring of ovarian cancer in an immunocompetent mouse model. Cancers 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Machado, S.A.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. Enhancement of ovarian tumor detection with αvβ3 integrin-targeted ultrasound molecular imaging agent in laying hens: A preclinical model of spontaneous ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2014, 24, 19–28. [Google Scholar] [CrossRef]
- Solass, W.; Horvath, P.; Struller, F.; Königsrainer, I.; Beckert, S.; Königsrainer, A.; Weinreich, F.-J.; Schenk, M. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum 2016, 1, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Hirsch, P.; Preston, N.; Tomlinson, A. Ascites in Ovarian Cancer Patients, Management of (Scientific Impact Paper No. 45); Royal College of Obstetricians & Gynaecologists: London, UK, 2014. [Google Scholar]
- Cui, J.; Bjornmalm, M.; Ju, Y.; Caruso, F. Nanoengineering of poly(ethylene glycol) particles for stealth and targeting. Langmuir 2018, 34, 10817–10827. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, G.R.; Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis-mission possible? Adv. Drug Deliv. Rev. 2017, 108, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Sadzuka, Y.; Hirota, S.; Sonobe, T. Intraperitoneal administration of doxorubicin encapsulating liposomes against peritoneal dissemination. Toxicol. Lett. 2000, 116, 51–59. [Google Scholar] [CrossRef]
- Mirahmadi, N.; Babaei, M.H.; Vali, A.M.; Dadashzadeh, S. Effect of liposome size on peritoneal retention and organ distribution after intraperitoneal injection in mice. Int. J. Pharm. 2010, 383, 7–13. [Google Scholar] [CrossRef]
- Pham, B.T.T.; Colvin, E.K.; Pham, N.T.H.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and clearance of stable superparamagnetic maghemite iron oxide nanoparticles in mice following intraperitoneal administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-m.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [Green Version]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O.; et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef]
- Griffeth, L.K. Use of PET/CT scanning in cancer patients: Technical and practical considerations. Proc. Bayl. Univ. Med. Cent. 2005, 18, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for bioimaging. Adv. Colloid Interface Sci. 2006, 123–126, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Roxbury, D.; Jena, P.V.; Shamay, Y.; Horoszko, C.P.; Heller, D.A. Cell membrane proteins modulate the carbon nanotube optical bandgap via surface charge accumulation. ACS Nano 2016, 10, 499–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
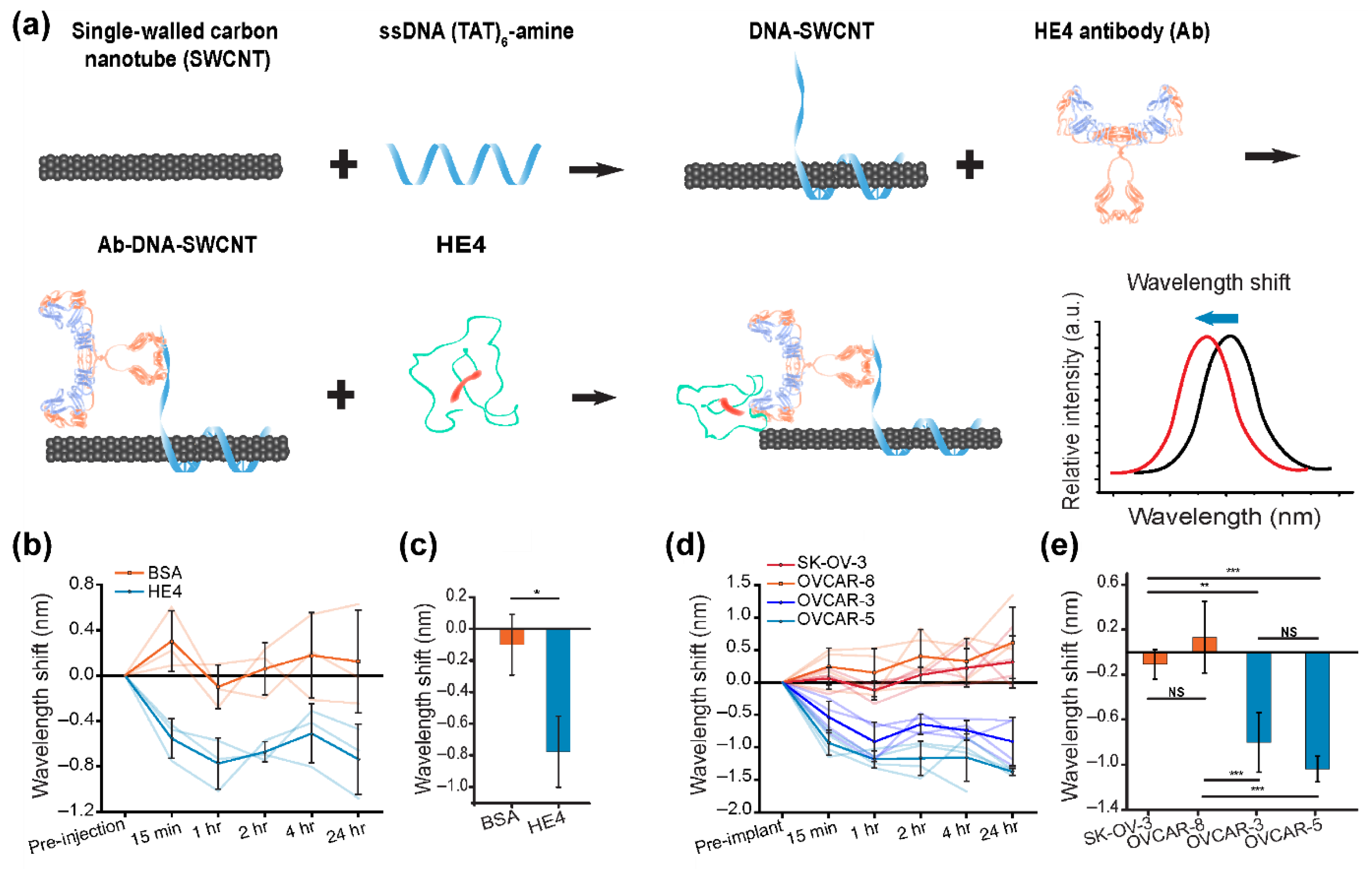
- Williams, R.M.; Lee, C.; Galassi, T.V.; Harvey, J.D.; Leicher, R.; Sirenko, M.; Dorso, M.A.; Shah, J.; Olvera, N.; Dao, F.; et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci. Adv. 2018, 4, eaaq1090. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-i/ii windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Zavaleta, C.L.; Kircher, M.F.; Gambhir, S.S. Raman’s “effect” on molecular imaging. J. Nucl. Med. 2011, 52, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, C.; Kishore, S.A.; Kircher, M.F. Surface-enhanced raman spectroscopy: A new modality for cancer imaging. J. Nucl. Med. 2015, 56, 1295–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.-H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef]
- Galassi, T.V.; Antman-Passig, M.; Yaari, Z.; Jessurun, J.; Schwartz, R.E.; Heller, D.A. Long-term in vivo biocompatibility of single-walled carbon nanotubes. PLoS ONE 2020, 15, e0226791. [Google Scholar] [CrossRef]
- Harvey, J.D.; Jena, P.V.; Baker, H.A.; Zerze, G.H.; Williams, R.M.; Galassi, T.V.; Roxbury, D.; Mittal, J.; Heller, D.A. A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 2017, 1, 0041. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.D.; Baker, H.A.; Ortiz, M.V.; Kentsis, A.; Heller, D.A. HIV detection via a carbon nanotube RNA sensor. ACS Sens. 2019, 4, 1236–1244. [Google Scholar] [CrossRef]
- Williams, R.M.; Lee, C.; Heller, D.A. A fluorescent carbon nanotube sensor detects the metastatic prostate cancer biomarker uPA. ACS Sens. 2018, 3, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Heller, D.A.; Jena, P.V.; Pasquali, M.; Kostarelos, K.; Delogu, L.G.; Meidl, R.E.; Rotkin, S.V.; Scheinberg, D.A.; Schwartz, R.E.; Terrones, M.; et al. Banning carbon nanotubes would be scientifically unjustified and damaging to innovation. Nat. Nanotechnol. 2020, 15, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K.B.; Annapragada, A. Nir-ii fluorescence imaging using indocyanine green nanoparticles. Sci. Rep. 2018, 8, 14455. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. Nir-ii nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, S.; Li, Y.; Luo, Q.; Lin, J.; Hu, L.; Fan, L. Downshifting nanoprobes with follicle stimulating hormone peptide fabrication for highly efficient nir ii fluorescent bioimaging guided ovarian tumor surgery. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Q.; Yuan, W.; Li, Z.; Xu, Y.; Feng, W.; Xu, C.; Li, F. Ultrabright nir-ii emissive polymer dots for metastatic ovarian cancer detection. Adv. Sci. 2021, 8, 2000441. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C.; Oseledchyk, A.; Nicolson, F.; Berisha, N.; Pal, S.; Kircher, M.F. Surface-enhanced resonance raman scattering nanoprobe ratiometry for detecting microscopic ovarian cancer via folate receptor targeting. J. Vis. Exp. 2019, 145, e58389. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Andreou, C.; Wall, M.A.; Kircher, M.F. Folate-targeted surface-enhanced resonance raman scattering nanoprobe ratiometry for detection of microscopic ovarian cancer. ACS Nano 2017, 11, 1488–1497. [Google Scholar] [CrossRef]
- Andreou, C.; Neuschmelting, V.; Tschaharganeh, D.-F.; Huang, C.-H.; Oseledchyk, A.; Iacono, P.; Karabeber, H.; Colen, R.R.; Mannelli, L.; Lowe, S.W.; et al. Imaging of liver tumors using surface-enhanced raman scattering nanoparticles. ACS Nano 2016, 10, 5015–5026. [Google Scholar] [CrossRef] [PubMed]
- Karabeber, H.; Huang, R.; Iacono, P.; Samii, J.M.; Pitter, K.; Holland, E.C.; Kircher, M.F. Guiding brain tumor resection using surface-enhanced raman scattering nanoparticles and a hand-held raman scanner. ACS Nano 2014, 8, 9755–9766. [Google Scholar] [CrossRef] [Green Version]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality mri-photoacoustic-raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef]
- Köse, G.; Darguzyte, M.; Kiessling, F. Molecular ultrasound imaging. Nanomaterials 2020, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three decades of ultrasound contrast agents: A review of the past, present and future improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tempany, C.M.C.; Zou, K.H.; Silverman, S.G.; Brown, D.L.; Kurtz, A.B.; McNeil, B.J. Staging of advanced ovarian cancer: Comparison of imaging modalities—Report from the radiological diagnostic oncology group. Radiology 2000, 215, 761–767. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Hallett, R.; Ryan, A.; Burnell, M.; Sharma, A.; Lewis, S.; Davies, S.; Philpott, S.; Lopes, A.; et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009, 10, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Meng, X.; Dou, T.; Sun, H. Diagnostic accuracy of transvaginal ultrasound examination for assigning a specific diagnosis to adnexal masses: A meta-analysis. Exp. Med. 2020, 20, 265. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V. Microbubble formulations: Synthesis, stability, modeling and biomedical applications. Ultrasound Med. Biol. 2019, 45, 301–343. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Edassery, S.L.; McNeal, S.R.; Bahr, J.M.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. Enhancement of ovarian tumor detection by dr6-targeted ultrasound imaging agents in laying hen model of spontaneous ovarian cancer. Int. J. Gynecol. Cancer 2016, 26, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Adur, M.K.; Utterback, C.W.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. Interleukin 16- (il-16-) targeted ultrasound imaging agent improves detection of ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer. Biomed. Res. Int. 2015, 2015, 567459. [Google Scholar] [CrossRef] [Green Version]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Machado, S.A.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. VEGFR2-targeted ultrasound imaging agent enhances the detection of ovarian tumors at early stage in laying hens, a preclinical model of spontaneous ovarian cancer. Ultrason. Imaging 2015, 37, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Bonomo, L.; Testa, A.C.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound molecular imaging with BR55 in patients with breast and ovarian lesions: First-in-human results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef]
- Yin, T.; Wang, P.; Zheng, R.; Zheng, B.; Cheng, D.; Zhang, X.; Shuai, X. Nanobubbles for enhanced ultrasound imaging of tumors. Int. J. Nanomed. 2012, 7, 895–904. [Google Scholar]
- Wu, H.; Abenojar, E.C.; Perera, R.; De Leon, A.C.; An, T.; Exner, A.A. Time-intensity-curve analysis and tumor extravasation of nanobubble ultrasound contrast agents. Ultrasound Med. Biol. 2019, 45, 2502–2514. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hernandez, C.; Yuan, H.X.; Lilly, J.; Kota, P.; Zhou, H.; Wu, H.; Exner, A.A. Ultrasound molecular imaging of ovarian cancer with ca-125 targeted nanobubble contrast agents. Nanomedicine 2017, 13, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Zhu, Y.; Zhang, J.; Shen, L.; Huang, S.; Fang, S. Improving ovarian cancer imaging with LHRH-NBS: An experimental study. Arch. Gynecol. Obstet. 2016, 294, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.A.-T.; Wang, X.; Peter, K.; Kempe, K.; Corrie, S.R. Dynamic solid-state ultrasound contrast agent for monitoring ph fluctuations in vivo. ACS Sens. 2020, 5, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Wang, F.; Luo, X.; Zhang, Y.; Guo, J.; Shi, W.; Wang, C. Rational design of magnetic nanorattles as contrast agents for ultrasound/magnetic resonance dual-modality imaging. ACS Appl. Mater. Interfaces 2014, 6, 12581–12587. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Guo, X.; Zhang, D.; Zheng, Y.; Zheng, H.; Shi, J. Double-scattering/reflection in a single nanoparticle for intensified ultrasound imaging. Sci. Rep. 2015, 5, 8766. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Dong, Y.; Zhang, Y.; Ru, D.; Wu, Z.; Zhang, J.; Shen, M.; Duan, Y.; Sun, Y. Gsh-sensitive Pt(IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell for precise theranostics against ovarian cancer. Theranostics 2019, 9, 1047–1065. [Google Scholar] [CrossRef]
- Guo, X.; Mei, J.; Jing, Y.; Wang, S. Curcumin-loaded nanoparticles with low-intensity focused ultrasound-induced phase transformation as tumor-targeted and Ph-sensitive theranostic nanoplatform of ovarian cancer. Nanoscale Res. Lett. 2020, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Zhu, S.; Chen, C.; Xie, W.; Xiao, L.; Zhu, Y.; Hao, L.; Wang, Z.; Sun, J.; et al. Dual-mode imaging and therapeutic effects of drug-loaded phase-transition nanoparticles combined with near-infrared laser and low-intensity ultrasound on ovarian cancer. Drug Deliv. 2018, 25, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, L.V. Photoacoustic tomography: Fundamentals, advances and prospects. Contrast Media Mol. Imaging 2011, 6, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liang, Y.; Wang, L. Single-shot photoacoustic microscopy of hemoglobin concentration, oxygen saturation, and blood flow in sub-microseconds. Photoacoustics 2020, 17, 100156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hsu, S.-W.; Gonzalez-Pech, N.; Jhunjhunwala, A.; Chen, F.; Hariri, A.; Grassian, V.; Tao, A.; Jokerst, J.V. Copper sulfide nanodisks and nanoprisms for photoacoustic ovarian tumor imaging. Part. Part. Syst. Charact. 2019, 36, 1900171. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.-T.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
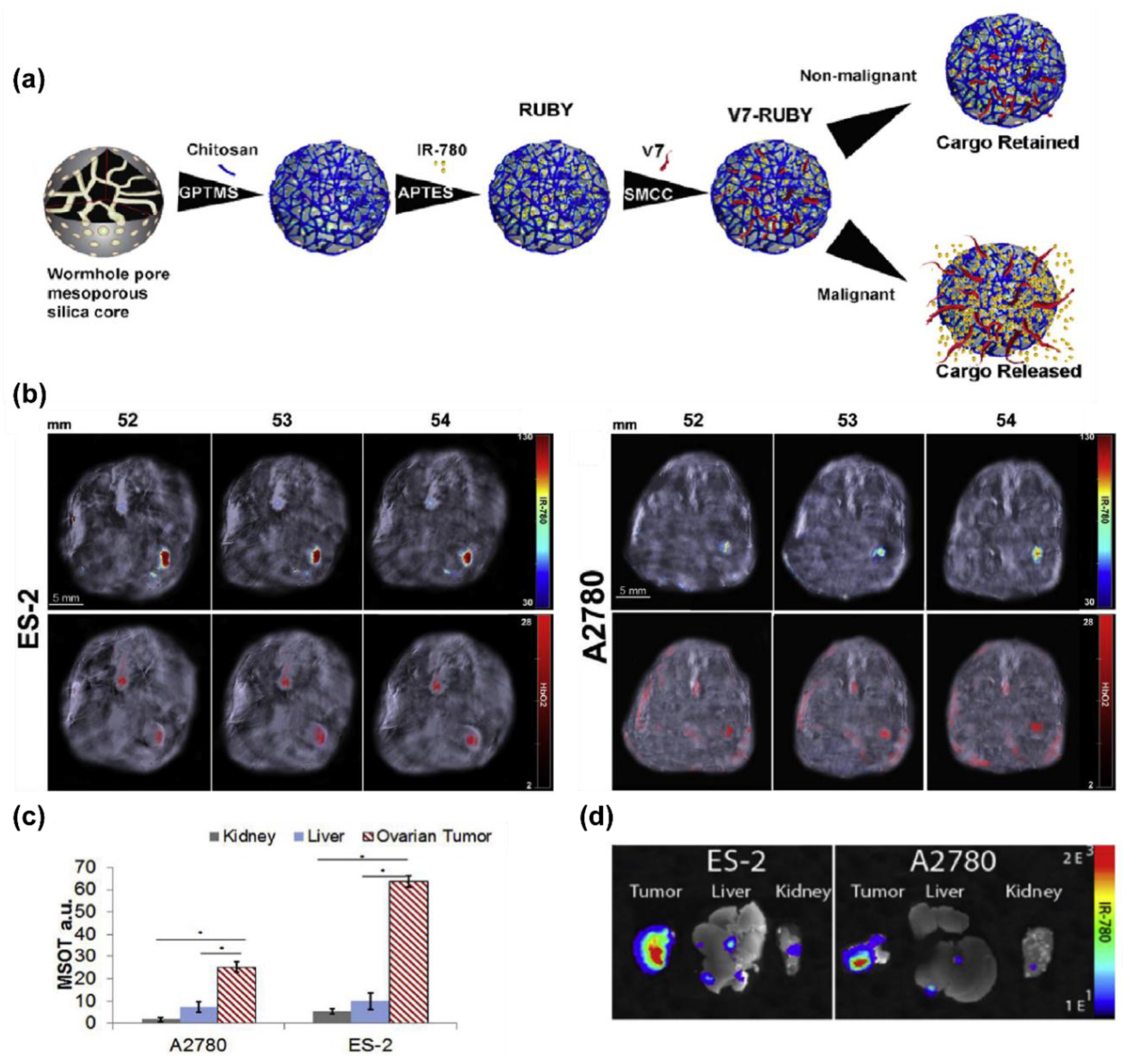
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef]
- Borg, R.E.; Rochford, J. Molecular photoacoustic contrast agents: Design principles & applications. Photochem. Photobiol. 2018, 94, 1175–1209. [Google Scholar]
- Ma, R.; Wu, Q.; Si, T.; Chang, S.; Xu, R.X. Oxygen and indocyanine green loaded microparticles for dual-mode imaging and sonodynamic treatment of cancer cells. Ultrason. Sonochem. 2017, 39, 197–207. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Sun, J.; Zhu, S.; Chen, C.; Xie, W.; Zheng, J.; Zhu, Y.; Xiao, L.; Hao, L.; et al. Folate-targeted and oxygen/indocyanine green-loaded lipid nanoparticles for dual-mode imaging and photo-sonodynamic/photothermal therapy of ovarian cancer in vitro and in vivo. Mol. Pharm. 2019, 16, 4104–4120. [Google Scholar] [CrossRef]
- Armanetti, P.; Flori, A.; Avigo, C.; Conti, L.; Valtancoli, B.; Petroni, D.; Doumett, S.; Cappiello, L.; Ravagli, C.; Baldi, G.; et al. Spectroscopic and photoacoustic characterization of encapsulated iron oxide super-paramagnetic nanoparticles as a new multiplatform contrast agent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 248–253. [Google Scholar] [CrossRef]
- Chakraborty, A.; Boer, J.C.; Selomulya, C.; Plebanski, M. Amino acid functionalized inorganic nanoparticles as cutting-edge therapeutic and diagnostic agents. Bioconjug. Chem. 2018, 29, 657–671. [Google Scholar] [CrossRef]
- Luo, H.; Xu, X.; Ye, M.; Sheng, B.; Zhu, X. The prognostic value of her2 in ovarian cancer: A meta-analysis of observational studies. PLoS ONE 2018, 13, e0191972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berchuck, A.; Kamel, A.; Whitaker, R.; Kerns, B.; Olt, G.; Kinney, R.; Soper, J.T.; Dodge, R.; Clarke-Pearson, D.L.; Marks, P. Overexpression of her-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990, 50, 4087–4091. [Google Scholar] [PubMed]
- Xi, L.; Satpathy, M.; Zhao, Q.; Qian, W.; Yang, L.; Jiang, H. Her-2/neu targeted delivery of a nanoprobe enables dual photoacoustic and fluorescence tomography of ovarian cancer. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satpathy, M.; Wang, L.; Zielinski, R.; Qian, W.; Lipowska, M.; Capala, J.; Lee, G.Y.; Xu, H.; Wang, Y.A.; Mao, H.; et al. Active targeting using her-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic her-2 positive ovarian tumors. Small 2014, 10, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Ding, N.; Sano, K.; Kanazaki, K.; Shimizu, Y.; Watanabe, H.; Namita, T.; Shiina, T.; Ono, M.; Saji, H. Sensitive photoacoustic/magnetic resonance dual imaging probe for detection of malignant tumors. J. Pharm. Sci. 2020, 109, 3153–3159. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Huang, T.; Song, J.; Wang, Y. A sandwich nanostructure of gold nanoparticle coated reduced graphene oxide for photoacoustic imaging-guided photothermal therapy in the second nir window. Front. Bioeng. Biotechnol. 2020, 8, 655. [Google Scholar] [CrossRef]
- Yoo, B.; Pagel, M.D. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. J. Virtual Libr. 2008, 13, 1733–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
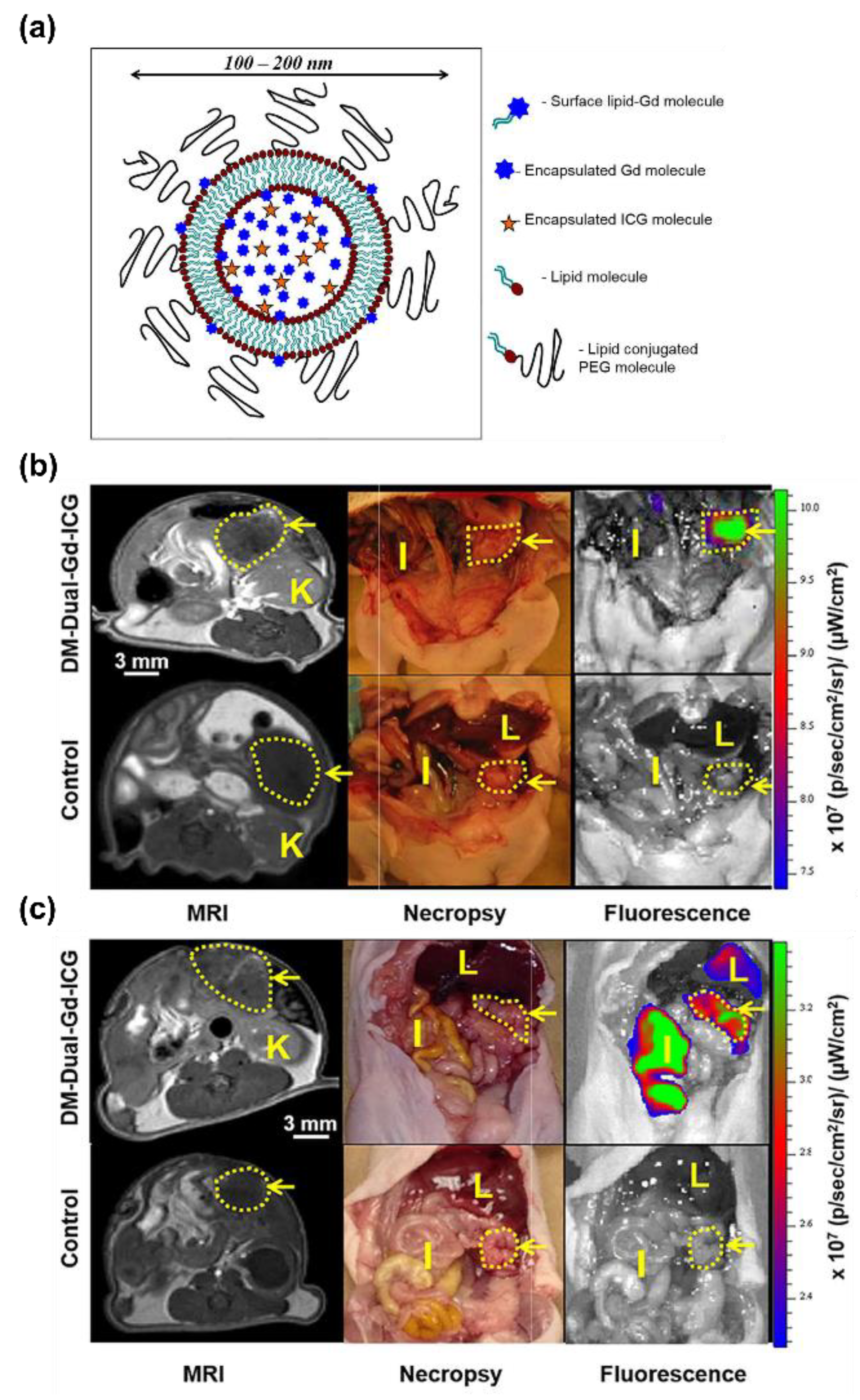
- Ravoori, M.K.; Singh, S.; Bhavane, R.; Sood, A.K.; Anvari, B.; Bankson, J.; Annapragada, A.; Kundra, V. Multimodal magnetic resonance and near-infrared-fluorescent imaging of intraperitoneal ovarian cancer using a dual-mode-dual-gadolinium liposomal contrast agent. Sci. Rep. 2016, 6, 38991. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, X.; Cai, L.; Zuo, F.; Zhao, M.; Wang, Q.; Zhang, S.; Xu, K.; Li, J. Targeted mri and chemotherapy of ovarian cancer with clinic available nano-drug based nanoprobe. Biomed. Pharmacother. 2020, 130, 110585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Zhang, M.; Wu, Z.; Xu, Y.-J.; Lin, J.; Ling, D.; Sheng, Y.; Lu, Y.; Wu, Q.; et al. A liposomal curcumol nanocomposite for magnetic resonance imaging and endoplasmic reticulum stress-mediated chemotherapy of human primary ovarian cancer. J. Mater. Chem. B 2019, 7, 2938–2947. [Google Scholar] [CrossRef]
- Ganta, S.; Singh, A.; Kulkarni, P.; Keeler, A.W.; Piroyan, A.; Sawant, R.R.; Patel, N.R.; Davis, B.; Ferris, C.; O’Neal, S.; et al. EGFR targeted theranostic nanoemulsion for image-guided ovarian cancer therapy. Pharm. Res. 2015, 32, 2753–2763. [Google Scholar]
- Ganta, S.; Singh, A.; Rawal, Y.; Cacaccio, J.; Patel, N.R.; Kulkarni, P.; Ferris, C.F.; Amiji, M.M.; Coleman, T.P. Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer. Drug Deliv. 2016, 23, 968–980. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive mri contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Wu, A.; Chen, X. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Satpathy, M.; Wang, L.; Zielinski, R.J.; Qian, W.; Wang, Y.A.; Mohs, A.M.; Kairdolf, B.A.; Ji, X.; Capala, J.; Lipowska, M.; et al. Targeted drug delivery and image-guided therapy of heterogeneous ovarian cancer using her2-targeted theranostic nanoparticles. Theranostics 2019, 9, 778–795. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Hu, Y.; Shen, M.; Shi, X.; Zhang, G. Folic acid-targeted iron oxide nanoparticles as contrast agents for magnetic resonance imaging of human ovarian cancer. J. Ovarian Res. 2016, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Amirshaghaghi, A.; Yan, L.; Miller, J.; Daniel, Y.; Stein, J.M.; Busch, T.M.; Cheng, Z.; Tsourkas, A. Chlorin e6-coated superparamagnetic iron oxide nanoparticle (spion) nanoclusters as a theranostic agent for dual-mode imaging and photodynamic therapy. Sci. Rep. 2019, 9, 2613. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.-Y.; Jin, K.-T.; Wang, S.-B.; Wang, H.-J.; Tong, X.-M.; Huang, D.-S.; Mou, X.-Z. Molecular imaging of cancer with nanoparticle-based theranostic probes. Contrast Media Mol. Imaging 2017, 2017, 1026270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, P.; Cronin, C.G.; Blake, M.A. Role of pet/ct in ovarian cancer. Am. J. Roentgenol. 2010, 194, W464–W470. [Google Scholar] [CrossRef] [PubMed]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1079–1091. [Google Scholar] [CrossRef]
- Fletcher, N.L.; Houston, Z.H.; Simpson, J.D.; Veedu, R.N.; Thurecht, K.J. Designed multifunctional polymeric nanomedicines: Long-term biodistribution and tumour accumulation of aptamer-targeted nanomaterials. Chem. Commun. 2018, 54, 11538–11541. [Google Scholar] [CrossRef]
- Castellucci, P.; Perrone, A.M.; Picchio, M.; Ghi, T.; Farsad, M.; Nanni, C.; Messa, C.; Meriggiola, M.C.; Pelusi, G.; Al-Nahhas, A.; et al. Diagnostic accuracy of 18f-fdg pet/ct in characterizing ovarian lesions and staging ovarian cancer: Correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl. Med. Commun. 2007, 28, 589–595. [Google Scholar] [CrossRef]
- Virarkar, M.; Ganeshan, D.; Gulati, A.T.; Palmquist, S.; Iyer, R.; Bhosale, P. Diagnostic performance of pet/ct and pet/mr in the management of ovarian carcinoma—a literature review. Abdom. Radiol. 2021, 46, 2323–2349. [Google Scholar] [CrossRef]
- Suppiah, S.; Chang, W.L.; Hassan, H.A.; Kaewput, C.; Asri, A.A.A.; Saad, F.F.A.; Nordin, A.J.; Vinjamuri, S. Systematic review on the accuracy of positron emission tomography/computed tomography and positron emission tomography/magnetic resonance imaging in the management of ovarian cancer: Is functional information really needed? World J. Nucl. Med. 2017, 16, 176–185. [Google Scholar] [CrossRef]
- Farkas, R.; Siwowska, K.; Ametamey, S.M.; Schibli, R.; van der Meulen, N.P.; Müller, C. 64cu- and 68ga-based pet imaging of folate receptor-positive tumors: Development and evaluation of an albumin-binding NODAGA–Folate. Mol. Pharm. 2016, 13, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sevak, K.K.; Monette, S.; Carlin, S.D.; Knight, J.C.; Wuest, F.R.; Sala, E.; Zeglis, B.M.; Lewis, J.S. Preclinical 89zr immuno-pet of high-grade serous ovarian cancer and lymph node metastasis. J. Nucl. Med. 2016, 57, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Lin, Q.; Jin, C.S.; Jiang, W.; Huang, H.; Ding, L.; Muhanna, N.; Irish, J.C.; Wang, F.; Chen, J.; et al. A pegylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano 2015, 9, 4484–4495. [Google Scholar] [CrossRef] [PubMed]
- Körhegyi, Z.; Rózsa, D.; Hajdu, I.; Bodnár, M.; Kertész, I.; Kerekes, K.; Kun, S.; Kollár, J.; Varga, J.; Garai, I.; et al. Synthesis of (68)ga-labeled biopolymer-based nanoparticle imaging agents for positron-emission tomography. Anticancer Res. 2019, 39, 2415–2427. [Google Scholar] [CrossRef]
- Ma, W.; Fu, F.; Zhu, J.; Huang, R.; Zhu, Y.; Liu, Z.; Wang, J.; Conti, P.S.; Shi, X.; Chen, K. 64cu-labeled multifunctional dendrimers for targeted tumor pet imaging. Nanoscale 2018, 10, 6113–6124. [Google Scholar] [CrossRef]
- Kashfi-Sadabad, R.; Gonzalez-Fajardo, L.; Hargrove, D.; Ahmadi, B.; Munteanu, D.; Shahbazmohamadi, S.; Jay, M.; Lu, X. Engineering multifunctional gold decorated dendritic mesoporous silica/tantalum oxide nanoparticles for intraperitoneal tumor-specific delivery. Part. Part. Syst. Charact. 2019, 36, 1900058. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Guo, D.; Wang, Z.; Song, W.; Chen, W.; Zhou, J. Synthesis and evaluation of perfluorooctylbromide nanoparticles modified with a folate receptor for targeting ovarian cancer: In vitro and in vivo experiments. Int. J. Clin. Exp. Med. 2015, 8, 10122–10131. [Google Scholar]
- Jakhmola, A.; Anton, N.; Vandamme, T.F. Inorganic nanoparticles based contrast agents for X-ray computed tomography. Adv. Healthc. Mater. 2012, 1, 413–431. [Google Scholar] [CrossRef]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.C.; Mottillo, E.P.; Baxa, A.C.; Heng, H.H.Q.; Doyon-Reale, N.; Gregoire, L.; Lancaster, W.D.; Rabah, R.; Schmelz, E.M. Sequential molecular and cellular events during neoplastic progression: A mouse syngeneic ovarian cancer model. Neoplasia 2005, 7, 944–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCloskey, C.W.; Goldberg, R.L.; Carter, L.E.; Gamwell, L.F.; Al-Hujaily, E.M.; Collins, O.; Macdonald, E.A.; Garson, K.; Daneshmand, M.; Carmona, E.; et al. A new spontaneously transformed syngeneic model of high-grade serous ovarian cancer with a tumor-initiating cell population. Front. Oncol. 2014, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, E.; Huynh, G.; Wilson, K.; Plebanski, M.; Corrie, S. The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers. Biomedicines 2021, 9, 1554. https://doi.org/10.3390/biomedicines9111554
Henderson E, Huynh G, Wilson K, Plebanski M, Corrie S. The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers. Biomedicines. 2021; 9(11):1554. https://doi.org/10.3390/biomedicines9111554
Chicago/Turabian StyleHenderson, Edward, Gabriel Huynh, Kirsty Wilson, Magdalena Plebanski, and Simon Corrie. 2021. "The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers" Biomedicines 9, no. 11: 1554. https://doi.org/10.3390/biomedicines9111554
APA StyleHenderson, E., Huynh, G., Wilson, K., Plebanski, M., & Corrie, S. (2021). The Development of Nanoparticles for the Detection and Imaging of Ovarian Cancers. Biomedicines, 9(11), 1554. https://doi.org/10.3390/biomedicines9111554






