The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
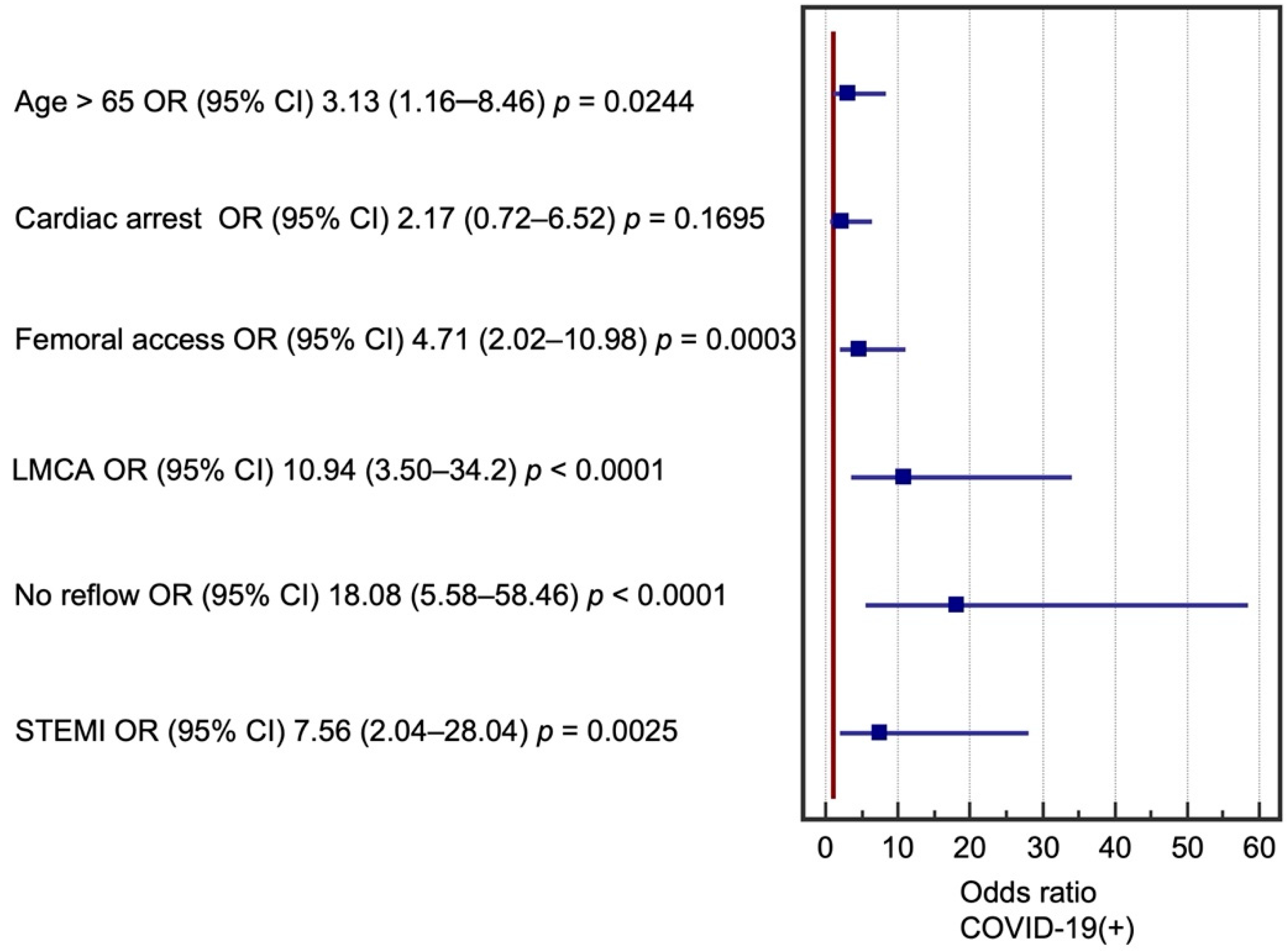
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
- During the pandemic, COVID-19(+) patients diagnosed with ACS underwent invasive cardiology procedures on par with COVID-19(−) patients, although the overall number of procedures has decreased, according to previous studies.
- The organization of the emergency medical rescue allowed COVID-19(+) patients with ACS to reach invasive diagnostic centers in a shorter time than non-infected patients.
- The development of thromboembolic processes in COVID-19 patients results in an unfavorable course of coronary angioplasty (no-reflow phenomenon) and promotes higher perioperative mortality.
- COVID-19(+) patients arrive at the cardiology centers quickly, have significantly developed thromboembolisms, and must be treated with appropriate drugs. They have a worse prognosis and higher perioperative mortality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 in Poland—Statistics. Available online: https://www.medicover.pl/koronawirus/statystyki/ (accessed on 10 September 2021).
- WHO Guidelines on Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease is Suspected. A Systematic Review of Asymptomatic Infections. 2020. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 10 September 2021).
- WHO Announcement. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 12 September 2021).
- Diagnosis and treatment plan of Corona Virus Disease 2019 (tentative sixth edition). Glob. Health J. 2020, 4, 1–5. [CrossRef] [PubMed]
- WHO Guidelines on Laboratory Diagnostics for Novel Coronavirus. 2020. Available online: https://www.who.int/publications/i/item/10665-331501 (accessed on 20 September 2021).
- Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Gurgoglione, F.; Niccoli, G. Myocardial and Microvascular Injury Due to Coronavirus Disease 2019. Eur. Cardiol. Rev. 2020, 15, e52. [Google Scholar] [CrossRef]
- Pandey, D.; Bansal, S.; Goyal, S.; Garg, A.; Sethi, N.; Pothiyill, D.I.; Sreelakshmi, E.S.; Sayyad, M.G.; Sethi, R. Psychological impact of mass quarantine on population during pandemics—The COVID-19 Lock-Down (COLD) study. PLoS ONE 2020, 15, e0240501. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Kardiol. Pol. 2018, 76, 229–313. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 544. [Google Scholar] [CrossRef] [PubMed]
- Legutko, J.; Dobrzycki, S.; Gąsior, M. Interventional Treatment of Myocardial Infarction in Poland during the COVID-19 Pandemic. Annex to the Position Polish Society of Cardiology “Cardiology during the COVID-19 Epidemic”. 2020. Available online: https://ptkardio.pl/resources/data/pliki/22/poste%CC%A8powanie_w_dobie_covid19_final.pdf?download=true (accessed on 20 September 2021).
- Siudak, Z.; Grygier, M.; Wojakowski, W.; Malinowski, K.P.; Witkowski, A.; Gasior, M.; Dudek, D.; Bartus, S. Clinical and procedural characteristics of COVID-19 patients treated with percutaneous coronary interventions. Catheter. Cardiovasc. Interv. 2020, 96, E568–E575. [Google Scholar] [CrossRef]
- COVID-19 Daily Stats. Available online: https://tvn24.pl/polska/koronawirus-w-polsce-mapa-zakazen-ile-szczepien-ile-nowych-przypadkow-wykryto-28-wrzesnia-2021-4344739 (accessed on 29 September 2021).
- Siudak, Z.; Dudek, D.; Grygier, M.; Araszkiewicz, A.; Dabrowski, M.; Kusa, J.; Hawranek, M.; Huczek, Z.; Kralisz, P.; Roleder, T.; et al. Interventional cardiology in Poland in 2020—Impact of the COVID-19 pandemic. Annual summary report of the Association of Cardiovascular Interventions of the Polish Cardiac Society and Jagiellonian University Medical College. Postepy Kardiol. Interwencyjnej 2021, 17, 131–134. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabro, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Papafaklis, M.I.; Katsouras, C.S.; Tsigkas, G.; Toutouzas, K.; Davlouros, P.; Hahalis, G.N.; Kousta, M.S.; Styliadis, I.G.; Triantafyllou, K.; Pappas, L.; et al. “Missing” acute coronary syndrome hospitalizations during the COVID-19 era in Greece: Medical care avoidance combined with a true reduction in incidence? Clin. Cardiol. 2020, 43, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Hess, S.; Marchandot, B.; Sato, C.; Truong, D.P.; Kim, N.T.; Weiss, A.; Jesel, L.; Ohlmann, P.; Morel, O. Clinical features of patients with acute coronary syndrome during the COVID-19 pandemic. J. Thromb. Thrombolysis 2021, 52, 95–104. [Google Scholar] [CrossRef]
- Koutsoukis, A.; Delmas, C.; Roubille, F.; Bonello, L.; Schurtz, G.; Manzo-Silberman, S.; Puymirat, E.; Elbaz, M.; Bouisset, F.; Meunier, P.A.; et al. Acute Coronary Syndrome in the Era of SARS-CoV-2 Infection: A Registry of the French Group of Acute Cardiac Care. CJC Open 2021, 3, 311–317. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: The pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Position Polish Society of Cardiology “Cardiology during the COVID-19 Epidemic”. Available online: https://ptkardio.pl/aktualnosci/510-kardiologia_podczas_epidemii_covid19_stanowisko_polskiego_towarzystwa_kardiologicznego (accessed on 27 September 2021).
- Sielski, J.; Kazirod-Wolski, K.; Siudak, Z. Risk of perioperative death and sudden cardiac arrest: A study of 113,456 cases from the National Registry of Invasive Cardiology Procedures (ORPKI) for estimation of the perioperative prognosis. Kardiol. Pol. 2021. [Google Scholar] [CrossRef]
- Tokarek, T.; Siudak, Z.; Dziewierz, A.; Rakowski, T.; Krycinska, R.; Siwiec, A.; Dudek, D. Clinical outcomes in nonagenarians undergoing a percutaneous coronary intervention: Data from the ORPKI Polish National Registry 2014–2016. Coron. Artery Dis. 2018, 29, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Kite, T.A.; Ludman, P.F.; Gale, C.P.; Wu, J.; Caixeta, A.; Mansourati, J.; Sabate, M.; Jimenez-Quevedo, P.; Candilio, L.; Sadeghipour, P.; et al. International Prospective Registry of Acute Coronary Syndromes in Patients With COVID-19. J. Am. Coll. Cardiol. 2021, 77, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Guler, A.; Gurbak, I.; Panc, C.; Guner, A.; Erturk, M. Frequency and predictors of no-reflow phenomenon in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. Acta Cardiol. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Peterson, E.D. Acute coronary syndromes in women and men. Nat. Rev. Cardiol. 2016, 13, 471–480. [Google Scholar] [CrossRef]
- Davis, M.; Diamond, J.; Montgomery, D.; Krishnan, S.; Eagle, K.; Jackson, E. Acute coronary syndrome in young women under 55 years of age: Clinical characteristics, treatment, and outcomes. Clin. Res. Cardiol. 2015, 104, 648–655. [Google Scholar] [CrossRef] [PubMed]



| Variable | Total | COVID(−) | COVID(+) | p-Value |
|---|---|---|---|---|
| Clinical Factors | ||||
| n | 47,940 | 44,952 (93.8) | 2988 (6.2) | <0.0001 |
| Sex (male) | 30,874 (64.6) | 28,814 (64.3) | 2060 (69.4) | <0.0001 |
| Age, median (Q1–Q3) | 67 (60–74) | 67 (60–74) | 66 (60–74) | 0.0043 |
| Age (>65 years) | 27,257 (56.9) | 25,708 (57.2) | 1549 (51.8) | <0.0001 |
| Diabetes (n, %) | 10,740 (22.4) | 10,097 (22.5) | 643 (21.5) | 0.2316 |
| Previous stroke (n, %) | 1474 (3.1) | 1368 (3.0) | 106 (3.5) | 0.1221 |
| Previous MI (n, %) | 10,713 (22.3) | 10,241 (22.8) | 472 (15.8) | <0.0001 |
| Previous PCI (n, %) | 12,326 (25.7) | 11,867 (26.4) | 459 (15.4) | <0.0001 |
| Previous CABG (n, %) | 2407 (5.0) | 2303 (5.1) | 104 (3.5) | 0.0001 |
| Smoking (n, %) | 10,587 (22.1) | 9850 (21.9) | 737 (24.7) | 0.0004 |
| Psoriasis (n, %) | 303 (0.6) | 287 (0.6) | 16 (0.5) | 0.5081 |
| Hypertension (n, %) | 32,249 (67.3) | 30,474 (67.8) | 1775 (59.5) | <0.0001 |
| Kidney disease (n, %) | 2728 (5.7) | 2552 (5.7) | 176 (5.9) | 0.5583 |
| COPD (n, %) | 1702 (3.6) | 1580 (3.5) | 122 (4.1) | 0.1041 |
| Prehospital Management | ||||
| Acute coronary syndrome (n, %) | 0.3390 | |||
| STEMI | 11,746 (24.5%) | 10,312 (22.9%) | 1434 (48.0%) | |
| NSTEMI | 13,600 (28.4%) | 12,682 (28.2%) | 918 (30.7%) | |
| Unstable angina | 22,594 (47.1%) | 21,958 (48.8%) | 636 (21.3%) | |
| Time from pain to first contact (n, %) | 0.3390 | |||
| <12 h | 16,064 (78.4) | 14,555 (78.4) | 1509 (79.9) | |
| 12–48 h | 4318 (21.3) | 3946 (21.3) | 372 (19.7) | |
| >48 h | 74 (0.4) | 65 (0.4) | 9 (0.5) | |
| Time from pain to inflation or angiogram (n, %) | <0.0001 | |||
| <12 h | 12,156 (58.4) | 10,898 (57.7) | 1258 (65.2) | |
| 12–48 h | 6024 (28.9) | 5548 (29.4) | 476 (24.7) | |
| >48 h | 2648 (12.7) | 2454 (13.0) | 194 (7.3) | |
| Time from first contact to inflation or angiogram (n, %) | <0.0001 | |||
| <12 h | 16,460 (79.6) | 14,697 (78.6) | 1763 (79.6) | |
| 12–48 h | 3340 (16.1) | 3145 (16.8) | 3340 (16.1) | |
| >48 h | 884 (4.3) | 845 (4.5) | 884 (4.3) | |
| Direct transport to catheterization lab (n, %) | 4259 (8.9) | 3622 (8.1) | 637 (21.3) | <0.0001 |
| Cardiac arrest at baseline | 726 (1.5) | 489 (1.1) | 237 (7.9) | <0.0001 |
| Variable | Total | COVID(−) | COVID(+) | p-Value |
|---|---|---|---|---|
| Pharmacological Factors | ||||
| ASA (n, %) | 17,607 (36.7) | 15,877 (35.3) | 1730 (57.9) | <0.0001 |
| UFH (n, %) | 11,116 (23.2) | 9994 (22.2) | 1122 (37.6) | <0.0001 |
| LMWH (n, %) | 1396 (2.9) | 1246 (2.8) | 150 (5.0) | <0.0001 |
| P2Y12 inhibitor (n, %) | 16,066 | 14,926 (92.9) | 1140 (7.1) | <0.0001 |
| clopidogrel | 9086 (56.6) | 8577 (57.5) | 509 (44.6) | |
| prasugrel, and ticagrelor | 6980 (43.4) | 6349 (42.5) | 631 (55.4) | |
| Thrombolysis (n, %) (47,940) | 11 (0.02) | 11 (0.02) | 0 | 0.3925 |
| GPI IIb/IIIa during angiogram (21,146) (n, %) | 4373 (20.7) | 3976 (19.8) | 397 (36.4) | <0.0001 |
| Bivalirudin (n, %) | 4 (0.008) | 4 (0.009) | 0 | 0.6061 |
| Periprocedural Factors | ||||
| IVUS (n, %) | 492 (1.0) | 468 (1.0) | 24 (0.8) | 0.2115 |
| OCT (n, %) | 35 (0.07) | 32 (0.07) | 3 (0.1) | 0.5670 |
| Vascular access (n, %) | <0.0001 | |||
| Radial | 42,088 (88.8) | 39,558 (89.0) | 2530 (85.7) | |
| Femoral | 5307 (11.2) | 4884 (11.0) | 423 (14.3) | |
| FFR (n, %) | 1376 (2.9) | 1341 (3.0) | 35 (1.2) | <0.0001 |
| Total amount of contrast used during procedure, mL (IQR) | 120 (80–170) | 120 (70–170) | 130 (100–180) | <0.0001 |
| Total radiation dose, mGy (IQR) | 404 (198–771) | 398 (191–760) | 491 (255–869) | <0.0001 |
| Variable | Total | COVID(−) | COVID(+) | p-Value |
|---|---|---|---|---|
| Critical Stenosis of Coronary Artery and Implanted Stents | ||||
| RCA (n, %) | 9941 (31.9) | 9280 (32.1) | 661 (28.8) | 0.0011 |
| LMCA (n, %) | 1034 (3.3) | 952 (3.3) | 82 (3.6) | 0.4702 |
| LAD (n, %) | 12,254 (39.3) | 11,178 (38.7) | 1076 (47.0) | <0.0001 |
| SvG (n, %) | 398 (1.3) | 371 (1.3) | 27 (1.2) | 0.6610 |
| LIMA/RIMA (n, %) | 58 (0.2) | 57 (0.2) | 1 (0.04) | 0.1002 |
| Bifurcation (n, %) | 3352 (10.8) | 3109 (10.8) | 243 (10.6) | 0.8061 |
| DES (n, %) | 27,325 (87.7) | 25,451 (88.1) | 1874 (81.9) | <0.0001 |
| BVS (n, %) | 29 (0.09) | 29 (0.1) | 0 | 0.1290 |
| BMS (n, %) | 51 (0.2) | 49 (0.2) | 2 (0.09) | 0.3473 |
| Number of implanted stents (n, %) | <0.0001 | |||
| 0 | 3737 (12.1) | 3351 (11.6) | 416 (18.2) | |
| 1 | 22,314 (71.6) | 20,752 (71.9) | 1562 (68.2) | |
| ≥2 | 5086 (16.3) | 4772 (16.5) | 314 (13.7) | |
| Complications During Procedures | ||||
| Total (n, %) | 350 (0.7) | 309 (0.7) | 41 (1.4) | <0.001 |
| Death (n, %) | 199 (0.4) | 172 (0.4) | 27 (0.9) | <0.0001 |
| Stroke (n, %) | 8 (0.02) | 8 (0.02) | 0 | 0.4658 |
| Dissection (n, %) | 34 (0.07) | 30 (0.07) | 4 (0.1) | 0.1822 |
| Bleeding at the puncture site (n, %) | 24 (0.05) | 21 (0.05) | 3 (0.1) | 0.2020 |
| Allergic reaction (n, %) | 9 (0.02) | 8 (0.02) | 1 (0.03) | 0.5449 |
| No reflow (n, %) | 342 (0.9) | 317 (0.9) | 25 (1.3) | 0.1098 |
| Variable | COVID(−) | COVID(+) | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Sex (male) | 0.69 (0.51–0.94) | 0.0176 | 1.55 (0.62–3.85) | 0.3443 |
| Age (>65 years) | 2.48 (1.74–3.53) | <0.0001 | 3.28 (1.32–8.16) | 0.0105 |
| Diabetes | 1.26 (0.90–1.77) | 0.1785 | 0.45 (0.14–1.51) | 0.1976 |
| Previous stroke | 3.30 (1.96–5.53) | <0.0001 | 2.20 (0.51–9.40) | 0.2883 |
| Previous MI | 0.84 (0.57–1.22) | 0.3456 | 1.53 (0.61–3.81) | 0.3613 |
| Previous PCI | 0.42 (0.28–0.67) | 0.0002 | 0.68(0.21–2.29) | 0.5407 |
| Previous CABG | 1.39 (0.77–2.51) | 0.2715 | * | |
| Smoking | 0.72 (0.48–1.08) | 0.1101 | 0.53 (0.18–1.53) | 0.2407 |
| Psoriasis | 2.78 (0.88–8.76) | 0.0806 | * | |
| Hypertension | 0.74 (0.55–1.01) | 0.0587 | 0.63 (0.30–1.35) | 0.2355 |
| Kidney disease | 2.20 (1.38–3.51) | 0.0010 | 2.82 (0.97–8.25) | 0.0582 |
| COPD | 1.15 (0.78–2.98) | 0.2237 | 0.90 (1.12–6.11) | 0.9203 |
| STEMI (vs. NSTEMI and UA) | 6.5 (4.74–8.9) | <0.0001 | 8.8 (2.64–29.29) | 0.0004 |
| Time from pain to first contact, (<12 h, 12–48 h, >48 h) | 0.93 (0.61–1.42) | 0.7250 | 1.27 (0.42–3.79) | 0.6743 |
| Time from pain to inflation or angiogram, (<12 h, 12–48 h, >48 h) | 0.69 (0.52–0.91) | 0.0085 | 1.12 (0.58–2.17) | 0.7416 |
| Time from first contact to inflation or angiogram, (<12 h, 12–48 h, >48 h) | 0.41 (0.24–0.70) | 0.0012 | 0.43 (0.07–2.78) | 0.3719 |
| Direct transfer to catheterization lab | 3.96 (2.81–5.59) | <0.0001 | 1.06 (0.42–2.63) | 0.9083 |
| Cardiac arrest at baseline | 17.86 (11.73–27.21) | <0.0001 | 3.38 (1.35–8.45) | 0.0093 |
| Variable | COVID(−) | COVID(+) | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| ASA | 2.75 (2.02–3.73) | <0.0001 | 1.06 (0.49–2.29) | 0.8856 |
| UFH | 2.41 (1.77–3.27) | <0.0001 | 1.15 (0.53–2.48) | 0.7311 |
| LMWH | 1.50 (0.70–3.20) | 0.3021 | 0.73 (0.10–5.39) | 0.7540 |
| Prasugrel and ticagrelor(vs. clopidogrel) | 1.58 (0.84–2.96) | 0.1561 | 1.08 (0.24–4.83) | 0.9238 |
| Thrombolysis | * | * | ||
| GPI Iib/IIIa during angiogram | 12.22 (6.17–24.21) | <0.0001 | 12.47 (1.53–31.53) | 0.0184 |
| Bivalirudin | * | * | ||
| IVUS | 1.12 (0.28–4.52) | 0.8649 | * | |
| OCT | * | * | ||
| Vascular access (femoral) | 9.16 (6.76–12.44) | <0.0001 | 7.71 (3.58–16.60) | <0.0001 |
| FFR | * | * | ||
| Total amount of contrast, ml | 1.005 (1.004–1.006) | <0.0001 | 1.004 (0.999–1.008) | 0.0637 |
| Total radiation dose, mGy | 1.001 (1.000–1.001) | <0.0001 | 1.0002 (0.9996–1.0007) | 0.2860 |
| COVID(−) | COVID(+) | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| RCA | 0.43 (0.28–0.65) | 0.0001 | 0.91 (0.38–2.18) | 0.8331 |
| LMCA | 9.73 (6.75–14.04) | <0.0001 | 8.59 (3.36–22.01) | <0.0001 |
| LAD | 1.87 (1.37–2.55) | 0.0001 | 1.32 (0.61–2.87) | 0.4800 |
| SvG | 0.97 (0.24–3.94) | 0.9616 | * | |
| LIMA/RIMA | 3.21 (0.44–23.32) | 0.2493 | * | |
| Bifurcation | 1.11 (0.69–1.79) | 0.6713 | 0.70 (0.16–2.98) | 0.6280 |
| DES | 0.24 (0.17–0.33) | <0.0001 | 0.60 (0.25–1.43) | 0.2464 |
| BVS | * | * | ||
| BMS | 3.73 (0.51–27.20) | 0.1937 | * | |
| Number of implanted stents | 0.46 (0.34–0.61) | <0.0001 | 0.90 (0.45–1.79) | 0.7676 |
| 0 | ||||
| 1 | ||||
| ≥2 | ||||
| Complications | ||||
| Stroke | * | * | ||
| Dissection | 9.02 (1.22–66.62) | 0.0308 | * | |
| Bleeding at the puncture site | * | * | ||
| Allergic reaction | * | * | ||
| No reflow (n, %) | 11.50 (6.77–19.53) | <0.0001 | 19.03 (6.70–54.06) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaziród-Wolski, K.; Sielski, J.; Sidło, J.; Januszek, R.; Siudak, Z. The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry. Biomedicines 2021, 9, 1813. https://doi.org/10.3390/biomedicines9121813
Kaziród-Wolski K, Sielski J, Sidło J, Januszek R, Siudak Z. The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry. Biomedicines. 2021; 9(12):1813. https://doi.org/10.3390/biomedicines9121813
Chicago/Turabian StyleKaziród-Wolski, Karol, Janusz Sielski, Jacek Sidło, Rafał Januszek, and Zbigniew Siudak. 2021. "The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry" Biomedicines 9, no. 12: 1813. https://doi.org/10.3390/biomedicines9121813
APA StyleKaziród-Wolski, K., Sielski, J., Sidło, J., Januszek, R., & Siudak, Z. (2021). The Most Relevant Factors Affecting the Perioperative Death Rate in Patients with Acute Coronary Syndrome and COVID-19, Based on Annual Follow-Up in the ORPKI Registry. Biomedicines, 9(12), 1813. https://doi.org/10.3390/biomedicines9121813








