Hyperspectral Imaging for the Evaluation of Microcirculatory Tissue Oxygenation and Perfusion Quality in Haemorrhagic Shock: A Porcine Study
Abstract
:1. Introduction
2. Methods
2.1. Sample Size Calculation, Randomisation, and Blinding
2.2. Animal Preparation and Anaesthesia
2.3. Ventilatory Settings
2.4. Haemodynamic Monitoring
3. Experimental Protocol
3.1. Surgical Procedure
3.2. Shock Induction
3.3. Haemodynamic Management and Intervention
3.4. Euthanasia
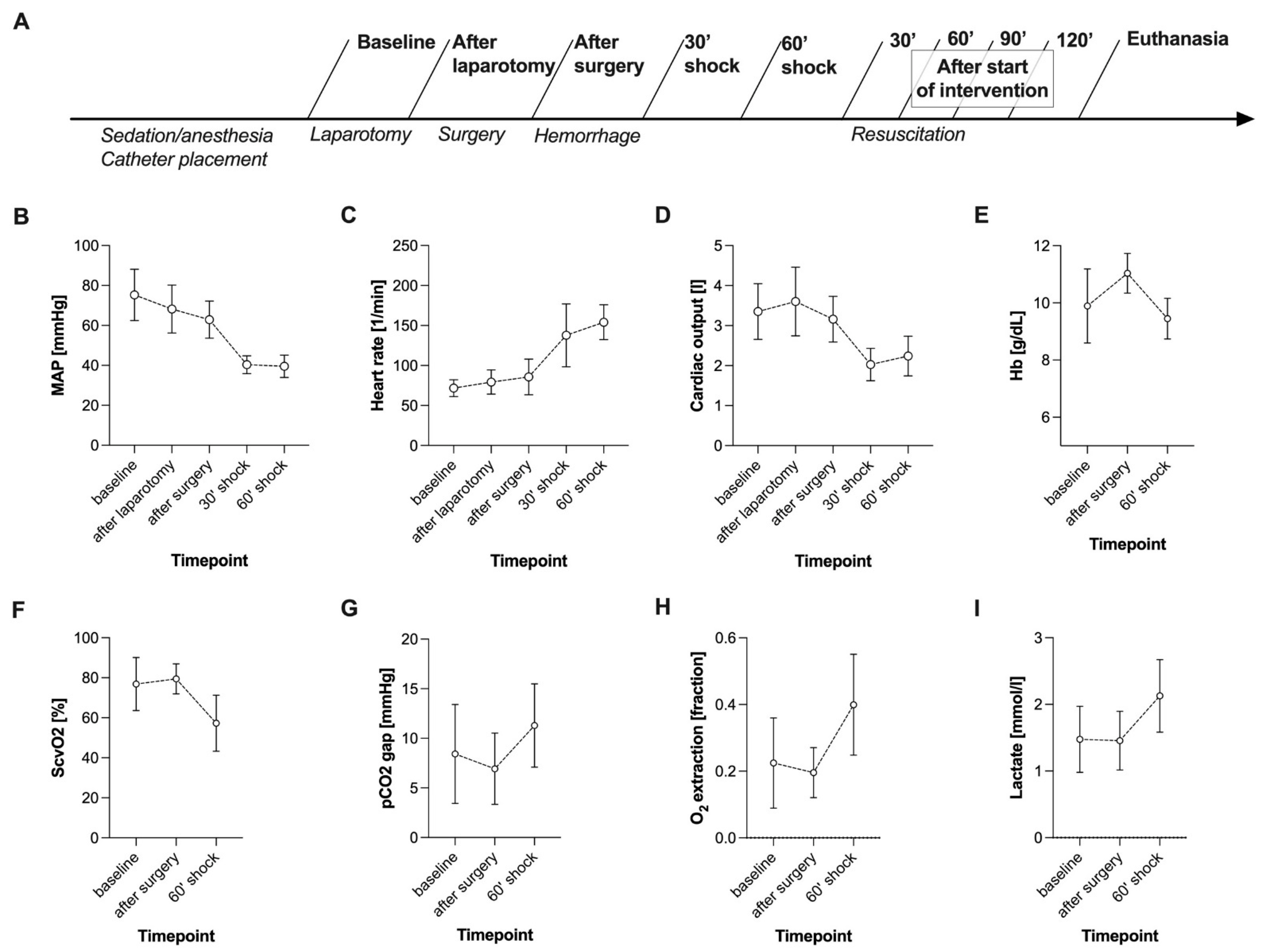
3.5. HSI Measurements
- -
- Tissue oxygenation (StO2, wavelength range: 500–650 and 700–815 nm): haemoglobin oxygen saturation in the capillary system of superficial tissue (penetration depth up to 1 mm) indicated in percent (0–100%);
- -
- NIR perfusion index (NIR, wavelength range: 655–735 and 825–925 nm): haemoglobin oxygen saturation in the capillary system of deeper tissue layers (penetration depth up to 4–6 mm) indicated in predefined arbitrary units (0–100);
- -
- Tissue haemoglobin index (THI, wavelength range: 530–590 and 785–825 nm): distribution of deoxygenated and oxygenated haemoglobin in the measured tissue (penetration depth up to 1–3 mm) indicated in predefined arbitrary units (0–100);
- -
- Tissue water index (TWI, wavelength range: 880–900 and 955–980 nm): relative water content of the tissue (penetration depth up to 1–3 mm) indicated in predefined arbitrary units (0–100).
3.6. Data Collection Time Points
3.7. Statistical Methods
4. Results
4.1. Haemorrhagic Shock Induced Global and HSI-Measured Tissue Oxygenation Deficiency
4.2. MAP Targets Were Not Consistently Achieved during Fluid and Vasopressor Therapy


4.3. Fluid Resuscitation Restored Global and HSI-Measured Tissue Oxygenation
4.4. Fluid Administration Increased HSI-Measured Tissue Water Content
4.5. Norepinephrine Administration Aggravated Global and HSI Tissue Oxygenation Deficiency Dose-Dependently

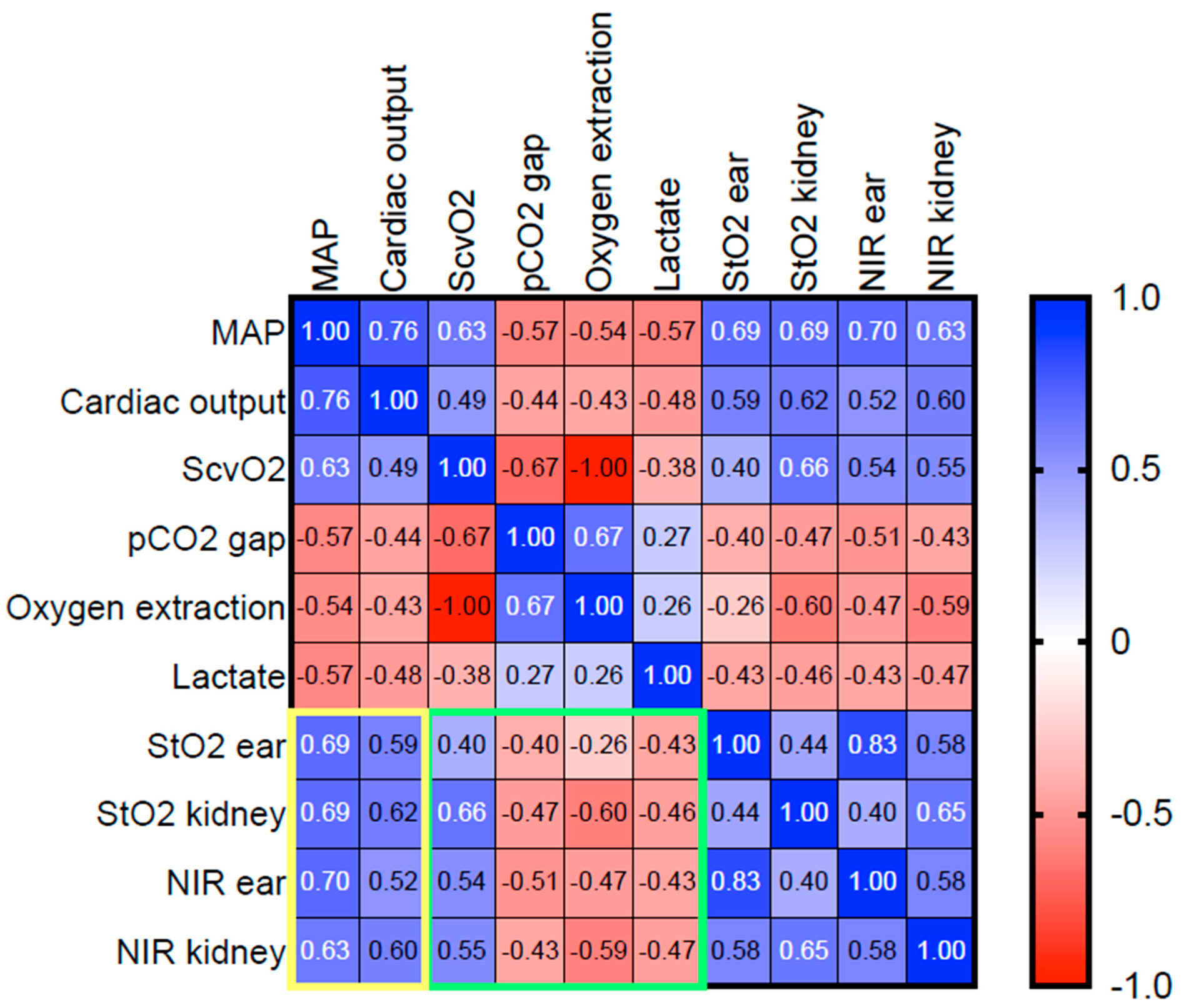
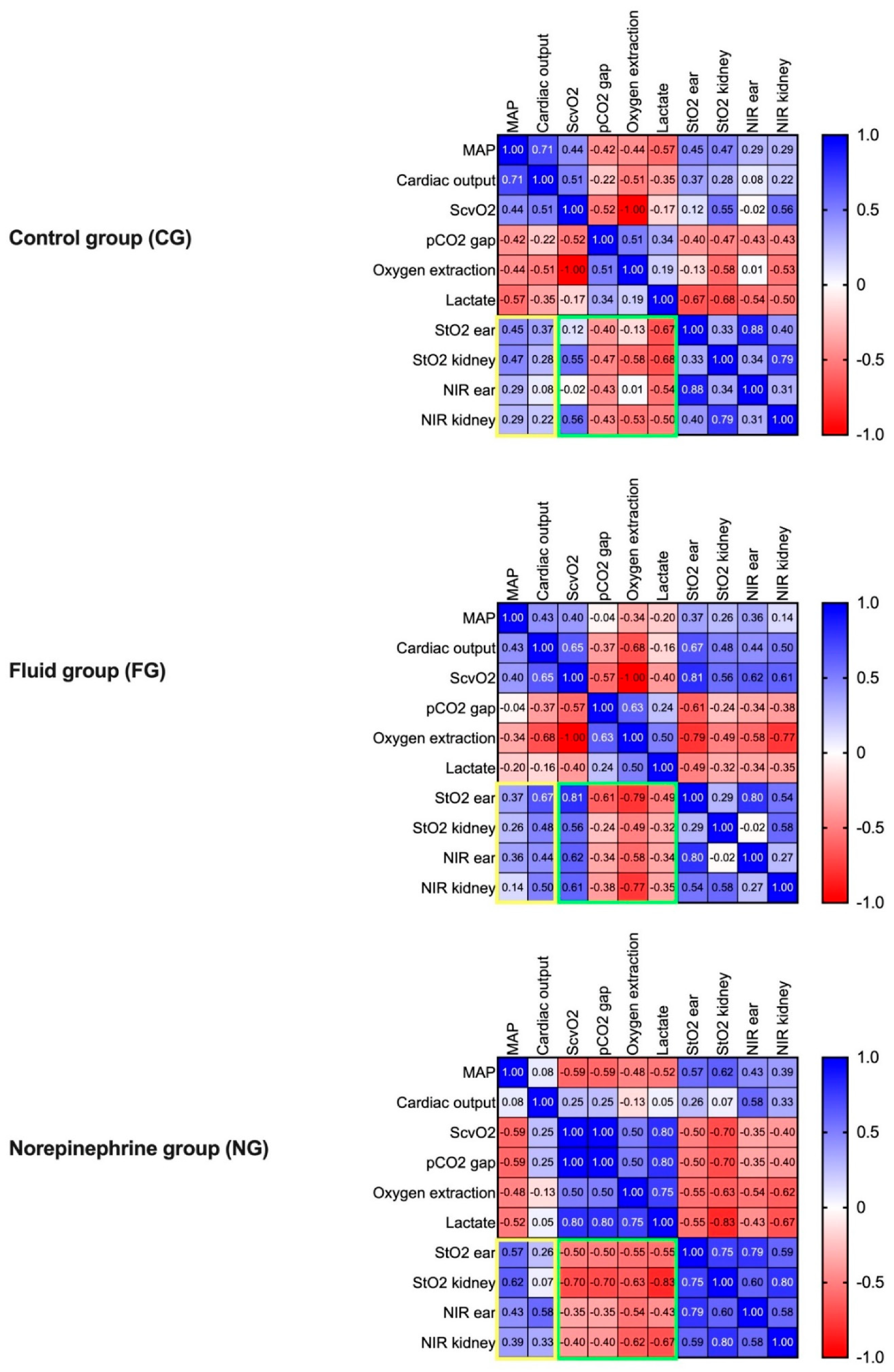
4.6. Macrohaemodynamic Variables and Global Markers of Tissue Oxygenation Correlated with HSI Tissue Oxygenation Parameters during Resuscitation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Backer, D.; Foulon, P. Minimizing catecholamines and optimizing perfusion. Crit. Care 2019, 23, 149. [Google Scholar] [CrossRef] [Green Version]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19, S8. [Google Scholar] [CrossRef] [Green Version]
- Huber, W.; Zanner, R.; Schneider, G.; Schmid, R.; Lahmer, T. Assessment of Regional Perfusion and Organ Function: Less and Non-invasive Techniques. Front. Med. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Holmer, A.; Tetschke, F.; Marotz, J.; Malberg, H.; Markgraf, W.; Thiele, C.; Kulcke, A. Oxygenation and perfusion monitoring with a hyperspectral camera system for chemical based tissue analysis of skin and organs. Physiol. Meas. 2016, 37, 2064–2078. [Google Scholar] [CrossRef]
- Schwandner, F.; Hinz, S.; Witte, M.; Philipp, M.; Schafmayer, C.; Grambow, E. Intraoperative Assessment of Gastric Sleeve Oxygenation Using Hyperspectral Imaging in Esophageal Resection: A Feasibility Study. Visc. Med. 2020, 37, 165–170. [Google Scholar] [CrossRef]
- Studier-Fischer, A.; Seidlitz, S.; Sellner, J.; Wiesenfarth, M.; Ayala, L.; Özdemir, B.; Odenthal, J.; Knödler, S.; Kowalewski, K.F.; Haney, C.M.; et al. Spectral organ fingerprints for intraoperative tissue classification with hyperspectral imaging. bioRxiv 2021, 469943. [Google Scholar] [CrossRef]
- Nickel, F.; Studier-Fischer, A.; Özdemir, B.; Odenthal, J.; Müller, L.R.; Knödler, S.; Kowalewski, K.F.; Camplisson, I.; Allers, M.M.; Dietrich, M.; et al. Optimization of anastomotic technique and gastric conduit perfusion with hyperspectral imaging in an experimental model for minimally invasive esophagectomy. bioRxiv 2021, 462901. [Google Scholar] [CrossRef]
- Kulcke, A.; Holmer, A.; Wahl, P.; Siemers, F.; Wild, T.; Daeschlein, G. A compact hyperspectral camera for measurement of perfusion parameters in medicine. Biomed. Tech. Eng. 2018, 63, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Langner, I.; Wild, T.; von Podewils, S.; Sicher, C.; Kiefer, T.; Jünger, M. Hyperspectral imaging as a novel diagnostic tool in microcirculation of wounds. Clin. Hemorheol. Microcirc. 2017, 67, 467–474. [Google Scholar] [CrossRef]
- Sucher, R.; Athanasios, A.; Köhler, H.; Wagner, T.; Brunotte, M.; Lederer, A.; Gockel, I.; Seehofer, D. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int. J. Surg. Case Rep. 2019, 62, 108–111. [Google Scholar] [CrossRef]
- Dietrich, M.; Marx, S.; von der Forst, M.; Bruckner, T.; Schmitt, F.; Fiedler, M.; Nickel, F.; Studier-Fischer, A.; Müller-Stich, B.; Hackert, T.; et al. Bedside hyperspectral imaging indicates a microcirculatory sepsis pattern—An observational study. Microvasc. Res. 2021, 136, 104164. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Cancio, L.C.; Batchinsky, A.I.; Mansfield, J.R.; Panasyuk, S.; Hetz, K.; Martini, D.; Jordan, B.S.; Tracey, B.; Freeman, J.E. Hyperspectral Imaging: A New Approach to the Diagnosis of Hemorrhagic Shock. J. Trauma Inj. Infect. Crit. Care 2006, 60, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Langeland, H.; Lyng, O.; Aadahl, P.; Skjærvold, N. The coherence of macrocirculation, microcirculation, and tissue metabolic response during nontraumatic hemorrhagic shock in swine. Physiol. Rep. 2017, 5, e13216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, S.D.; Naumann, D.N.; Hopkins, P.; Mellis, C.; Riozzi, P.; Sartini, S.; Mamuza, J.; Harris, T.; Midwinter, M.J.; Wendon, J. Microcirculatory Impairment Is Associated With Multiple Organ Dysfunction Following Traumatic Hemorrhagic Shock. Crit. Care Med. 2018, 46, e889–e896. [Google Scholar] [CrossRef]
- Kazune, S.; Caica, A.; Volceka, K.; Suba, O.; Rubins, U.; Grabovskis, A. Relationship of mottling score, skin microcirculatory perfusion indices and biomarkers of endothelial dysfunction in patients with septic shock: An observational study. Crit. Care 2019, 23, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öveges, N.; László, I.; Tánczos, K.; Németh, M.; Lebák, G.; Tudor-Drobjewski, B.-A.; Érces, D.; Kaszaki, J.; Rudas, L.; Huber, W.; et al. Mean arterial pressure targeted fluid resuscitation may lead to fluid overload: A bleeding-resuscitation animal experiment. PLoS ONE 2018, 13, e0196188. [Google Scholar] [CrossRef]
- Annane, D.; Ouanes-Besbes, L.; De Backer, D.; Du, B.; Gordon, A.C.; Hernandez, G.; Olsen, K.M.; Osborn, T.M.; Peake, S.; Russell, J.A.; et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 2018, 44, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Gan, T.J. Peri-operative fluid management to enhance recovery. Anaesthesia 2016, 71, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Meier-Hellmann, A.; Reinhart, K. Effects of catecholamines on regional perfusion and oxygenation in critically ill patients. Acta Anaesthesiol. Scand. 1995, 39, 239–248. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Frick, R.W.; Goetze, E.; Gielisch, M.; Al-Nawas, B.; Kämmerer, P.W. Hyperspectral analysis for perioperative perfusion monitoring—a clinical feasibility study on free and pedicled flaps. Clin. Oral Investig. 2021, 25, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients—A Systematic Review and Meta-Analysis of Observational Studies. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, D.-W.; Qu, J.; Zeng, X.-A.; Pu, H.; Ma, J. Feasibility of using hyperspectral imaging to predict moisture content of porcine meat during salting process. Food Chem. 2014, 152, 197–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, M.; Özdemir, B.; Gruneberg, D.; Petersen, C.; Studier-Fischer, A.; von der Forst, M.; Schmitt, F.C.F.; Fiedler, M.O.; Nickel, F.; Müller-Stich, B.P.; et al. Hyperspectral Imaging for the Evaluation of Microcirculatory Tissue Oxygenation and Perfusion Quality in Haemorrhagic Shock: A Porcine Study. Biomedicines 2021, 9, 1829. https://doi.org/10.3390/biomedicines9121829
Dietrich M, Özdemir B, Gruneberg D, Petersen C, Studier-Fischer A, von der Forst M, Schmitt FCF, Fiedler MO, Nickel F, Müller-Stich BP, et al. Hyperspectral Imaging for the Evaluation of Microcirculatory Tissue Oxygenation and Perfusion Quality in Haemorrhagic Shock: A Porcine Study. Biomedicines. 2021; 9(12):1829. https://doi.org/10.3390/biomedicines9121829
Chicago/Turabian StyleDietrich, Maximilian, Berkin Özdemir, Daniel Gruneberg, Clara Petersen, Alexander Studier-Fischer, Maik von der Forst, Felix C. F. Schmitt, Mascha O. Fiedler, Felix Nickel, Beat Peter Müller-Stich, and et al. 2021. "Hyperspectral Imaging for the Evaluation of Microcirculatory Tissue Oxygenation and Perfusion Quality in Haemorrhagic Shock: A Porcine Study" Biomedicines 9, no. 12: 1829. https://doi.org/10.3390/biomedicines9121829
APA StyleDietrich, M., Özdemir, B., Gruneberg, D., Petersen, C., Studier-Fischer, A., von der Forst, M., Schmitt, F. C. F., Fiedler, M. O., Nickel, F., Müller-Stich, B. P., Brenner, T., Weigand, M. A., Uhle, F., & Schmidt, K. (2021). Hyperspectral Imaging for the Evaluation of Microcirculatory Tissue Oxygenation and Perfusion Quality in Haemorrhagic Shock: A Porcine Study. Biomedicines, 9(12), 1829. https://doi.org/10.3390/biomedicines9121829








