Tissue Engineering of the Urethra: From Bench to Bedside
Abstract
:1. Introduction
2. Methods and Study Selection
3. Results
3.1. Small Intestinal Submucosa Grafts (SIS)
3.2. Bladder-Derived Matrices
3.3. Acellular Dermis Graft
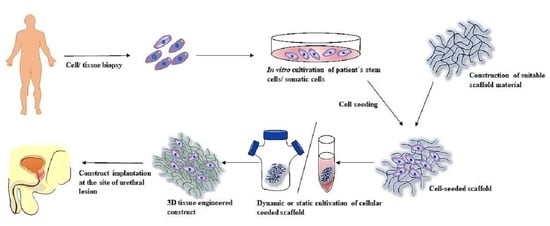
3.4. Tissue Engineering Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengupta, D.; Waldman, S.D.; Li, S. From in Vitro to in Situ Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 1537–1545. [Google Scholar] [CrossRef]
- Qiu, J.; Li, J.; Wang, G.; Zheng, L.; Ren, N.; Liu, H.; Tang, W.; Jiang, H.; Wang, Y. In Vitro Investigation on the Biodegradability and Biocompatibility of Genipin Cross-Linked Porcine Acellular Dermal Matrix with Intrinsic Fluorescence. ACS Appl. Mater. Interfaces 2013, 5, 344–350. [Google Scholar] [CrossRef]
- Barbagli, G.; Heidenreich, A.; Zugor, V.; Karapanos, L.; Lazzeri, M. Urothelial or Oral Mucosa Cells for Tissue-Engineered Urethroplasty: A Critical Revision of the Clinical Outcome. Asian J. Urol. 2020, 7, 18–23. [Google Scholar] [CrossRef]
- Verla, W.; Oosterlinck, W.; Spinoit, A.-F.; Waterloos, M. A Comprehensive Review Emphasizing Anatomy, Etiology, Diagnosis, and Treatment of Male Urethral Stricture Disease. Biomed. Res. Int. 2019, 2019, 9046430. [Google Scholar] [CrossRef] [PubMed]
- Pierik, F.H.; Burdorf, A.; Nijman, J.M.R.; de Muinck Keizer-Schrama, S.M.P.F.; Juttmann, R.E.; Weber, R.F.A. A High Hypospadias Rate in The Netherlands. Hum. Reprod. 2002, 17, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Mundy, A.R. Management of Urethral Strictures. Postgrad Med. J. 2006, 82, 489–493. [Google Scholar] [CrossRef]
- Davis, N.F.; Quinlan, M.R.; Bhatt, N.R.; Browne, C.; MacCraith, E.; Manecksha, R.; Walsh, M.T.; Thornhill, J.A.; Mulvin, D. Incidence, Cost, Complications and Clinical Outcomes of Iatrogenic Urethral Catheterization Injuries: A Prospective Multi-Institutional Study. J. Urol. 2016, 196, 1473–1477. [Google Scholar] [CrossRef]
- Steenkamp, J.W.; Heyns, C.F.; de Kock, M.L. Internal Urethrotomy versus Dilation as Treatment for Male Urethral Strictures: A Prospective, Randomized Comparison. J. Urol. 1997, 157, 98–101. [Google Scholar] [CrossRef]
- Al Taweel, W.; Seyam, R. Visual Internal Urethrotomy for Adult Male Urethral Stricture Has Poor Long-Term Results. Adv. Urol. 2015, 2015, 656459. [Google Scholar] [CrossRef] [Green Version]
- Redón-Gálvez, L.; Molina-Escudero, R.; Álvarez-Ardura, M.; Otaola-Arca, H.; Alarcón Parra, R.O.; Páez-Borda, Á. Predictors of Urethral Stricture Recurrence after Endoscopic Urethrotomy. Actas Urol. Esp. 2016, 40, 529–533. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Jiang, L.; Wang, L.; Wang, K. Safety and Efficacy of Laser and Cold Knife Urethrotomy for Urethral Stricture. Chin. Med. J. (Eng.) 2010, 123, 1589–1595. [Google Scholar]
- EAU. EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan 2021; EAU: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Mangera, A.; Patterson, J.M.; Chapple, C.R. A Systematic Review of Graft Augmentation Urethroplasty Techniques for the Treatment of Anterior Urethral Strictures. Eur. Urol. 2011, 59, 797–814. [Google Scholar] [CrossRef]
- Soave, A.; Dahlem, R.; Pinnschmidt, H.O.; Rink, M.; Langetepe, J.; Engel, O.; Kluth, L.A.; Loechelt, B.; Reiss, P.; Ahyai, S.A.; et al. Substitution Urethroplasty with Closure Versus Nonclosure of the Buccal Mucosa Graft Harvest Site: A Randomized Controlled Trial with a Detailed Analysis of Oral Pain and Morbidity. Eur. Urol. 2018, 73, 910–922. [Google Scholar] [CrossRef]
- Sinha, R.J.; Singh, V.; Sankhwar, S.N.; Dalela, D. Donor Site Morbidity in Oral Mucosa Graft Urethroplasty: Implications of Tobacco Consumption. BMC Urol. 2009, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, T.L.; Erickson, B.; Medendorp, A.; Gonzalez, C.M. Comparison of Donor Site Intraoral Morbidity after Mucosal Graft Harvesting for Urethral Reconstruction. Urology 2005, 66, 716–720. [Google Scholar] [CrossRef]
- Atala, A.; Guzman, L.; Retik, A.B. A Novel Inert Collagen Matrix for Hypospadias Repair. J. Urol. 1999, 162, 1148–1151. [Google Scholar] [CrossRef]
- Mantovani, F.; Trinchieri, A.; Castelnuovo, C.; Romanò, A.L.; Pisani, E. Reconstructive Urethroplasty Using Porcine Acellular Matrix. Eur. Urol. 2003, 44, 600–602. [Google Scholar] [CrossRef]
- el-Kassaby, A.W.; Retik, A.B.; Yoo, J.J.; Atala, A. Urethral Stricture Repair with an Off-the-Shelf Collagen Matrix. J. Urol. 2003, 169, 170–173; discussion 173. [Google Scholar] [CrossRef]
- le Roux, P.J. Endoscopic Urethroplasty with Unseeded Small Intestinal Submucosa Collagen Matrix Grafts: A Pilot Study. J. Urol. 2005, 173, 140–143. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Bullocks, J.M.; Basu, C.B.; Bienstock, A.; Link, R.; Kozovska, M.; Hollier, L.; Yuksel, E. Dermal Composite Flaps Reconstructed from Acellular Dermis: A Novel Method of Neourethral Reconstruction. Plast. Reconstr. Surg. 2005, 115, 96e–100e. [Google Scholar] [CrossRef] [PubMed]
- Donkov, I.I.; Bashir, A.; Elenkov, C.H.G.; Panchev, P.K. Dorsal Onlay Augmentation Urethroplasty with Small Intestinal Submucosa: Modified Barbagli Technique for Strictures of the Bulbar Urethra. Int. J. Urol. 2006, 13, 1415–1417. [Google Scholar] [CrossRef]
- Hauser, S.; Bastian, P.J.; Fechner, G.; Müller, S.C. Small Intestine Submucosa in Urethral Stricture Repair in a Consecutive Series. Urology 2006, 68, 263–266. [Google Scholar] [CrossRef]
- Fiala, R.; Vidlar, A.; Vrtal, R.; Belej, K.; Student, V. Porcine Small Intestinal Submucosa Graft for Repair of Anterior Urethral Strictures. Eur. Urol. 2007, 51, 1702–1708; discussion 1708. [Google Scholar] [CrossRef]
- Palminteri, E.; Berdondini, E.; Colombo, F.; Austoni, E. Small Intestinal Submucosa (SIS) Graft Urethroplasty: Short-Term Results. Eur. Urol. 2007, 51, 1695–1701; discussion 1701. [Google Scholar] [CrossRef]
- Fossum, M.; Svensson, J.; Kratz, G.; Nordenskjöld, A. Autologous in Vitro Cultured Urothelium in Hypospadias Repair. J. Pediatr. Urol. 2007, 3, 10–18. [Google Scholar] [CrossRef]
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-Engineered Buccal Mucosa Urethroplasty-Clinical Outcomes. Eur. Urol. 2008, 53, 1263–1269. [Google Scholar] [CrossRef]
- el-Kassaby, A.; AbouShwareb, T.; Atala, A. Randomized Comparative Study between Buccal Mucosal and Acellular Bladder Matrix Grafts in Complex Anterior Urethral Strictures. J. Urol. 2008, 179, 1432–1436. [Google Scholar] [CrossRef]
- Farahat, Y.A.; Elbahnasy, A.M.; El-Gamal, O.M.; Ramadan, A.R.; El-Abd, S.A.; Taha, M.R. Endoscopic Urethroplasty Using Small Intestinal Submucosal Patch in Cases of Recurrent Urethral Stricture: A Preliminary Study. J. Endourol. 2009, 23, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-Engineered Autologous Urethras for Patients Who Need Reconstruction: An Observational Study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Fossum, M.; Skikuniene, J.; Orrego, A.; Nordenskjöld, A. Prepubertal Follow-up after Hypospadias Repair with Autologous in Vitro Cultured Urothelial Cells. Acta Paediatr. 2012, 101, 755–760. [Google Scholar] [CrossRef]
- Palminteri, E.; Berdondini, E.; Fusco, F.; De Nunzio, C.; Salonia, A. Long-Term Results of Small Intestinal Submucosa Graft in Bulbar Urethral Reconstruction. Urology 2012, 79, 695–701. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Fu, Q.; Sa, Y.-L.; Zhang, J.; Song, L.-J.; Feng, C. Outcome of Small Intestinal Submucosa Graft for Repair of Anterior Urethral Strictures. Int. J. Urol. 2013, 20, 622–629. [Google Scholar] [CrossRef]
- Barbagli, G.; Akbarov, I.; Heidenreich, A.; Zugor, V.; Olianas, R.; Aragona, M.; Romano, G.; Balsmeyer, U.; Fahlenkamp, D.; Rebmann, U.; et al. Anterior Urethroplasty Using a New Tissue Engineered Oral Mucosa Graft: Surgical Techniques and Outcomes. J. Urol. 2018, 200, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Ram-Liebig, G.; Barbagli, G.; Heidenreich, A.; Fahlenkamp, D.; Romano, G.; Rebmann, U.; Standhaft, D.; van Ahlen, H.; Schakaki, S.; Balsmeyer, U.; et al. Results of Use of Tissue-Engineered Autologous Oral Mucosa Graft for Urethral Reconstruction: A Multicenter, Prospective, Observational Trial. EBioMedicine 2017, 23, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Mandal, T.K.; Dhanuka, S.; Choudhury, S.; Mukhopadhyay, B.C.; Kayal, A.; Majhi, T.K.; Mondal, M. Tissue Engineered Indigenous Pericardial Patch Urethroplasty: A Promising Solution to a Nagging Problem. Asian J. Urol. 2020, 7, 56–60. [Google Scholar] [CrossRef]
- Fisher, M.B.; Mauck, R.L. Tissue Engineering and Regenerative Medicine: Recent Innovations and the Transition to Translation. Tissue Eng. Part B Rev. 2013, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culenova, M.; Nicodemou, A.; Novakova, Z.V.; Debreova, M.; Smolinská, V.; Bernatova, S.; Ivanisova, D.; Novotna, O.; Vasicek, J.; Varga, I.; et al. Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells. Int. J. Mol. Sci. 2021, 22, 12503. [Google Scholar] [CrossRef]
- Dublin, N.; Stewart, L.H. Oral Complications after Buccal Mucosal Graft Harvest for Urethroplasty. BJU Int. 2004, 94, 867–869. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, M.-X.; Zhou, Z.; Zhang, K.; Zhou, J.; Zhao, Y.; Wang, Z.; Lu, M.-J. The Differentiation of Human Adipose-Derived Stem Cells towards a Urothelium-like Phenotype in Vitro and the Dynamic Temporal Changes of Related Cytokines by Both Paracrine and Autocrine Signal Regulation. PLoS ONE 2014, 9, e95583. [Google Scholar] [CrossRef] [Green Version]
- Versteegden, L.R.M.; de Jonge, P.K.J.D.; IntHout, J.; van Kuppevelt, T.H.; Oosterwijk, E.; Feitz, W.F.J.; de Vries, R.B.M.; Daamen, W.F. Tissue Engineering of the Urethra: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Eur. Urol. 2017, 72, 594–606. [Google Scholar] [CrossRef]
- Dorin, R.P.; Pohl, H.G.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized Urethral Replacement with Unseeded Matrices: What Is the Maximum Distance for Normal Tissue Regeneration? World J. Urol. 2008, 26, 323–326. [Google Scholar] [CrossRef]
- Barbagli, G.; Sansalone, S.; Djinovic, R.; Romano, G.; Lazzeri, M. Current Controversies in Reconstructive Surgery of the Anterior Urethra: A Clinical Overview. Int. Braz. J. Urol. 2012, 38, 307–316; discussion 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundy, A.R.; Andrich, D.E. Urethral Strictures. BJU Int. 2011, 107, 6–26. [Google Scholar] [CrossRef]
- Atala, A. Regenerative Bladder Augmentation Using Autologous Tissue-When Will We Get There? J. Urol. 2014, 191, 1204–1205. [Google Scholar] [CrossRef]
- Curry, J.I.; Reeves, B.; Stringer, M.D. Randomized Controlled Trials in Pediatric Surgery: Could We Do Better? J. Pediatr. Surg. 2003, 38, 556–559. [Google Scholar] [CrossRef]
- Faure, A.; Bouty, A.; Nyo, Y.L.; O’Brien, M.; Heloury, Y. Two-Stage Graft Urethroplasty for Proximal and Complicated Hypospadias in Children: A Retrospective Study. J. Pediatr. Urol. 2016, 12, 286.e1–286.e7. [Google Scholar] [CrossRef]
- Trommelmans, L.; Dierickx, K. Standard of Care in Clinical Research with Human Tissue Engineered Products (HTEPs). Am. J. Bioeth. 2009, 9, 44–45. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Karanicolas, P.J.; Thoma, A.; Simunovic, M.; Bhandari, M.; Devereaux, P.J.; Anvari, M.; Adili, A.; Guyatt, G. Randomized Controlled Trials of Surgical Interventions. Ann. Surg. 2010, 251, 409–416. [Google Scholar] [CrossRef]
- Oerlemans, A.J.M.; Feitz, W.F.J.; van Leeuwen, E.; Dekkers, W.J.M. Regenerative Urology Clinical Trials: An Ethical Assessment of Road Blocks and Solutions. Tissue Eng. Part B Rev. 2013, 19, 41–47. [Google Scholar] [CrossRef]
- Schroeck, F.R.; Jacobs, B.L.; Bhayani, S.B.; Nguyen, P.L.; Penson, D.; Hu, J. Cost of New Technologies in Prostate Cancer Treatment: Systematic Review of Costs and Cost Effectiveness of Robotic-Assisted Laparoscopic Prostatectomy, Intensity-Modulated Radiotherapy, and Proton Beam Therapy. Eur. Urol. 2017, 72, 712–735. [Google Scholar] [CrossRef]
- Multistakeholder Advanced Therapy Medicinal Products (ATMPs) Expert Meeting: Exploring Solutions to Foster ATMPs’ Development and Patient Access in Europe European Medicines Agency. Available online: https://www.ema.europa.eu/en/events/multistakeholder-advanced-therapy-medicinal-products-atmps-expert-meeting-exploring-solutions (accessed on 22 September 2021).
- Ram-Liebig, G.; Bednarz, J.; Stuerzebecher, B.; Fahlenkamp, D.; Barbagli, G.; Romano, G.; Balsmeyer, U.; Spiegeler, M.-E.; Liebig, S.; Knispel, H. Regulatory Challenges for Autologous Tissue Engineered Products on Their Way from Bench to Bedside in Europe. Adv. Drug Deliv. Rev. 2015, 82–83, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Regulation(EC) No. 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02007R1394-20190726&from=en (accessed on 22 September 2021).

| Material | Technique | Location | Follow-Up in Months (Mean) | Number of Patients | Results | Ref |
|---|---|---|---|---|---|---|
| collagen-based inert matrix - bladder submucosal graft | dorsal onlay | hypospadias - meatus penoscrotal 3 patients - meatus scrotal 1 patient | 22 | 4 | 1 patient with subglandular fistula repaired using standard techniques all 4 patients as success | [17] |
| SIS | dorsal onlay | complete urethral stricture | 16 | 1 | 100% success | [18] |
| bladder submucosa collagen based inert matrix | ventral onlay | N/A | 37 | 28 | 24 patients (86%) success 4 patients slight caliber decrease | [19] |
| Unseeded SIS | endoscopic urethroplasty | bulbar urethral strictures | 24 | 9 | 2 patients success (25%) 6 patients as failure 1 lost during follow up | [20] |
| acellular dermis (AlloDerm) + buccal mucosa | dorsal onlay + buccal mucosa ventral cover | 4 cm segment of ventral penile urethra | 6 | 1 | 100% success | [21] |
| SIS | dorsal onlay technique | bulbar urethras | 18 | 9 | 8 patients success (89%) | [22] |
| SIS | dorsal onlay substitution urethroplasty | 2 bulbar stricture 3 penile-bulbar stricture | 14 | 5 | 1 patient success (20%) | [23] |
| SIS | onlay urethroplasty | 10 patients bulbar urethra 31 patients bulbopenile area 9 patients distal penile urethra | 31.2 | 50 | 40 patients success (80%) | [24] |
| SIS | 14 patients dorsal inlay, 1 patient ventral onlay 5 patients dorsal onlay plus ventral onlay. | Anterior urethral stricture | 21 | 20 | 17 cases success (85%) | [25] |
| in vitro cultured urothelial cells on acellular dermis | onlay | scrotal or perineal hypospadias | 52 | 6 | 6 cases as success (100%) | [26] |
| autologous tissue-engineered buccal mucosa | dorsal onlay technique | urethral stricture secondary to to lichen sclerosus | 33.6 | 5 | 0 | [27] |
| acellular bladder matrix (BAMG) and buccal mucosa | ventral onlay | 11 patients bulbar stricture 7 pendulous stricture 12 combined | 25 | 30 | 2 patients lost during follow-up buccal mucosa 15 (100%) BAMG 10 patients success (66%) | [28] |
| SIS | SIS endoscopically placed | bulbar urethral stricture | 14.25 | 10 | 8 patients as success (80%) | [29] |
| seeded tubularised polyglycolic acid: poly(lactide-co-glycolide acid) scaffolds | urethral tubularised posterior urethroplasty | 3 patients posterior urethral disruption 2 patients with previous failed posterior urethral repairs | 71 | 5 | 100% success | [30] |
| seeded acellular dermis | ventral onlay | scrotal or perineal hypospadias | 87 | 6 | 100% success | [31] |
| SIS | dorsal/ventral or dorsal plus ventral onlay | bulbar strictures (non-obliterative) | 71 | 25 | 19 (76%) success | [32] |
| SIS | Augmentation urethroplasty Onlay and inlay technique | 8 patients bulbar urethra 9 patients bulbopenile area 10 patients distal penile urethra 1 patient after failed hypospadias repair | 24.8 | 28 | 24 patients success (85%) | [33] |
| TE autologous oral mucosa graft MukoCell® | ventral onlay, dorsal onlay, dorsal inlay and combined | penile in 3 (7.9%) cases, bulbar in 29 (76.3%), peno-bulbar in 6 (15.8%) | 55 | 38 | 32 patients (84.2%) as success | [34] |
| TE autologous oral mucosa graft MukoCell® | ventral onlay | any etiology, location, length and severity | 24 | 99 | success rate 70.8% (46 of 65) and 76.9% (30 of 39) | [35] |
| acellular TE bovine pericardial patch | dorsal onlay technique | long segment anterior urethral strictures (involving penile and/or bulbar urethra | 8 | 9 | 8 (88.9%) success | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorek, D.; Culenova, M.; Csobonyeiova, M.; Skuciova, V.; Danisovic, L.; Ziaran, S. Tissue Engineering of the Urethra: From Bench to Bedside. Biomedicines 2021, 9, 1917. https://doi.org/10.3390/biomedicines9121917
Pastorek D, Culenova M, Csobonyeiova M, Skuciova V, Danisovic L, Ziaran S. Tissue Engineering of the Urethra: From Bench to Bedside. Biomedicines. 2021; 9(12):1917. https://doi.org/10.3390/biomedicines9121917
Chicago/Turabian StylePastorek, Dusan, Martina Culenova, Maria Csobonyeiova, Veronika Skuciova, Lubos Danisovic, and Stanislav Ziaran. 2021. "Tissue Engineering of the Urethra: From Bench to Bedside" Biomedicines 9, no. 12: 1917. https://doi.org/10.3390/biomedicines9121917