The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension
Abstract
:1. Introduction
2. Oxidative Stress and Hypertension
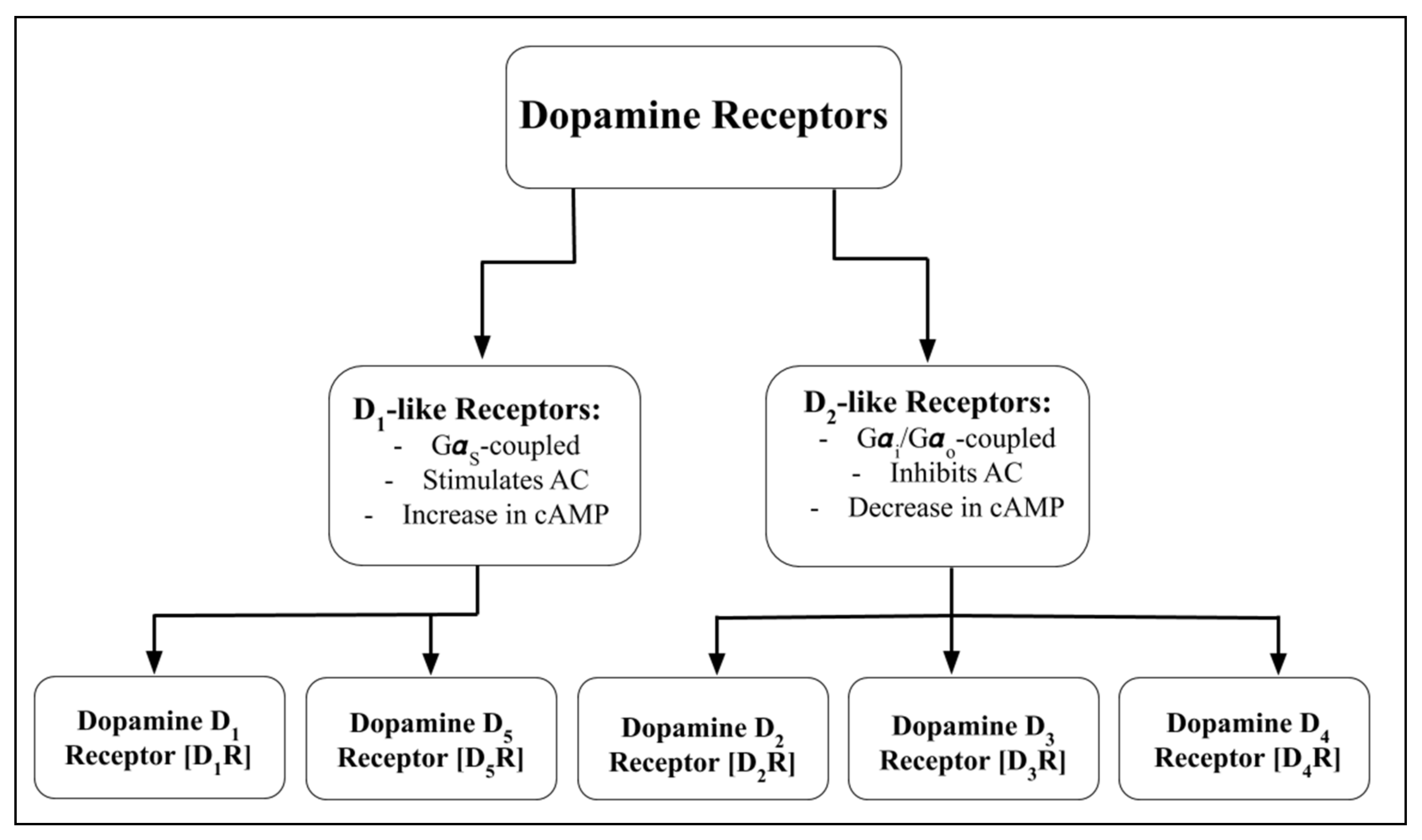
3. Renal Dopaminergic System
4. Impaired Dopamine Receptor Function and Hypertension
5. Renal Dopamine D1 Receptor [D1R], Oxidative Stress, and Hypertension
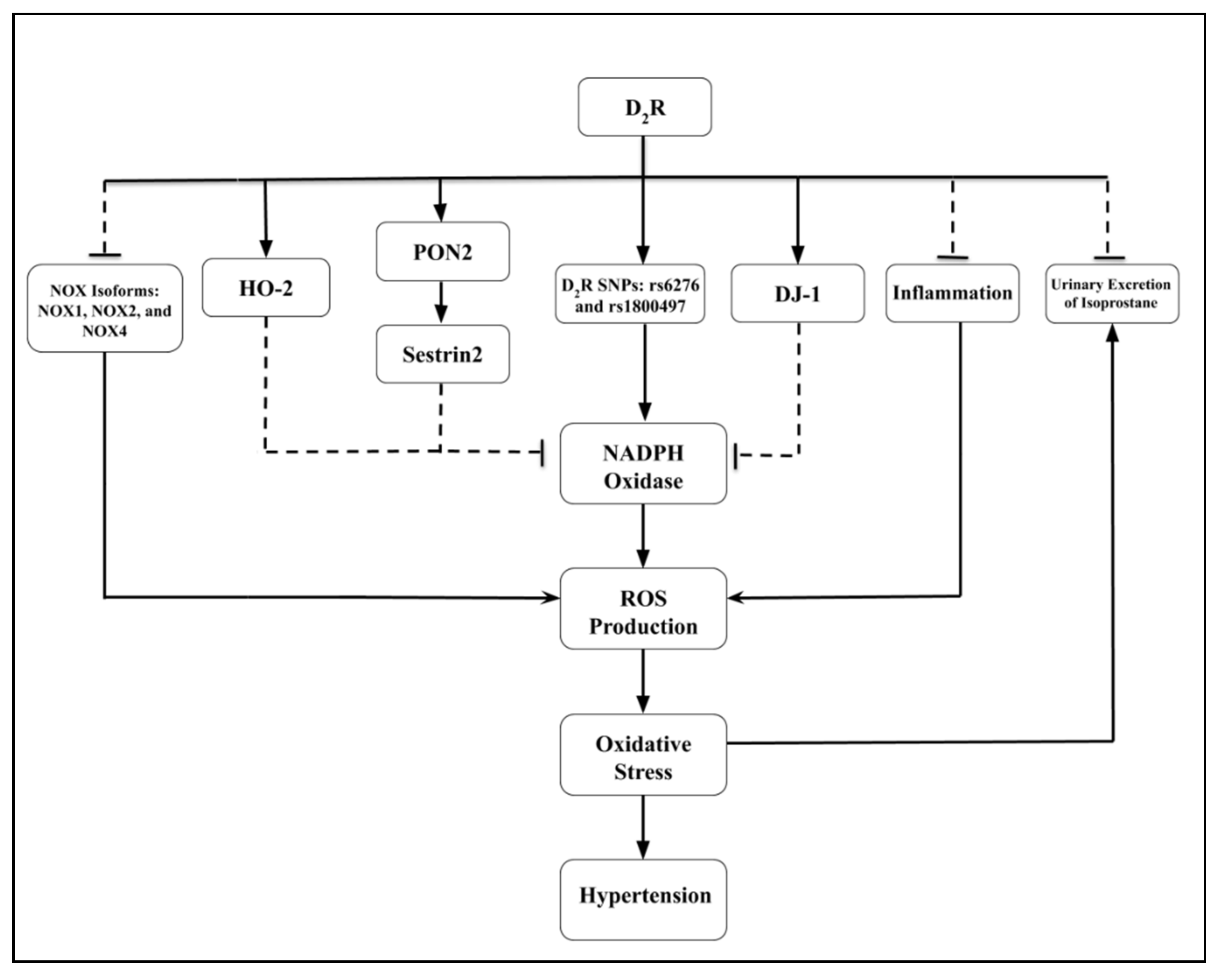
6. Renal Dopamine D2 Receptor [D2R], Oxidative Stress, and Hypertension
7. Renal Dopamine D3 Receptor [D3R], Oxidative Stress, and Hypertension
8. Renal Dopamine D4 Receptor [D4R], Oxidative Stress, and Hypertension
9. Renal Dopamine D5 Receptor [D5R], Oxidative Stress, and Hypertension
10. Renal Dopaminergic and Renin-Angiotensin Systems Interaction in Oxidative Stress and Inflammation
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luft, F.C. Molecular genetics of human Hypertension. Curr. Opin. Cardiol. 2020, 35, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Padmanabhan, S.; Iwashima, Y.; Yamagishi, K.; Goto, A. Gene and environmental interactions according to the components of lifestyle modifications in hypertension guidelines. Environ. Health Prev. Med. 2019, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Giorgini, P.; Di Giosia, P.; Grassi, D.; Rubenfire, M.; Brook, R.D.; Ferri, C. Air Pollution Exposure and Blood Pressure: An Updated Review of the Literature. Curr. Pharm. Des. 2016, 22, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Riggs, D.W.; Conklin, D.J. Environmental Determinants of Hypertension and Diabetes Mellitus: Sounding Off About the Effects of Noise. J. Am. Heart Assoc. 2020, 9, e016048. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Geladari, E.; Kounatidis, D. Microbiome and hypertension: Where are we now? J. Cardiovasc. Med. (Hagerstown) 2020, 21, 83–88. [Google Scholar] [CrossRef]
- Baumgartner, J.; Schauer, J.J.; Ezzati, M.; Lu, L.; Cheng, C.; Patz, J.A.; Bautista, L.E. Indoor air pollution and blood pressure in adult women living in rural China. Environ. Health Perspect. 2011, 119, 1390–1395. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Ye, Z.; Zheng, S.; Ren, H.; Zeng, J.; Wang, X.; Jose, P.A.; Chen, K.; Zeng, C. Long-Term Exposure of Fine Particulate Matter Causes Hypertension by Impaired Renal D1 Receptor-Mediated Sodium Excretion via Upregulation of G-Protein-Coupled Receptor Kinase Type 4 Expression in Sprague-Dawley Rats. J. Am. Heart Assoc. 2018, 7, e007185. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Asico, L.D.; Zanos, P.; Mahabeleshwar, G.H.; Singh Gangwar, R.; Xia, C.; Duan, L.; Cisse, Y.M.; Rengasamy, P.; Jose, P.A.; et al. Alpha2B-Adrenergic Receptor Overexpression in the Brain Potentiate Air Pollution-induced Behavior and Blood Pressure Changes. Toxicol. Sci. 2019, 169, 95–107. [Google Scholar] [CrossRef]
- Ye, Z.; Lu, X.; Deng, Y.; Wang, X.; Zheng, S.; Ren, H.; Zhang, M.; Chen, T.; Jose, P.A.; Yang, J.; et al. In Utero Exposure to Fine Particulate Matter Causes Hypertension Due to Impaired Renal Dopamine D1 Receptor in Offspring. Cell. Physiol. Biochem. 2018, 46, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, D.J.; Mattson, D.L. Inflammatory macrophages in the kidney contribute to salt–Sensitive Hypertension. Am. J. Physiol. Ren. Physiol. 2020, 318, F544–F548. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, S.; Luft, F.C.; Titze, J.; Kitada, K. Sodium Handling and Interaction in Numerous Organs. Am. J. Hypertens. 2020, 33, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Robles-Vera, I.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Jiménez, R.; Moleón, J.; González-Correa, C.; Romero, M.; Yang, T.; Raizada, M.K.; et al. Mycophenolate Improves Brain-Gut Axis Inducing Remodeling of Gut Microbiota in DOCA–Salt Hypertensive Rats. Antioxidants 2020, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jose, P.A.; Zeng, C. Gastrointestinal–Renal Axis: Role in the Regulation of Blood Pressure. J. Am. Heart Assoc. 2017, 6, e005536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 63. [Google Scholar] [CrossRef] [Green Version]
- Soares-da–Silva, P.; Cabral, J.M.; Magalhães, D.; Fraga, S.; Magro, F. Amine neurotransmitters, inflammation and epithelial sodium transport. Exp. Physiol. 2016, 101, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.Y.; Li, Y.; Li, L.S.; Li, X.F.; Zheng, L.F.; Zhang, X.L.; Fan, R.F.; Song, J.; Hong, F.; Zhang, Y.; et al. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl. Res. 2013, 161, 486–494. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Wang, S.; Fan, X.; Wu, G.; Yang, H.; Yin, H.; Yang, S.; Harris, R.C. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J. Clin. Investig. 2011, 121, 2845–2854. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Yang, Y.; Yang, J.; Asico, L.D.; Chen, W.; Felder, R.A.; Armando, I.; Jose, P.A.; Yang, Z. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E1–E10. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Siragy, H.M.; Felder, R.A.; Carey, R.M. Intrarenal dopamine production and distribution in the rat. Physiological control of sodium excretion. Hypertension 1997, 29, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, S.S.; Lokhandwala, M.F. Stimulation of renal dopamine production during acute volume expansion requires the presence of intact vagi but not renal nerves. Clin. Exp. Hypertens. A 1992, 14, 1169–1187. [Google Scholar] [CrossRef]
- Asico, L.D.; Eisner, G.M.; Jose, P.A. Renal nerves and D1-dopamine receptor-mediated natriuresis. Clin. Exp. Hypertens. 1998, 20, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Luippold, G.; Osswald, H.; Mühlbauer, B. Renal effects of exogenous dopamine: Modulation by renal nerves and dopamine receptor antagonists. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 358, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lokhandwala, M.F. Role of endogenous dopamine in the natriuretic response to various degrees of iso-osmotic volume expansion in rats. Clin. Exp. Hypertens. A 1991, 13, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Oates, N.S.; Ball, S.G.; Perkins, C.M.; Lee, M.R. Plasma and urine dopamine in man given sodium chloride in the diet. Clin. Sci. (Lond.) 1979, 56, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Hansell, P.; Fasching, A. The effect of dopamine receptor blockade on natriuresis is dependent on the degree of hypervolemia. Kidney Int. 1991, 39, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, M.E.; Albertoni Borghese, M.F.; Majowicz, M.P.; Ortiz, M.C.; Loidl, F.; Rey-Funes, M.; Di Ciano, L.A.; Ibarra, F.R. Concerted regulation of renal plasma flow and glomerular filtration rate by renal dopamine and NOS I in rats on high salt intake. Physiol. Rep. 2017, 5, e13202. [Google Scholar] [CrossRef]
- Barendregt, J.N.; Muizert, Y.; van Nispen tot Pannerden, L.L.; Chang, P.C. Intrarenal production of dopamine and natriuresis following DOPA and saline infusions in healthy human volunteers. J. Hum. Hypertens. 1995, 9, 187–194. [Google Scholar]
- Du, D.D.; Yoshinaga, M.; Sonoda, M.; Kawakubo, K.; Uehara, Y. Blood pressure reduction by Japanese traditional Miso is associated with increased diuresis and natriuresis through dopamine system in Dahl salt–Sensitive rats. Clin. Exp. Hypertens. 2014, 36, 359–366. [Google Scholar] [CrossRef]
- Vered, Y.; Grosskopf, I.; Palevitch, D.; Harsat, A.; Charach, G.; Weintraub, M.S.; Graff, E. The influence of Vicia faba (broad bean) seedlings on urinary sodium excretion. Planta Med. 1997, 63, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.M.; Cesar, T.S.; Lonce, S.; Ferguson, M.C.; Robertson, D. An increase in renal dopamine does not stimulate natriuresis after fava bean ingestion. Am. J. Clin. Nutr. 2013, 97, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennis, R.C.; Asico, L.D.; Armando, I.; Yang, J.; Feranil, J.B.; Jurgens, J.A.; Escano, C.S.; Yu, P., Jr.; Wang, X.; Sibley, D.R.; et al. Dopamine D₁-like receptors regulate the α₁A-adrenergic receptor in human renal proximal tubule cells and D₁-like dopamine receptor knockout mice. Am. J. Physiol. Ren. Physiol. 2014, 307, F1238–F1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, M.C. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern. Ther. Health Med. 2007, 13, S128–S133. [Google Scholar]
- Kawano, Y.; Kawasaki, T.; Kawazoe, N.; Abe, I.; Uezono, K.; Ueno, M.; Fukiyama, K.; Omae, T. Circadian variations of urinary dopamine, norepinephrine, epinephrine and sodium in normotensive and hypertensive subjects. Nephron. 1990, 55, 277–282. [Google Scholar] [CrossRef]
- Sulyok, E.; Gyódi, G.; Ertl, T.; Bódis, J.; Hartmann, G. The influence of NaCl supplementation on the postnatal development of urinary excretion of noradrenaline, dopamine, and serotonin in premature infants. Pediatr. Res. 1985, 19, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Vanpée, M.; Herin, P.; Lagercrantz, H.; Aperia, A. Effect of extreme prematurity on renal dopamine and norepinephrine excretion during the neonatal period. Pediatr. Nephrol. 1997, 11, 46–48. [Google Scholar] [CrossRef]
- Lakatua, D.J.; Nicolau, G.Y.; Bogdan, C.; Plinga, L.; Jachimowicz, A.; Sackett-Lundeen, L.; Petrescu, E.; Ungureanu, E.; Haus, E. Chronobiology of catecholamine excretion in different age groups. Prog. Clin. Biol. Res. 1987, 227B, 31–50. [Google Scholar]
- Gerlo, E.A.; Schoors, D.F.; Dupont, A.G. Age- and sex-related differences for the urinary excretion of norepinephrine, epinephrine, and dopamine in adults. Clin. Chem. 1991, 37, 875–878. [Google Scholar] [CrossRef]
- Young, J.B.; Troisi, R.J.; Weiss, S.T.; Parker, D.R.; Sparrow, D.; Landsberg, L. Relationship of catecholamine excretion to body size, obesity, and nutrient intake in middle-aged and elderly men. Am. J. Clin. Nutr. 1992, 56, 827–834. [Google Scholar] [CrossRef]
- Romero-Vecchione, E.; Vásquez, J.; Lema, G.; Guerrero, H.; Rosa, F.; Bermúdez, M. Low urinary dopamine excretion associated to low sodium excretion in normotensive Piaroa Amazonian ethnia compared to urban subjects. Investig. Clin. 1995, 36, 61–71. [Google Scholar]
- Chan, T.Y.; Critchley, J.A.; Ho, C.S.; Chan, J.C.; Tomlinson, B. Urinary dopamine outputs do not rise in healthy Chinese subjects during gradually increasing oral sodium intake over 8 days. J. Auton. Pharmacol. 1996, 16, 155–159. [Google Scholar] [PubMed]
- Critchley, J.A.; Makarananda, K.; Balali-Mood, M.; Sriwatanakul, K.; Lee, M.R. FurTher. ethnic differences in the renal sodium-dopamine relationship: Its uncoupling in Iranian but not in Thai normotensive subjects. J. Hypertens. Suppl. 1988, 6, S623–S625. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Takeshita, E.; Saruta, T.; Nagano, S.; Sekihara, T. Urinary dopamine excretion in normotensive subjects with or without family history of Hypertension. J. Hypertens. 1986, 4, 57–60. [Google Scholar] [CrossRef]
- Dazai, Y.; Iwata, T.; Hiwada, K. Augmentation of the renal tubular dopaminergic activity by oral calcium supplementation in patients with essential Hypertension. Am. J. Hypertens. 1993, 6, 933–937. [Google Scholar] [CrossRef]
- Ball, S.G.; Oats, N.S.; Lee, M.R. Urinary dopamine in man and rat: Effects of inorganic salts on dopamine excretion. Clin. Sci. Mol. Med. 1978, 55, 167–173. [Google Scholar] [CrossRef] [Green Version]
- López-Contreras, A.J.; Galindo, J.D.; López-García, C.; Castells, M.T.; Cremades, A.; Peñafiel, R. Opposite sexual dimorphism of 3,4-dihydroxyphenylalanine decarboxylase in the kidney and small intestine of mice. J. Endocrinol. 2008, 196, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, F.; Jose, P.A.; Ecelbarger, C.M. Reduction of renal dopamine receptor expression in obese Zucker rats: Role of sex and angiotensin II. Am. J. Physiol. Ren. Physiol. 2010, 299, F1164–F1170. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Iimura, O.; Yamaji, I.; Shibata, S.; Nishimura, M.; Aoki, K.; Nozawa, A.; Hasegawa, T.; Honma, C.; Kobayakawa, H. The pathophysiological role of water–Sodium balance and renal dopaminergic activity in overweight patients with essential Hypertension. J. Clin. Hypertens. 1987, 3, 3–11. [Google Scholar] [CrossRef]
- Sakamoto, T.; Chen, C.; Lokhandwala, M.F. Lack of renal dopamine production during acute volume expansion in Dahl salt–Sensitive rats. Clin. Exp. Hypertens. 1994, 16, 197–206. [Google Scholar] [CrossRef]
- Escano, C.S.; Armando, I.; Wang, X.; Asico, L.D.; Pascua, A.; Yang, Y.; Wang, Z.; Lau, Y.S.; Jose, P.A. Renal dopaminergic defect in C57Bl/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1660–R1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, S.; Asico, L.D.; Jose, P.A.; Konkalmatt, P. Renal hydrogen peroxide production prevents salt–Sensitive Hypertension. J. Am. Heart Assoc. 2020, 9, e013818. [Google Scholar] [CrossRef] [PubMed]
- Combe, R.; Mudgett, J.; El Fertak, L.; Champy, M.F.; Ayme-Dietrich, E.; Petit-Demouliere, B.; Sorg, T.; Herault, Y.; Madwed, J.B.; Monassier, L. How Does Circadian Rhythm Impact Salt Sensitivity of Blood Pressure in Mice? A Study in Two Close C57Bl/6 Substrains. PLoS ONE 2016, 11, e0153472. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Serrão, P.; Vieira-Coelho, M.A.; Pestana, M. Differences in the renal dopaminergic system activity between Wistar rats from two suppliers. Acta Physiol. Scand. 2003, 178, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Loperena, R.; Harrison, D. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.R.; Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Rosón, M.I.; Rodríguez Fermepin, M.; Fernández, B.E. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J. Nephrol. 2015, 4, 196–212. [Google Scholar] [CrossRef]
- Cuevas, S.; Villar, V.A.; Jose, P.A.; Armando, I. Renal dopamine receptors, oxidative stress, and Hypertension. Int. J. Mol. Sci. 2013, 14, 17553–17572. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Lokhandwala, M.F. Renal Dopamine Oxidation and Inflammation in High Salt Fed Rats. J. Am. Heart Assoc. 2020, 9, e014977. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Szeliga, M. Peroxiredoxins in Neurodegenerative Diseases. Antioxidants 2020, 9, 1203. [Google Scholar] [CrossRef]
- Foret, M.K.; Lincoln, R.; Do Carmo, S.; Cuello, A.C.; Cosa, G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757–12787. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative Stress in Chronic Kidney Disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejri, I.; Agapouda, A.; Grimm, A.; Eckert, A. Mitochondria-and Oxidative Stress-Targeting Substances in Cognitive Decline–Related Disorders: From Molecular Mechanisms to Clinical Evidence. Oxid. Med. Cell. Longev. 2019, 2019, 9695412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, O.M.; Gorman, G.S.; Lightowlers, R.N.; Turnbull, D.M. Mitochondrial Diseases: Hope for the Future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Lerman, A.; Lerman, L.O. Enhancing Mitochondrial Health to Treat Hypertension. Curr. Hypertens. Rep. 2018, 20, 89. [Google Scholar] [CrossRef]
- Cowley, A.W., Jr.; Abe, M.; Mori, T.; O’Connor, P.M.; Ohsaki, Y.; Zheleznova, N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and Hypertension. Am. J. Physiol. Ren. Physiol. 2015, 308, F179–F197. [Google Scholar] [CrossRef] [Green Version]
- Knock, G.A. NADPH oxidase in the vasculature: Expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in Hypertension. Free Radic. Biol. Med. 2019, 145, 385–427. [Google Scholar] [CrossRef]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Vicente, A.; Hong, N.; Garvin, J.L. Effects of reactive oxygen species on renal tubular transport. Am. J. Physiol. Ren. Physiol. 2019, 317, F444–F455. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Wilcox, C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [Green Version]
- Fellner, R.C.; Cook, A.K.; O’Connor, P.M.; Zhang, S.; Pollock, D.M.; Inscho, E.W. High–Salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am. J. Physiol. Ren. Physiol. 2014, 307, F33–F40. [Google Scholar] [CrossRef]
- Textor, S.C.; Gloviczki, M.L.; Flessner, M.F.; Calhoun, D.A.; Glockner, J.; Grande, J.P.; McKusick, M.A.; Cha, S.S.; Lerman, L.O. Association of filtered sodium load with medullary volumes and medullary hypoxia in hypertensive African Americans as compared with whites. Am. J. Kidney Dis. 2012, 59, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Al–Solaiman, Y.; Jesri, A.; Zhao, Y.; Morrow, J.D.; Egan, B.M. Low–Sodium DASH reduces oxidative stress and improves vascular function in salt–Sensitive humans. J. Hum. Hypertens. 2009, 23, 826–835. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Klawitter, J.; Chonchol, M.; Bassett, C.J.; Racine, M.L.; Seals, D.R. Effect of dietary sodium restriction on human urinary metabolomic profiles. Clin. J. Am. Soc. Nephrol. 2015, 10, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Murzabekova, G.; Egemnazarov, B.; Kraut, S.; Eisele, H.J.; Dumitrascu, R.; Heitmann, J.; Seimetz, M.; Witzenrath, M.; Ghofrani, H.A.; et al. Arterial hypertension in a murine model of sleep apnea: Role of NADPH oxidase 2. J. Hypertens. 2014, 32, 300–305. [Google Scholar] [CrossRef]
- Welch, W.J.; Chabrashvili, T.; Solis, G.; Chen, Y.; Gill, P.S.; Aslam, S.; Wang, X.; Ji, H.; Sandberg, K.; Jose, P.; et al. Role of Extracellular Superoxide Dismutase in the Mouse Angiotensin Slow Pressor Response. Hypertension 2006, 48, 934–941. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Cuevas, S.; Villar, V.A.; Escano, C.D.; Asico, L.; Yu, P.; Grandy, D.K.; Felder, R.A.; Armando, I.; et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic. Biol. Med. 2012, 53, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, S.; Zhang, Y.; Yang, Y.; Escano, C.; Asico, L.; Jones, J.E.; Armando, I.; Jose, P.A. Role of renal DJ-1 in the pathogenesis of hypertension associated with increased reactive oxygen species production. Hypertension 2012, 59, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cuevas, S.; Yang, S.; Villar, V.A.; Escano, C.; Asico, L.; Yu, P.; Jiang, X.; Weinman, E.J.; Armando, I.; et al. Sestrin2 decreases renal oxidative stress, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of reactive oxygen species production. Hypertension 2014, 64, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Harris, R. Antihypertensive mechanisms of intra-renal dopamine. Curr. Opin. Nephrol. Hypertens. 2015, 24, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Carey, R.M. The intrarenal renin-angiotensin and dopaminergic systems: Control of renal sodium excretion and blood pressure. Hypertension 2013, 61, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Konkalmatt, P.; Felder, R.A.; Jose, P.A. The renal dopaminergic system: Novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl. Res. 2015, 165, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.; Coffman, T.M. The kidney and hypertension: Novel insights from transgenic models. Curr. Opin. Nephrol. Hypertens. 2012, 21, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Taveira-da–Silva, R.; da Silva Sampaio, L.; Vieyra, A.; Einicker-Lamas, M. L-Tyr-Induced Phosphorylation of Tyrosine Hydroxylase at Ser40: An Alternative Route for Dopamine Synthesis and Modulation of Na+/K+-ATPase in Kidney Cells. Kidney Blood Press Res. 2019, 44. [Google Scholar] [CrossRef] [PubMed]
- Carranza, A.; Nowicki, S.; Barontini, M.; Armando, I. L-Dopa uptake and dopamine production in proximal tubular cells are regulated by β(2)-adrenergic receptors. Am. J. Physiol. Ren. Physiol. 2000, 279, F77–F83. [Google Scholar] [CrossRef] [Green Version]
- Wolfovitz, E.; Grossman, E.; Folio, C.J.; Keiser, H.R.; Kopin, I.J.; Goldstein, D.S. Derivation of urinary dopamine from plasma dihydroxyphenylalanine in humans. Clin. Sci. (Lond.) 1993, 84, 549–557. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamaji, Y.; Kitajima, W.; Saruta, T. Aromatic L-amino acid decarboxylase activity along the rat nephron. Am. J. Physiol. 1990, 258, F28–F33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Siragy, H.M.; Felder, R.A.; Carey, R.M. Preferential release of renal dopamine into the tubule lumen: Effect of chronic sodium loading. Clin. Exp. Hypertens. 1997, 19, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Allison, S.; Fader, D.; Claflin, V.; Baizer, L. Bovine dopamine β-hydroxylase cDNA. Complete coding sequence and expression in mammalian cells with vaccinia virus vector. J. Biol. Chem. 1990, 265, 1021–1028. [Google Scholar] [CrossRef]
- Catelas, D.; Serrão, M.; Soares-Da–Silva, P. Effects of nepicastat upon dopamine-β-hydroxylase activity and dopamine and norepinephrine levels in the rat left ventricle, kidney, and adrenal gland. Clin. Exp. Hypertens. 2019, 42, 118–125. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Soares-da–Silva, P. The activity of MAO A and B in rat renal cells and tubules. Life Sci. 1998, 62, 727–737. [Google Scholar] [CrossRef]
- Wang, Y.; Berndt, T.J.; Gross, J.M.; Peterson, M.A.; So, M.J.; Knox, F.G. Effect of inhibition of MAO and COMT on intrarenal dopamine and serotonin and on renal function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R248–R254. [Google Scholar] [CrossRef] [Green Version]
- Quelhas–Santos, J.; Serrão, M.P.; Soares–Silva, I.; Fernandes-Cerqueira, C.; Simões–Silva, L.; Pinho, M.J.; Remião, F.; Sampaio-Maia, B.; Desir, G.V.; Pestana, M. Renalase regulates peripheral and central dopaminergic activities. Am. J. Physiol. Ren. Physiol. 2015, 308, F84–F91. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, F.R.; Armando, I.; Nowicki, S.; Carranza, A.; De Luca Sarobe, V.; Arrizurieta, E.E.; Barontini, M. Dopamine is metabolised by different enzymes along the rat nephron. Pflug. Arch. 2005, 450, 185–191. [Google Scholar] [CrossRef]
- Correa, A.H.; Choi, M.R.; Gironacci, M.; Aprile, F.; Fernández, B.E. Atrial natriuretic factor decreases renal dopamine turnover and catabolism without modifying its release. Regul. Pept. 2008, 146, 238–242. [Google Scholar] [CrossRef]
- Soares-da–Silva, P.; Fernandes, M.H.; Pestana, M. A comparative study on the synthesis of dopamine in the human, dog and rat kidney. Acta Physiol. Scand. 1993, 148, 347–351. [Google Scholar] [CrossRef]
- Akama, H.; Noshiro, T.; Sano, N.; Watanabe, T.; Trigg, L.; Kotsonis, P.; Majewski, H.; McGrath, B.P.; Miura, Y.; Abe, K. Effects of isotonic saline loading on renal tubular and neurogenic dopamine release in conscious rabbits. Clin. Exp. Pharmacol. Physiol. 1995, 22, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Shimizu, K.; Way, D.; Secombe, J.; McGrath, B.P. The dopamine prodrug, gludopa, decreases both renal and extrarenal noradrenaline spillover in conscious rabbits. Clin. Exp. Pharmacol. Physiol. 1993, 20, 365–368. [Google Scholar] [CrossRef] [PubMed]
- De Brito Gariepy, H.; Carayon, P.; Ferrari, B.; Couture, R. Contribution of the central dopaminergic system in the anti-hypertensive effect of kinin B1 receptor antagonists in two rat models of Hypertension. Neuropeptides 2010, 44, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Hoffman, A.; Chang, P.C.; Keiser, H.R.; Goldstein, D.S. Increased spillover of dopa into arterial blood during dietary salt loading. Clin. Sci. (Lond.) 1990, 78, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yin, Y.; Li, Q.; Yu, J.; Liu, W.; Wang, D.; Cheng, Q.; Xie, S.; Cheng, X.; Qiu, L. Validation of an improved liquid chromatography tandem mass spectrometry method for rapid and simultaneous analysis of plasma catecholamine and their metabolites. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1129, 121805. [Google Scholar] [CrossRef] [PubMed]
- Miramontes-Gonzalez, J.P.; Hightower, C.M.; Zhang, K.; Kurosaki, H.; Schork, A.J.; Biswas, N.; Vaingankar, S.; Mahata, M.; Lipkowitz, M.S.; Nievergelt, C.M.; et al. A new common functional coding variant at the DDC gene change renal enzyme activity and modify renal dopamine function. Sci. Rep. 2019, 9, 5055. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [Green Version]
- Soares-da–Silva, P.; Pestana, M.; Fernandes, M.H. Involvement of tubular sodium in the formation of dopamine in the human renal cortex. J. Am. Soc. Nephrol. 1993, 3, 1591–1599. [Google Scholar]
- Vieira-Coelho, M.A.; Soares-da–Silva, P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundam. Clin. Pharmacol. 1993, 7, 235–243. [Google Scholar] [CrossRef]
- Baines, A.D. Functional effects of proximal tubular dopamine production. Am. J. Hypertens. 1990, 3, 68S–71S. [Google Scholar] [CrossRef]
- DeFeo, M.; Jadhav, A.; Lokhandwala, M. Dietary Sodium Intake and Urinary Dopamine and Sodium Excretion During the Course of Blood Pressure Development in Dahl Salt–Sensitive and Salt–Resistant Rats. Clin. Exp. Hypertens. A 1987, 9, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Hansell, P.; Ande’n, N.E.; Grabowska-Ande’n, M.; Ulfendahl, H.R. Atrial natriuretic factor, urinary catechol compounds and electrolyte excretion in rats during normal hydration and isotonic volume expansion. Influence of dopamine receptor blockade. Acta Physiol. Scand. 1988, 134, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Stull, R.; Eisenhofer, G.; Gill, J.R., Jr. Urinary excretion of dihydroxyphenylalanine and dopamine during alterations of dietary salt intake in humans. Clin. Sci. (Lond.) 1989, 76, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kuchel, O.; Kuchel, G. Peripheral dopamine in pathophysiology of Hypertension Interaction with aging and lifestyle. Hypertension 1991, 18, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armando, I.; Nowicki, S.; Aguirre, J.; Barontini, M. A decreased tubular uptake of dopa results in defective renal dopamine production in aged rats. Am. J. Physiol. 1995, 268, F1087–F1092. [Google Scholar] [CrossRef]
- Voorhess, M.L. Urinary catecholamine excretion by healthy children. I. Daily excretion of dopamine, norepinephrine, epinephrine, and 3-methoxy-4-hydroxymandelic acid. Pediatrics 1967, 39, 252–257. [Google Scholar]
- Vieira-Coelho, M.A.; Hussain, T.; Kansra, V.; Serrao, M.P.; Guimaraes, J.T.; Pestana, M.; Soares-Da–Silva, P.; Lokhandwala, M.F. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension 1999, 34, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.L.; Jayanthi, S.; McCoy, M.T.; Beauvais, G.; Cai, N.S. Dopamine D1 Receptors, Regulation of Gene Expression in the Brain, and Neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 526–538. [Google Scholar] [CrossRef]
- Saklayen, S.S.; Mabrouk, O.S.; Pehek, E.A. Negative Feedback Regulation of Nigrostriatal Dopamine Release: Mediation by Striatal D1 Receptors. J. Pharmacol. Exp. Ther. 2004, 311, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Lokhandwala, M.F. Dopamine receptors and Hypertension. Curr. Hypertens. Rep. 2008, 10, 268–275. [Google Scholar] [CrossRef]
- Armando, I.; Villar, V.A.; Jose, P.A. Dopamine and renal function and blood pressure regulation. Compr. Physiol. 2011, 1, 1075–1117. [Google Scholar] [CrossRef]
- Stansley, B.J.; Yamamoto, B.K. L-Dopa and Brain Serotonin System Dysfunction. Toxics 2015, 3, 75–88. [Google Scholar] [CrossRef]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.K.; Bhatnagar, P.; Chauhan, L.K.; Saxena, P.N.; Arun, J.; Chaudhari, B.P.; Patel, D.K.; et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef]
- Van den Buuse, M. Pressor responses to brain dopaminergic stimulation. Clin. Exp. Pharmacol. Physiol. 1997, 24, 764–769. [Google Scholar] [CrossRef]
- Sawamura, T.; Nakada, T. Role of dopamine in the striatum, renin-angiotensin system and renal sympathetic nerve on the development of two-kidney, one-clip Goldblatt Hypertension. J. Urol. 1996, 155, 1108–1111. [Google Scholar] [CrossRef]
- Moore, T.L.; Killiany, R.J.; Rosene, D.L.; Prusty, S.; Hollander, W.; Moss, M.B. Hypertension-induced changes in monoamine receptors in the prefrontal cortex of rhesus monkeys. Neuroscience 2003, 120, 177–189. [Google Scholar] [CrossRef]
- Fujita, S.; Adachi, K.; Lee, J.; Uchida, T.; Koshikawa, N.; Cools, A.R. Decreased postsynaptic dopaminergic and cholinergic functions in the ventrolateral striatum of spontaneously hypertensive rat. Eur. J. Pharmacol. 2004, 484, 75–82. [Google Scholar] [CrossRef]
- Bek, M.; Fischer, K.G.; Greiber, S.; Hupfer, C.; Mundel, P.; Pavenstädt, H. Dopamine depolarizes podocytes via a D1-like receptor. Nephrol. Dial. Transplant. 1999, 14, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Zhang, X.; Hu, J.; Gao, T.; Chen, J.; Xu, C.; Wei, C. Dopamine 1 receptor activation protects mouse diabetic podocytes injury via regulating the PKA/NOX-5/p38 MAPK axis. Exp. Cell Res. 2020, 388, 111849. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Vaughan, C.J.; Aherne, A.M.; Botkin, S.J.; Wang, Z.Q.; Felder, R.A.; Carey, R.M. Expression of the dopamine D3 receptor protein in the rat kidney. Hypertension 1998, 32, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Shultz, P.J.; Sedor, J.R.; Abboud, H.E. Dopaminergic stimulation of cAMP accumulation in cultured rat mesangial cells. Am. J. Physiol. 1987, 253, H358–H364. [Google Scholar] [CrossRef]
- Barili, P.; Ricci, A.; Baldoni, E.; Mignini, F.; Amenta, F. Pharmacological characterisation and autoradiographic localisation of a putative dopamine D3 receptor in the rat kidney. Eur. J. Pharmacol. 1997, 338, 89–95. [Google Scholar] [CrossRef]
- Pizzinat, N.; Marchal-Victorion, S.; Maurel, A.; Ordener, C.; Bompart, G.; Parini, A. Substrate-dependent regulation of MAO-A in rat mesangial cells: Involvement of dopamine D2-like receptors. Am. J. Physiol. Ren. Physiol. 2003, 284, F167–F174. [Google Scholar] [CrossRef] [Green Version]
- Grupp, C.; Begher, M.; Cohen, D.; Raghunath, M.; Franz, H.E.; Müller, G.A. Isolation and characterization of the lower portion of the thin limb of Henle in primary culture. Am. J. Physiol. Ren. Physiol. 1998, 274, F775–F782. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, C.; Villar, V.A.; Chen, S.Y.; Konkalmatt, P.; Wang, X.; Asico, L.D.; Jones, J.E.; Yang, Y.; Sanada, H.; et al. Human GRK4γ142V Variant Promotes Angiotensin II Type I Receptor-Mediated Hypertension via Renal Histone Deacetylase Type 1 Inhibition. Hypertension 2016, 67, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Sanada, H.; Jones, J.E.; Jose, P.A. Genetics of salt–Sensitive Hypertension. Curr. Hypertens. Rep. 2011, 13, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Soma, M.; Nakayama, T.; Kanmatsuse, K. Dopamine D1 receptor gene polymorphism is associated with essential Hypertension. Hypertension 2000, 36, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Fung, M.M.; Rana, B.K.; Tang, C.M.; Shiina, T.; Nievergelt, C.M.; Rao, F.; Salem, R.M.; Waalen, J.; Ziegler, M.G.; Insel, P.A.; et al. Dopamine D1 receptor (DRD1) genetic polymorphism: Pleiotropic effects on heritable renal traits. Kidney Int. 2009, 76, 1070–1080. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, F.E.; Drago, J.; Felder, R.A.; Printz, M.P.; Eisner, G.M.; Robillard, J.E.; Sbley, D.R.; Westphal, H.J.; Jose, P.A. Role of the D1A dopamine receptor in the pathogenesis of genetic Hypertension. J. Clin. Investig. 1996, 97, 2283–2288. [Google Scholar] [CrossRef]
- Li, X.X.; Bek, M.; Asico, L.D.; Yang, Z.; Grandy, D.K.; Goldstein, D.S.; Rubinstein, M.; Eisner, G.M.; Jose, P.A. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension 2001, 38, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Ueda, A.; Ozono, R.; Oshima, T.; Yano, A.; Kambe, M.; Teranishi, Y.; Katsuki, M.; Chayama, K. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am. J. Hypertens. 2003, 16, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Asico, L.D.; Ladines, C.; Fuchs, S.; Accili, D.; Carey, R.M.; Semeraro, C.; Pocchiari, F.; Felder, R.A.; Eisner, G.M.; Jose, P.A. Disruption of the dopamine D3 receptor gene produces renin-dependent Hypertension. J. Clin. Investig. 1998, 102, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.L.; Tulis, D.A.; Keeler, B.E.; Virag, J.A.; Lust, R.M.; Clemens, S. The dopamine D3 receptor knockout mouse mimics aging-related changes in autonomic function and cardiac fibrosis. PLoS ONE 2013, 8, e74116. [Google Scholar] [CrossRef]
- Bek, M.J.; Wang, X.; Asico, L.D.; Jones, J.E.; Zheng, S.; Li, X.; Eisner, G.M.; Grandy, D.K.; Carey, R.M.; Soares-da-Silva, P.; et al. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension 2006, 47, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Hollon, T.R.; Bek, M.J.; Lachowicz, J.E.; Ariano, M.A.; Mezey, E.; Ramachandran, R.; Wersinger, S.R.; Soares-da–Silva, P.; Liu, Z.F.; Grinberg, A.; et al. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J. Neurosci. 2002, 22, 10801–10810. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, T.; Pech, B.; Tappe, M.; Gross, G.; Mühlbauer, B.; Luippold, G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens. Res. 2007, 30, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Konkalmatt, P.R.; Asico, L.D.; Zhang, Y.; Yang, Y.; Drachenberg, C.; Zheng, X.; Han, F.; Jose, P.A.; Armando, I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight 2016, 1, e85888. [Google Scholar] [CrossRef]
- Asico, L.; Zhang, X.; Jiang, J.; Cabrera, D.; Escano, C.S.; Sibley, D.R.; Wang, X.; Yang, Y.; Mannon, R.; Jones, J.E.; et al. Lack of renal dopamine D5 receptors promotes Hypertension. J. Am. Soc. Nephrol. 2011, 22, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Jose, P.A.; Eisner, G.M.; Robillard, J.E. Renal hemodynamics and natriuresis induced by the dopamine-1 agonist, SKF 82526. Am. J. Med. Sci. 1987, 294, 181–186. [Google Scholar] [CrossRef]
- Gildea, J.J.; Kemp, B.A.; Howell, N.L.; Van Sciver, R.E.; Carey, R.M.; Felder, R.A. Inhibition of renal caveolin-1 reduces natriuresis and produces hypertension in sodium-loaded rats. Am. J. Physiol. Ren. Physiol. 2011, 300, F914–F920. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Felder, R.A.; Carey, R.M. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension 1999, 33, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Di Ciano, L.A.; Azurmendi, P.J.; Colombero, C.; Levin, G.; Oddo, E.M.; Arrizurieta, E.E.; Nowicki, S.; Ibarra, F.R. Defective renal dopamine function and sodium-sensitive hypertension in adult ovariectomized Wistar rats: Role of the cytochrome P-450 pathway. Am. J. Physiol. Ren. Physiol. 2015, 308, F1358–F1368. [Google Scholar] [CrossRef] [Green Version]
- Felder, R.A.; Seikaly, M.G.; Cody, P.; Eisner, G.M.; Jose, P.A. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 1990, 15, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Yan, Q.; Wan, L.; Weinbaum, S.; Weinstein, A.M.; Wang, T. Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport. Am. J. Physiol. Ren. Physiol. 2012, 303, F386–F395. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Bobulescu, I.A.; Quiñones, H.; Gisler, S.M.; Moe, O.W. Dopamine reduces cell surface Na+/H+ exchanger-3 protein by decreasing NHE3 exocytosis and cell membrane recycling. Am. J. Physiol. Ren. Physiol. 2017, 313, F1018–F1025. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, R.; Gomes, P.; Soares-da-Silva, P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol. Biochem. 2004, 14, 91–100. [Google Scholar] [CrossRef]
- Albrecht, F.E.; Xu, J.; Moe, O.W.; Hopfer, U.; Simonds, W.F.; Orlowski, J.; Jose, P.A. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1064–R1073. [Google Scholar] [CrossRef]
- Kocinsky, H.S.; Girardi, A.C.; Biemesderfer, D.; Nguyen, T.; Mentone, S.; Orlowski, J.; Aronson, P.S. Use of phospho–Specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am. J. Physiol. Ren. Physiol. 2005, 289, F249–F258. [Google Scholar] [CrossRef] [Green Version]
- Weinman, E.; Biswas, R.; Steplock, D.; Douglass, T.; Cunningham, R.; Shenolikar, S. Sodium–Hydrogen Exchanger Regulatory Factor 1 (NHERF-1) Transduces Signals That Mediate Dopamine Inhibition of Sodium-Phosphate Co-transport in Mouse Kidney. J. Biol. Chem. 2010, 285, 13454–13460. [Google Scholar] [CrossRef] [Green Version]
- Kunimi, M.; Seki, G.; Hara, C.; Taniguchi, S.; Uwatoko, S.; Goto, A.; Kimura, S.; Fujita, T. Dopamine inhibits renal Na+:HCO3-cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000, 57, 534–543. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Weinbaum, S.; Weinstein, A.M. Regulation of glomerulotubular balance: Flow-activated proximal tubule function. Pflug. Arch. 2017, 469, 643–654. [Google Scholar] [CrossRef]
- Gildea, J.J.; Xu, P.; Kemp, B.A.; Carlson, J.M.; Tran, H.T.; Bigler Wang, D.; Langouët-Astrié, C.J.; McGrath, H.E.; Carey, R.M.; Jose, P.A.; et al. Sodium bicarbonate cotransporter NBCe2 gene variants increase sodium and bicarbonate transport in human renal proximal tubule cells. PLoS ONE 2018, 13, e0189464. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, R.; Jose, P.A.; Soares-da–Silva, P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3- exchanger in immortalized SHR proximal tubular epithelial cells. Am. J. Physiol. Ren. Physiol. 2004, 286, F1120–F1126. [Google Scholar] [CrossRef]
- Aperia, A. 2011 Homer Smith Award: To serve and protect: Classic and novel roles for Na+, K+-adenosine triphosphatase. J. Am. Soc. Nephrol. 2012, 23, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Gildea, J.J.; Shah, I.T.; Van Sciver, R.E.; Israel, J.A.; Enzensperger, C.; McGrath, H.E.; Jose, P.A.; Felder, R.A. The cooperative roles of the dopamine receptors, D1R and D5R, on the regulation of renal sodium transport. Kidney Int. 2014, 86, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, A.R.; Eisner, G.M.; Armando, I.; Browning, S.; Pezzullo, J.C.; Rhee, L.; Dajani, M.; Carey, R.M.; Jose, P.A. The Renin-Angiotensin and Renal Dopaminergic Systems Interact in Normotensive Humans. J. Am. Soc. Nephrol. 2016, 27, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Luo, Y.; Escano, C.S.; Yang, Z.; Asico, L.; Li, H.; Jones, J.E.; Armando, I.; Lu, Q.; Sibley, D.R.; et al. Upregulation of renal sodium transporters in D5 dopamine receptor-deficient mice. Hypertension 2010, 55, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Diaz, A.D.; Lokhandwala, M. Kidney dopamine D(1)-like receptors and angiotensin 1-7 interaction inhibits renal Na(+) transporters. Am. J. Physiol. Ren. Physiol. 2019, 317, F949–F956. [Google Scholar] [CrossRef]
- Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Lee, B.M.; Carranza, A.; Del Mauro, J.S.; Pandolfo, M.; Gironacci, M.M.; Gorzalczany, S.; Toblli, J.E.; et al. Atrial Natriuretic Peptide Stimulates Dopamine Tubular Transport by Organic Cation Transporters: A Novel Mechanism to Enhance Renal Sodium Excretion. PLoS ONE 2016, 11, e0157487. [Google Scholar] [CrossRef] [Green Version]
- Crambert, S.; Sjöberg, A.; Eklöf, A.C.; Ibarra, F.; Holtbäck, U. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2010, 299, F49–F54. [Google Scholar] [CrossRef] [Green Version]
- Gildea, J.J.; Xu, P.; Kemp, B.A.; Carey, R.M.; Jose, P.A.; Felder, R.A. The Dopamine D(1) Receptor and Angiotensin II Type-2 Receptor are Required for Inhibition of Sodium Transport Through a Protein Phosphatase 2A Pathway. Hypertension 2019, 73, 1258–1265. [Google Scholar] [CrossRef]
- Chen, Y.; Asico, L.D.; Zheng, S.; Villar, V.A.; He, D.; Zhou, L.; Zeng, C.; Jose, P.A. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension 2013, 62, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Robbesaul, G.D.; Gorzalczany, S.B.; Carranza, A.; Trida, V.; Fernández, B.E.; Choi, M.R. Acute infusion of angiotensin II regulates organic cation transporters function in the kidney: Its impact on the renal dopaminergic system and sodium excretion. Hypertens. Res. 2020. [Google Scholar] [CrossRef]
- Trivedi, M.; Narkar, V.A.; Hussain, T.; Lokhandwala, M.F. Dopamine recruits D1A receptors to Na-K-ATPase-rich caveolar plasma membranes in rat renal proximal tubules. Am. J. Physiol. Ren. Physiol. 2004, 287, F921–F931. [Google Scholar] [CrossRef]
- Villar, V.A.; Armando, I.; Sanada, H.; Frazer, L.C.; Russo, C.M.; Notario, P.M.; Lee, H.; Comisky, L.; Russell, H.A.; Yang, Y.; et al. Novel role of sorting nexin 5 in renal D1 dopamine receptor trafficking and function: Implications for hypertension. FASEB J. 2013, 27, 1808–1819. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Lokhandwala, M.F. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin. Exp. Hypertens. A 1992, 14, 615–628. [Google Scholar] [CrossRef]
- Yu, P.; Asico, L.D.; Luo, Y.; Andrews, P.; Eisner, G.M.; Hopfer, U.; Felder, R.A.; Jose, P.A. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in Hypertension. Kidney Int. 2006, 70, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, D.; Ragsdale, N.; Boyd, D.; Felder, R.; Carey, R. Differential Human Renal Tubular Responses to Dopamine Type 1 Receptor Stimulation Are Determined by Blood Pressure Status. Hypertension 1997, 29, 115–122. [Google Scholar] [CrossRef]
- Cosentino, M.; Rasini, E.; Colombo, C.; Marino, F.; Blandini, F.; Ferrari, M.; Samuele, A.; Lecchini, S.; Nappi, G.; Frigo, G. Dopaminergic modulation of oxidative stress and apoptosis in human peripheral blood lymphocytes: Evidence for a D1-like receptor-dependent protective effect. Free Radic. Biol. Med. 2004, 36, 1233–1240. [Google Scholar] [CrossRef]
- Acquier, A.B.; Mori Sequeiros García, M.; Gorostizaga, A.B.; Paz, C.; Mendez, C.F. Reactive oxygen species mediate dopamine-induced signaling in renal proximal tubule cells. FEBS Lett. 2013, 587, 3254–3260. [Google Scholar] [CrossRef]
- Yu, P.; Han, W.; Villar, V.A.; Li, H.; Arnaldo, F.B.; Concepcion, G.P.; Felder, R.A.; Quinn, M.T.; Jose, P.A. Dopamine D1 receptor-mediated inhibition of NADPH oxidase activity in human kidney cells occurs via protein kinase A-protein kinase C cross talk. Free Radic. Biol. Med. 2011, 50, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Li, H.; Villar, V.A.; Pascua, A.M.; Dajani, M.I.; Wang, X.; Natarajan, A.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension 2008, 51, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yang, Y.; Yu, P.; Yang, J.; Jiang, X.; Villar, V.A.; Sibley, D.R.; Jose, P.A.; Zeng, C. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radic. Res. 2015, 49, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Lokhandwala, M.F. Transcription factor Nrf2 protects renal dopamine D1 receptor function during oxidative stress. Hypertension 2013, 62, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Lau, Y.S.; Lokhandwala, M.F. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt–Sensitive hypertension in Sprague-Dawley rats. Hypertension 2008, 51, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Fazili, F.R.; Lokhandwala, M.F. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappa B and protein kinase C. J. Am. Soc. Nephrol. 2007, 18, 1446–1457. [Google Scholar] [CrossRef]
- Asghar, M.; George, L.; Lokhandwala, M.F. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Ren. Physiol. 2007, 293, F914–F919. [Google Scholar] [CrossRef] [Green Version]
- Tapia, E.; García-Arroyo, F.; Silverio, O.; Rodríguez-Alcocer, A.N.; Jiménez–Flores, A.B.; Cristobal, M.; Arellano, A.S.; Soto, V.; Osorio-Alonso, H.; Molina-Jijón, E.; et al. Mycophenolate mofetil and curcumin provide comparable therapeutic benefit in experimental chronic kidney disease: Role of Nrf2-Keap1 and renal dopamine pathways. Free Radic. Res. 2016, 50, 781–792. [Google Scholar] [CrossRef]
- Marwaha, A.; Lokhandwala, M.F. Tempol reduces oxidative stress and restores renal dopamine D1-like receptor- G protein coupling and function in hyperglycemic rats. Am. J. Physiol. Ren. Physiol. 2006, 291, F58–F66. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.A.; Lokhandwala, M.F. Transcriptional Regulation of Renal Dopamine D1 Receptor Function During Oxidative Stress. Hypertension 2015, 65, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Konkalmatt, P.; Mokashi, C.; Kumar, M.; Zhang, Y.; Ko, A.; Farino, Z.J.; Asico, L.D.; Xu, G.; Gildea, J.; et al. Dopamine D2 receptor modulates Wnt expression and control of cell proliferation. Sci. Rep. 2019, 9, 16861. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Konkalmatt, P.; Yang, Y.; Gildea, J.; Jones, J.E.; Cuevas, S.; Felder, R.A.; Jose, P.A.; Armando, I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension 2014, 63, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cuevas, S.; Asico, L.D.; Escano, C.; Yang, Y.; Pascua, A.M.; Wang, X.; Jones, J.E.; Grandy, D.; Eisner, G.; et al. Deficient Dopamine D2 Receptor Function Causes Renal Inflammation Independently of High Blood Pressure. PLoS ONE 2012, 7, e38745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Jiang, X.; Qin, C.; Cuevas, S.; Jose, P.A.; Armando, I. Dopamine D2 receptors’ effects on renal inflammation are mediated by regulation of PP2A function. Am. J. Physiol. Ren. Physiol. 2016, 310, F128–F134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, I.S.; George, S.R.; Seeman, P. The human dopamine D2(Longer) receptor has a high-affinity state and inhibits adenylyl cyclase. Brain Res. Mol. Brain Res. 2000, 77, 281–284. [Google Scholar] [CrossRef]
- Bolan, E.A.; Kivell, B.; Jaligam, V.; Oz, M.; Jayanthi, L.D.; Han, Y.; Sen, N.; Urizar, E.; Gomes, I.; Devi, L.A.; et al. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol. Pharmacol. 2007, 71, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.U.; Mrzljak, L.; Gutierrez, A.; de la Calle, A.; Goldman–Rakic, P.S. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 7731–7736. [Google Scholar] [CrossRef] [Green Version]
- Ozono, R.; Ueda, A.; Oishi, Y.; Yano, A.; Kambe, M.; Katsuki, M.; Oshima, T. Dopamine D2 Receptor Modulates Sodium Handling via Local Production of Dopamine in the Kidney. J. Cardiovasc. Pharmacol. 2003, 42, S75–S80. [Google Scholar] [CrossRef]
- Armando, I.; Asico, L.D.; Wang, X.; Jones, J.E.; Serrão, M.P.; Cuevas, S.; Grandy, D.K.; Soares-da–Silva, P.; Jose, P.A. Antihypertensive effect of etamicastat in dopamine D2 receptor-deficient mice. Hypertens. Res. 2018, 41, 489–498. [Google Scholar] [CrossRef]
- Gao, D.Q.; Canessa, L.M.; Mouradian, M.M.; Jose, P.A. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am. J. Physiol. 1994, 266, F646–F650. [Google Scholar] [CrossRef]
- Zaika, O.L.; Mamenko, M.; Palygin, O.; Boukelmoune, N.; Staruschenko, A.; Pochynyuk, O. Direct inhibition of basolateral Kir4.1/5.1 and Kir4.1 channels in the cortical collecting duct by dopamine. Am. J. Physiol. Ren. Physiol. 2013, 305, F1277–F1287. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.X.; Cuevas, C.A.; Su, X.T.; Wu, P.; Gao, Z.X.; Lin, D.H.; McCormick, J.A.; Yang, C.L.; Wang, W.H.; Ellison, D.H. Potassium intake modulates the thiazide–Sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018, 93, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Su, X.T.; Klett, N.J.; Sharma, A.; Allen, C.N.; Wang, W.H.; Yang, C.L.; Ellison, D.H. Distal convoluted tubule Cl- concentration is modulated via K+ channels and transporters. Am. J. Physiol. Ren. Physiol. 2020, 319, F534–F540. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, F.; Cohen, H.T.; Satoh, T.; Katz, A.I. Dopamine inhibits Na/K-ATPase in single tubules and cultured cells from distal nephron. Pflug. Arch. 1992, 421, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, A.; Aperia, A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am. J. Physiol. 1990, 259, F924–F928. [Google Scholar] [CrossRef]
- IUPHAR/BPS Guide to Pharmacology: Nemonapride Page. Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=983 (accessed on 10 December 2020).
- IUPHAR/BPS Guide to Pharmacology: Sulpiride Page. Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=5501 (accessed on 10 December 2020).
- Shin, Y.; Kumar, U.; Patel, Y.; Patel, S.; Sidhu, A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J. Hypertens. 2003, 21, 199–207. [Google Scholar] [CrossRef]
- Yang, J.; Villar, V.; Jose, P.A.; Zeng, C. Renal Dopamine Receptors and Oxidative Stress: Role in Hypertension. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Armando, I.; Wang, X.; Villar, V.A.; Jones, J.E.; Asico, L.D.; Escano, C.; Jose, P.A. Reactive oxygen species dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension 2007, 49, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Charvin, D.; Vanhoutte, P.; Pagès, C.; Borrelli, E.; Caboche, J. Unraveling a role for dopamine in Huntington’s disease: The dual role of reactive oxygen species and D2 receptor stimulation. Proc. Natl. Acad. Sci. USA 2005, 102, 12218–12223. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, C.; Hamrick, W.C.; Sedaka, R.; Jagarlamudi, S.; Asico, L.D.; Jose, P.A.; Cuevas, S. Uncoupling Protein 2 Increases Blood Pressure in DJ-1 Knockout Mice. J. Am. Heart Assoc. 2019, 8, e011856. [Google Scholar] [CrossRef]
- Han, F.; Konkalmatt, P.; Chen, J.; Gildea, J.; Felder, R.A.; Jose, P.A.; Armando, I. miR-217 Mediates the Protective Effects of the Dopamine D2 Receptor on Fibrosis in Human Renal Proximal Tubule Cells. Hypertension 2015, 65, 1118–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Guan, W.; Han, Y.; Ren, H.; Tang, X.; Zhang, H.; Liu, Y.; Fu, J.; He, D.; Asico, L.D.; et al. Stimulation of Dopamine D3 Receptor Attenuates Renal Ischemia–Reperfusion Injury via Increased Linkage with Gα12. Transplantation 2015, 99, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, A.; Elgueta, D.; Campos, J.; Chovar, O.; Falcón, P.; Matus, S.; Alfaro, I.; Bono, M.R.; Pacheco, R. Dopamine receptor D3 signalling in astrocytes promotes neuroinflammation. J. Neuroinflamm. 2019, 16, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Li, X.; Chen, Q.; He, J.; Dong, Y.; Wang, J.; Shen, S.; Jia, R.; Zang, Q.J.; Zhang, T.; et al. Associations between D3R expression in synovial mast cells and disease activity and oxidant status in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Y.; Li, G.; Wang, B.; Zhou, T.; Zhu, L.; Chen, T.; Chen, Y. The Dopamine Receptor D3 Regulates Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Int. J. Neuropsychopharm. 2018, 21, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Han, Y.; Zheng, S.; Kou, X.; Asico, L.D.; Huang, H.; Gao, Z.; Jose, P.A.; Zeng, C. Enhanced natriuresis and diuresis in wistar rats caused by the costimulation of renal dopamine D3 and angiotensin II type 2 receptors. Am. J. Hypertens. 2015, 28, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Ladines, C.A.; Zeng, C.; Asico, L.D.; Sun, X.; Pocchiari, F.; Semeraro, C.; Pisegna, J.; Wank, S.; Yamaguchi, I.; Eisner, G.M.; et al. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1071–R1078. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, Z.; Li, H.; Yu, P.; Zheng, S.; Wu, L.; Asico, L.D.; Hopfer, U.; Eisner, G.M.; Felder, R.A.; et al. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension 2006, 47, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, Y.; Wang, W.E.; Chen, C.; Ren, H.; Zheng, S.; Zhou, L.; Zeng, C. Effect of D3 dopamine receptor on dopamine D4 receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats and spontaneously hypertensive rats. J. Hypertens. 2016, 34, 1599–15606. [Google Scholar] [CrossRef]
- Huang, H.; Ren, H.; Chen, C.; Wang, X.; Yang, J.; Han, Y.; He, D.; Zhou, L.; Asico, L.D.; Jose, P.A.; et al. D3 dopamine receptor regulation of D5 receptor expression and function in renal proximal tubule cells. Hypertens. Res. 2012, 35, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Yang, Z.; Ren, H.; Zhang, Y.; Han, Y.; He, D.; Lu, Q.; Wang, X.; Wang, X.; Yang, C.; et al. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am. J. Hypertens. 2009, 22, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Fu, C.; Asico, L.D.; Villar, V.A.; Ren., H.; He, D.; Wang, Z.; Yang, J.; Jose, P.A.; Zeng, C. Role of Galpha(12)- and Galpha(13)-protein subunit linkage of D(3) dopamine receptors in the natriuretic effect of D(3) dopamine receptor in kidney. Hypertens. Res. 2011, 34, 1011–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luippold, G.; Zimmermann, C.; Mai, M.; Kloor, D.; Starck, D.; Gross, G.; Mühlbauer, B. Dopamine D(3) receptors and salt-dependent hypertension. J. Am. Soc. Nephrol. 2001, 12, 2272–2279. [Google Scholar]
- Everett, P.B.; Senogles, S.E. D3 dopamine receptor signals to activation of phospholipase D through a complex with Rho. J. Neurochem. 2010, 112, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Schiffrin, E.L. Ang II–Stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 1999, 34, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Rosin, C.; Colombo, S.; Calver, A.; Bates, T.; Skaper, S. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia 2005, 52, 336–343. [Google Scholar] [CrossRef]
- IUPHAR/BPS Guide to Pharmacology: Pramipexole Page. Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=953 (accessed on 10 December 2020).
- Lieberknecht, V.; Junqueira, S.C.; Cunha, M.P.; Barbosa, T.A.; de Souza, L.F.; Coelho, I.S.; Santos, A.R.; Rodrigues, A.L.; Dafré, A.L.; Dutra, R.C. Pramipexole, a Dopamine D2/D3 Receptor-Preferring Agonist, Prevents Experimental Autoimmune Encephalomyelitis Development in Mice. Mol. Neurobiol. 2017, 54, 1033–1045. [Google Scholar] [CrossRef]
- Shibagaki, K.; Okamoto, K.; Katsuta, O.; Nakamura, M. Beneficial protective effect of pramipexole on light-induced retinal damage in mice. Exp. Eye Res. 2015, 139, 64–72. [Google Scholar] [CrossRef]
- Le, W.D.; Jankovic, J.; Xie, W.; Appel, S.H. Antioxidant property of pramipexole independent of dopamine receptor activation in neuroprotection. J. Neural Transm. (Vienna) 2000, 107, 1165–1173. [Google Scholar] [CrossRef]
- Wang, X.; Johns, J.; Asico, L.; Armando, I.; Jose, P. High blood pressure but normal oxidative stress in D3 dopamine receptor deficient mice. FASEB J. 2013, 27, 955.11. [Google Scholar]
- Wang, W.; Cohen, J.A.; Wallrapp, A.; Trieu, K.G.; Barrios, J.; Shao, F.; Krishnamoorthy, N.; Kuchroo, V.K.; Jones, M.R.; Fine, A.; et al. Age–Related Dopaminergic Innervation Augments T Helper 2-Type Allergic Inflammation in the Postnatal Lung. Immunity 2019, 51, 1102–1118.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, A.W.; Peng, Y.P.; Liu, Y.; Huang, H.W.; Qiu, Y.H. Roles of dopamine receptor subtypes in mediating modulation of T lymphocyte function. Neuro Endocrinol. Lett. 2010, 31, 782–791. [Google Scholar] [PubMed]
- Guenova, E.; Skabytska, Y.; Hoetzenecker, W.; Weindl, G.; Sauer, K.; Tham, M.; Kim, K.W.; Park, J.H.; Seo, J.H.; Ignatova, D.; et al. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2015, 112, 2163–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Hirabayashi, M.; Ishige, K.; Kosuge, Y.; Kihara, T.; Ito, Y. Activation of dopamine D4 receptors is protective against hypoxia/reoxygenation-induced cell death in HT22 cells. J. Pharmacol. Sci. 2010, 114, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, S.M.; Sullivan, K.M.; Liu, F.; DiPaula, B.A.; Jose, P.A.; Kitchen, C.A.; Feldman, S.M.; Kelly, D.L. Blood Pressure and Heart Rate Changes During Clozapine Treatment. Psychiatr. Q. 2017, 88, 545–552. [Google Scholar] [CrossRef] [PubMed]
- IUPHAR/BPS Guide to Pharmacology: PD168,077 Page. Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=975 (accessed on 10 December 2020).
- Tang, L.; Zheng, S.; Ren, H.; He, D.; Zeng, C.; Wang, W. Activation of angiotensin II type 1 receptors increases D4 dopamine receptor expression in rat renal proximal tubule cells. Hypertens. Res. 2017, 40, 652–657. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Lu, X.; He, D.; Han, Y.; Wang, H.; Zeng, C.; Shi, W. Inhibition of D4 Dopamine Receptors on Insulin Receptor Expression and Effect in Renal Proximal Tubule Cells. J. Am. Heart Assoc. 2016, 5, e002448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Deng, K.; Wang, X.; Wang, Z.; Zheng, S.; Ren, H.; He, D.; Han, Y.; Asico, L.D.; Jose, P.A.; et al. Activation of D4 Dopamine Receptor Decreases Angiotensin II Type 1 Receptor Expression in Rat Renal Proximal Tubule Cells. Hypertension 2015, 65, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Schafer, J.A. Dopamine inhibits AVP-dependent Na+ transport and water permeability in rat CCD via a D4-like receptor. Am. J. Physiol. 1996, 271, F391–F400. [Google Scholar] [CrossRef]
- Ricci, A.; Marchal-Victorion, S.; Bronzetti, E.; Parini, A.; Amenta, F.; Tayebati, S.K. Dopamine D4 receptor expression in rat kidney: Evidence for pre- and postjunctional localization. J. Histochem. Cytochem. 2002, 50, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Lara, L.S.; McCormack, M.; Semprum-Prieto, L.C.; Shenouda, S.; Majid, D.S.; Kobori, H.; Navar, L.G.; Prieto, M.C. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II–Salt Hypertension. Am. J. Physiol. Ren. Physiol. 2012, 302, F85–F94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Campbell, R.C.; Warnock, D.G. Oxidative stress in hypertension and chronic kidney disease: Role of angiotensin II. Semin. Nephrol. 2004, 24, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, J.; Guan, W.; Han, Y.; Wang, W.E.; Wang, X.; Wang, H.; Jose, P.A.; Zeng, C. Activation of the D4 dopamine receptor attenuates proliferation and migration of vascular smooth muscle cells through downregulation of AT1a receptor expression. Hypertens. Res. 2015, 38, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Chen, Q.; Sagara, Y.; Schubert, D. The activation of dopamine D4 receptors inhibits oxidative stress-induced nerve cell death. J. Neurosci. 2001, 21, 6069–6076. [Google Scholar] [CrossRef] [Green Version]
- Bastianetto, S.; Danik, M.; Mennicken, F.; Williams, S.; Quirion, R. Prototypical antipsychotic drugs protect hippocampal neuronal cultures against cell death induced by growth medium deprivation. BMC Neurosci. 2006, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.B.; Cortez, A.P.; de Ávila, R.I.; de Carvalho, F.S.; Andrade, W.M.; da Cruz, A.F.; Reis, K.B.; Menegatti, R.; Lião, L.M.; Romeiro, L.; et al. The novel piperazine-containing compound LQFM018: Necroptosis cell death mechanisms, dopamine D(4) receptor binding and toxicological assessment. Biomed. Pharmacother. 2018, 102, 481–493. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, W.; Liu, X.; Wang, Z.; Liu, Y.; Felder, R.A.; Gildea, J.J.; Jose, P.A.; Qin, C.; Yang, Z. The Synergistic Roles of Cholecystokinin B and Dopamine D5 Receptors on the Regulation of Renal Sodium Excretion. PLoS ONE 2016, 11, e0146641. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, W.; Chen, W.; Jiang, X.; Zhang, Y.; Wang, Z.; Yang, J.; Jones, J.E.; Jose, P.A.; Yang, Z. Regulation of blood pressure, oxidative stress and AT1R by high salt diet in mutant human dopamine D5 receptor transgenic mice. Hypertens. Res. 2015, 38, 394–399. [Google Scholar] [CrossRef]
- Zeng, C.; Yang, Z.; Wang, Z.; Jones, J.; Wang, X.; Altea, J.; Mangrum, A.J.; Hopfer, U.; Sibley, D.R.; Eisner, G.M.; et al. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 2005, 45, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Armando, I.; Yu, P.; Escano, C.; Mueller, S.C.; Asico, L.; Pascua, A.; Lu, Q.; Wang, X.; Villar, V.A.; et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J. Clin. Investig. 2008, 118, 2180–2189. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Escano, C.S.; Asico, L.; Jones, J.E.; Jose, P.A. Upregulation of the thiazide-sensitive sodium chloride cotransporter in the kidney is associated with the hypertension in D3 dopamine receptor heterozygous (D3−/+) mice. FASEB J. 2009, 23, 605.13. [Google Scholar]
- Wang, X.; Asico, L.; Jones, J.E.; Escano, C.S.; Luo, Y.; Armando, I.; Jose, P.A. Profiling Protein Abundance of Renal Sodium Transporters in D4 Dopamine Receptor–Deficient Mice on Normal, High, or Low NaCl Intake. In Proceedings of the 61st Annual High Blood Pressure Research Conference, Phoenix, AZ, USA, 26–29 September 2007; p. 90. [Google Scholar]
- Plouffe, B.; Yang, X.; Tiberi, M. The third intracellular loop of D1 and D5 dopaminergic receptors dictates their subtype–Specific PKC-induced sensitization and desensitization in a receptor conformation-dependent manner. Cell Signal. 2012, 24, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Sunahara, R.K.; Guan, H.C.; O’Dowd, B.F.; Seeman, P.; Laurier, L.G.; Ng, G.; George, S.R.; Torchia, J.; Van Tol, H.H.; Niznik, H.B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature 1991, 350, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Whistler, J. Trafficking Properties of the D5 Dopamine Receptor. Traffic 2011, 12, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, Y.; Villar, V.A.; Asico, L.; Jones, J.E.; Yu, P.; Li, H.; Weinman, E.J.; Eisner, G.M.; Jose, P.A. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertension Res. 2013, 36, 684–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Asico, L.D.; Yu, P.; Wang, Z.; Jones, J.E.; Escano, C.S.; Wang, X.; Quinn, M.T.; Sibley, D.R.; Romero, G.G.; et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R96–R104. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Liu, X.; Wang, W.; Wang, Z.; Hu, Y.; Zhang, Y.; Zhang, Y.; Jose, P.A.; Wei, Q.; et al. Over-expression of a cardiac–Specific human dopamine D5 receptor mutation in mice causes a dilated cardiomyopathy through ROS over-generation by NADPH oxidase activation and Nrf2 degradation. Redox Biol. 2018, 19, 134–146. [Google Scholar] [CrossRef]
- Yang, Z.; Asico, L.D.; Yu, P.; Wang, Z.; Jones, J.E.; Bai, R.K.; Sibley, D.R.; Felder, R.A.; Jose, P.A. D5 dopamine receptor regulation of phospholipase D. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H55–H61. [Google Scholar] [CrossRef]
- Wang, S.; Tan, X.; Chen, P.; Zheng, S.; Ren, H.; Cai, J.; Zhou, L.; Jose, P.A.; Yang, J.; Zeng, C. Role of Thioredoxin 1 in Impaired Renal Sodium Excretion of hD5RF173L Transgenic Mice. J. Am. Heart Assoc. 2019, 8, e012192. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Barrios, F.; Prado, C.; Contreras, F.; Pacheco, R. Dopamine Receptor D5 Signaling Plays a Dual Role in Experimental Autoimmune Encephalomyelitis Potentiating Th17-Mediated Immunity and Favoring Suppressive Activity of Regulatory T-Cells. Front. Cell. Neurosci. 2018, 12, 192. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Hu, Y.; Wang, B.; Li, S.; Ma, C.; Liu, X.; Moynagh, P.N.; Zhou, J.; Yang, S. Dopamine Uses the DRD5-ARRB2-PP2A Signaling Axis to Block the TRAF6-Mediated NF-kappaB Pathway and Suppress Systemic Inflammation. Mol. Cell. 2020, 78, 42–56.e6. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, J.; Bozzo, L.; Roberto, A.; Pontarini, E.; Tentorio, P.; Hudspeth, K.; Lugli, E.; Mavilio, D. Dopamine inhibits the effector functions of activated NK cells via the upregulation of the D5 receptor. J. Immunol. 2014, 193, 2792–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.N.; Fatima, N.; Ali, R.; Hussain, T. Emerging Role of Angiotensin AT2 Receptor in Anti-Inflammation: An Update. Curr. Pharm. Des. 2020, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Novoa, U.; Moya, J.; Gabrielli, L.; Jalil, J.E.; García, L.; Chiong, M.; Lavandero, S.; Ocaranza, M.P. Angiotensin-(1-9) reduces cardiovascular and renal inflammation in experimental renin-independent Hypertension. Biochem. Pharmacol. 2018, 156, 357–370. [Google Scholar] [CrossRef]
- Skaaning Jensen, B.; Levavi–Sivan, B.; Fishburn., C.S.; Fuchs, S. Functional expression of the murine D2, D3 and D4 dopamine receptors in Xenopus laevis oocytes. FEBS Lett. 1997, 420, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Ljungstrom, T.; Grunnet, M.; Skaaning Jensen, B.S.; Olesen, S.-P. Functional coupling between heterologously expressed dopamine D(2) receptors and KCNQ channels. Pflug. Arch. 2003, 446, 684–694. [Google Scholar] [CrossRef]


| Endogenous Antioxidants | ||
|---|---|---|
| Enzymatic Antioxidants | Non-Enzymatic Antioxidants | |
| Antioxidants | Metabolic Antioxidants | Nutrient Antioxidants |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaddumi, W.N.; Jose, P.A. The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension. Biomedicines 2021, 9, 139. https://doi.org/10.3390/biomedicines9020139
Qaddumi WN, Jose PA. The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension. Biomedicines. 2021; 9(2):139. https://doi.org/10.3390/biomedicines9020139
Chicago/Turabian StyleQaddumi, Waleed N., and Pedro A. Jose. 2021. "The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension" Biomedicines 9, no. 2: 139. https://doi.org/10.3390/biomedicines9020139
APA StyleQaddumi, W. N., & Jose, P. A. (2021). The Role of the Renal Dopaminergic System and Oxidative Stress in the Pathogenesis of Hypertension. Biomedicines, 9(2), 139. https://doi.org/10.3390/biomedicines9020139







