Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases
Abstract
:1. Introduction
2. Physicochemical Characteristics of Curcumin
3. Curcumin Bioavailability: Metabolic Reactions and New Formulations
4. Relationship between Structural Properties and Biological Activity of Curcumin Derivatives
5. Curcumin Derivatives and Hybrids Molecules
5.1. Monocarbonyl Analogs of Curcumin (MACs)
5.2. C4-Substituted Curcumin Derivatives
5.3. Heterocyclic Derivatives
5.4. Tetrahydrocurcumins (THCs)
5.5. Curcumin-Like (CL) Compounds
5.6. Aromatic Ring Substitution: Methoxy and Hydroxy Groups
5.7. Aromatic Ring Substitution: Halogenated and Prenylated Derivatives
5.8. Other Aromatic Rings
5.9. Hemi-Curcuminoids
5.10. Calebin A Derivatives
5.11. Hybrid Compounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Pelletier, J. Examen chimique de la racine de Curcuma. J. Pharm. 1815, 1, 289–300. [Google Scholar]
- Ravindran, P.N.; Babu, K.N.; Sivaraman, K. Turmeric: The Genus Curcuma; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hatami, M.; Abdolahi, M.; Soveyd, N.; Djalali, M.; Togha, M.; Honarvar, N.M. Molecular Mechanisms of Curcumin in Neuroinflammatory Disorders: A Mini Review of Current Evidences. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. Biofactors (Oxf. Engl.) 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2015, 51, 466–479. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Dementia. 21 September 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 9 February 2021).
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharm. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s disease: Treatments in discovery and development. Nat. Neurosci. 2002, 5, 1055–1057. [Google Scholar] [CrossRef]
- Tarawneh, R.; Holtzman, D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef] [PubMed]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar]
- Mesulam, M.M.; Thompson, C.K.; Weintraub, S.; Rogalski, E.J. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain A J. Neurol. 2015, 138, 2423–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.C.; LeVine, H. The cerebral proteopathies. Mol. Neurobiol. 2000, 21, 83–95. [Google Scholar] [CrossRef]
- Nedelsky, N.B.; Todd, P.K.; Taylor, J.P. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim. Biophys. Acta 2008, 1782, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selkoe, D.J. Alzheimer’s disease: A central role for amyloid. J. Neuropathol. Exp. Neurol. 1994, 53, 438–447. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Glabe, C.G.; Kayed, R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 2006, 66, S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Lesné, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Zaidi, T.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA 2001, 98, 6923–6928. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.; Stankowski, J.N.; Carlomagno, Y.; Stetler, C.; Petrucelli, L. Acetylation: A new key to unlock tau’s role in neurodegeneration. Alzheimer’s Res. Ther. 2014, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates with Changes in Its Function Beyond Microtubule Stability. Front. Cell. Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef] [Green Version]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strang, K.H.; Croft, C.L.; Sorrentino, Z.A.; Chakrabarty, P.; Golde, T.E.; Giasson, B.I. Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J. Biol. Chem. 2018, 293, 2408–2421. [Google Scholar] [CrossRef] [Green Version]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef] [Green Version]
- Belostozky, A.; Richman, M.; Lisniansky, E.; Tovchygrechko, A.; Chill, J.H.; Rahimipour, S. Inhibition of tau-derived hexapeptide aggregation and toxicity by a self-assembled cyclic d,l-alpha-peptide conformational inhibitor. Chem. Commun. (Camb. Engl.) 2018, 54, 5980–5983. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Petersen, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D. Do neuronal inclusions kill the cell? J. Neural Transm. Suppl. 2000, 59, 91–93. [Google Scholar] [PubMed]
- Van De Nes, J.A.; Nafe, R.; Schlote, W. Non-tau based neuronal degeneration in Alzheimer’s disease -- an immunocytochemical and quantitative study in the supragranular layers of the middle temporal neocortex. Brain Res. 2008, 1213, 152–165. [Google Scholar] [CrossRef]
- Maeda, S.; Sahara, N.; Saito, Y.; Murayama, S.; Ikai, A.; Takashima, A. Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer’s disease. Neurosci. Res 2006, 54, 197–201. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Sarmiento, J.; Troncoso, J.; Jackson, G.R.; Kayed, R. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, K.R.; Remmers, C.; Fu, Y.; Brooker, S.; Kanaan, N.M.; Vana, L.; Ward, S.; Reyes, J.F.; Philibert, K.; Glucksman, M.J.; et al. Characterization of prefibrillar Tau oligomers in vitro and in Alzheimer disease. J. Biol. Chem. 2011, 286, 23063–23076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerson, J.E.; Castillo-Carranza, D.L.; Kayed, R. Advances in therapeutics for neurodegenerative tauopathies: Moving toward the specific targeting of the most toxic tau species. ACS Chem. Neurosci. 2014, 5, 752–769. [Google Scholar] [CrossRef]
- Gerson, J.E.; Kayed, R. Formation and propagation of tau oligomeric seeds. Front. Neurol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usenovic, M.; Niroomand, S.; Drolet, R.E.; Yao, L.; Gaspar, R.C.; Hatcher, N.G.; Schachter, J.; Renger, J.J.; Parmentier-Batteur, S. Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 14234–14250. [Google Scholar] [CrossRef] [Green Version]
- Mayeux, R.; Sano, M. Treatment of Alzheimer’s disease. N. Engl. J. Med. 1999, 341, 1670–1679. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Necula, M.; Kayed, R.; Milton, S.; Glabe, C.G. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007, 282, 10311–10324. [Google Scholar] [CrossRef] [Green Version]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Amyloid-β Aggregate Toxicity and Modulates Amyloid-β Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release Off. J. Control. Release Soc. 2019, 316, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimer’s Dis. JAD 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Wang, M.S.; Boddapati, S.; Emadi, S.; Sierks, M.R. Curcumin reduces α-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.A.; Smith, W.W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res. 2011, 63, 439–444. [Google Scholar] [CrossRef]
- Kim, G.Y.; Kim, K.H.; Lee, S.H.; Yoon, M.S.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Ahn, S.C.; Park, Y.C.; Park, Y.M. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimer’s Dis. JAD 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Lampe, V.; Milobedzka, J. Studien über Curcumin. Ber. Der Dtsch. Chem. Ges. 2006, 46, 2235–2240. [Google Scholar] [CrossRef]
- Pabon, H.J.J. A synthesis of curcumin and related compounds. Recueil Travaux Chimiques Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Krackov, M.H.; Bellis, H.E. Process for the Synthesis of Curcumin Related Compounds. US Patent 5,679,864, 21 October 1997. [Google Scholar]
- Babu, K.V.D.; Rajasekharan, K.N. Simplified condition for synthesis of Curcumin I and other curcuminoids. Org. Prep. Proced. Int. 1994, 26, 674–677. [Google Scholar] [CrossRef]
- Rao, E.V.; Sudheer, P. Revisiting curcumin chemistry part I: A new strategy for the synthesis of curcuminoids. Indian J. Pharm. Sci. 2011, 73, 262–270. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6374–6380. [Google Scholar] [CrossRef]
- Buadonpri, W.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Synthetic Curcumin Inhibits Carrageenan-Induced Paw Edema in Rats. J. Health Res. 2018, 23, 11–16. [Google Scholar]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is A Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]
- Parimita, S.P.; Ramshankar, Y.V.; Suresh, S.; Guru Row, T.N. Redetermination of curcumin: (1E,4Z,6E)-5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one. Acta Crystallogr. Sect. E 2007, 63, o860–o862. [Google Scholar] [CrossRef]
- Balasubramanian, K. Molecular Orbital Basis for Yellow Curry Spice Curcumin’s Prevention of Alzheimer’s Disease. J. Agric. Food Chem. 2006, 54, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. BAnal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Coll. Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Khurana, A.; Ho, C.-T. High Performance Liquid Chromatographic Analysis of Curcuminoids and Their Photo-oxidative Decomposition Compounds in Curcuma longa, L. J. Liq. Chromatogr. 1988, 11, 2295–2304. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.d.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef] [Green Version]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L. Curcumin-loaded chitosan-bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res. Lett. 2018, 13, 330. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Uehara, S.; Sawangrat, K.; Morishita, M.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of intestinal absorption of curcumin by cyclodextrins and the mechanisms underlying absorption enhancement. Int. J. Pharm. 2018, 535, 340–349. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin, a Biological Wonder Molecule: A Crystal Engineering Point of View. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 16953–16965. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, F.; Puangmalai, N.; Ellsworth, A.; Bucchieri, F.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Toxic Tau Oligomers Modulated by Novel Curcumin Derivatives. Sci. Rep. 2019, 9, 19011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pithadia, A.S.; Bhunia, A.; Sribalan, R.; Padmini, V.; Fierke, C.A.; Ramamoorthy, A. Influence of a curcumin derivative on hIAPP aggregation in the absence and presence of lipid membranes. Chem. Commun. (Camb. Engl.) 2016, 52, 942–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, R.M. Kinetics of amyloid formation and membrane interaction with amyloidogenic proteins. Biochim. Biophys. Acta 2007, 1768, 1923–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fändrich, M.; Schmidt, M.; Grigorieff, N. Recent progress in understanding Alzheimer’s β-amyloid structures. Trends Biochem. Sci. 2011, 36, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjernberg, L.O.; Näslund, J.; Lindqvist, F.; Johansson, J.; Karlström, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluti, F.; Rampa, A.; Gobbi, S.; Bisi, A. Small-molecule inhibitors/modulators of amyloid-β peptide aggregation and toxicity for the treatment of Alzheimer’s disease: A patent review (2010–2012). Expert Opin. Ther. Pat. 2013, 23, 581–596. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Buchete, N.-V.; Hummer, G. Structure and dynamics of parallel beta-sheets, hydrophobic core, and loops in Alzheimer’s A beta fibrils. Biophys. J. 2007, 92, 3032–3039. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.M.; Wang, Q.; Telivala, T.P.; Smith, R.J.; Lanzirotti, A.; Miklossy, J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 2006, 155, 30–37. [Google Scholar] [CrossRef]
- Huang, X.; Atwood, C.S.; Moir, R.D.; Hartshorn, M.A.; Tanzi, R.E.; Bush, A.I. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Aβ peptides. JBIC J. Biol. Inorg. Chem. 2004, 9, 954–960. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and Synthesis of Curcumin Analogues for in Vivo Fluorescence Imaging and Inhibiting Copper-Induced Cross-Linking of Amyloid Beta Species in Alzheimer’s Disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiala, M.; Liu, P.T.; Espinosa-Jeffrey, A.; Rosenthal, M.J.; Bernard, G.; Ringman, J.M.; Sayre, J.; Zhang, L.; Zaghi, J.; Dejbakhsh, S.; et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA 2007, 104, 12849–12854. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, S.; Franco, V.; Sorrentino, S.; Zucca, S.; Pandini, C.; Rota, P.; Bernuzzi, S.; Costa, A.; Sinforiani, E.; Pansarasa, O.; et al. Curcumin and Novel Synthetic Analogs in Cell-Based Studies of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Wang, L.; Ganguly, A.; Saikia, J.M.; Wagner, S.L.; Koo, E.H.; Roy, S. Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat. Neurosci. 2016, 19, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Konno, H.; Endo, H.; Ise, S.; Miyazaki, K.; Aoki, H.; Sanjoh, A.; Kobayashi, K.; Hattori, Y.; Akaji, K. Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. Bioorganic Med. Chem. Lett. 2014, 24, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Narlawar, R.; Pickhardt, M.; Leuchtenberger, S.; Baumann, K.; Krause, S.; Dyrks, T.; Weggen, S.; Mandelkow, E.; Schmidt, B. Curcumin-derived pyrazoles and isoxazoles: Swiss army knives or blunt tools for Alzheimer’s disease? ChemMedChem 2008, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Jiménez, J.S.; Sayas, C.L.; Bolós, M.; Zabala, J.C.; Rivas, G.; Hernández, F. Tau Structures. Front. Aging Neurosci. 2016, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Hu, X.; Zhou, L.; Tu, Y.; Shi, S.; Yao, T. Orientation-Inspired Perspective on Molecular Inhibitor of Tau Aggregation by Curcumin Conjugated with Ruthenium(II) Complex Scaffold. J. Phys. Chem. B 2020, 124, 2343–2353. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Bhide, M.K.; Kadam, R.M.; Mohan, H. Reactions of superoxide radicals with curcumin: Probable mechanisms by optical spectroscopy and EPR. Free Radic. Res. 2004, 38, 355–362. [Google Scholar] [CrossRef]
- Ferrari, E.; Benassi, R.; Saladini, M.; Orteca, G.; Gazova, Z.; Siposova, K. In vitro study on potential pharmacological activity of curcumin analogues and their copper complexes. Chem. Biol. Drug Des. 2017, 89, 411–419. [Google Scholar] [CrossRef]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuya, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, R.A.; Gonzales, A.M.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. A Chemical Analog of Curcumin as an Improved Inhibitor of Amyloid Abeta Oligomerization. PLoS ONE 2012, 7, e31869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the heart from the curcumin molecule: Monocarbonyl curcumin mimics (MACs). Molecules 2014, 20, 249–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-Y.; Chen, Y.; Li, Y.-P.; Chen, S.-H.; Tan, J.-H.; Ou, T.-M.; Gu, L.-Q.; Huang, Z.-S. Design, synthesis, and biological evaluation of curcumin analogues as multifunctional agents for the treatment of Alzheimer’s disease. Bioorganic Med. Chem. 2011, 19, 5596–5604. [Google Scholar] [CrossRef] [PubMed]
- Ramshini, H.; mohammad-zadeh, M.; Ebrahim-Habibi, A. Inhibition of amyloid fibril formation and cytotoxicity by a chemical analog of Curcumin as a stable inhibitor. Int. J. Biol. Macromol. 2015, 78, 396–404. [Google Scholar] [CrossRef]
- Ao, G.-Z.; Chu, X.-J.; Ji, Y.-Y.; Wang, J.-W. Antioxidant properties and PC12 cell protective effects of a novel curcumin analogue (2E,6E)-2,6-bis(3,5- dimethoxybenzylidene)cyclohexanone (MCH). Int. J. Mol. Sci. 2014, 15, 3970–3988. [Google Scholar] [CrossRef] [Green Version]
- Orteca, G.; Tavanti, F.; Bednarikova, Z.; Gazova, Z.; Rigillo, G.; Imbriano, C.; Basile, V.; Asti, M.; Rigamonti, L.; Saladini, M.; et al. Curcumin derivatives and Aβ-fibrillar aggregates: An interactions’ study for diagnostic/therapeutic purposes in neurodegenerative diseases. Bioorganic Med. Chem. 2018, 26, 4288–4300. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, U.; Rasmussen, P.B.; Lawesson, S.-O. Synthesis of Naturally Occurring Curcuminoids and Related Compounds. Liebigs Ann. Der Chem. 1985, 1985, 1557–1569. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Abeta-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 31, 4179–4185. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N.; Dhanalakshmi, C.; Manivasagam, T.; Essa, M.M. CNB-001, a novel pyrazole derivative mitigates motor impairments associated with neurodegeneration via suppression of neuroinflammatory and apoptotic response in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2014, 220, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Akaishi, T.; Schubert, D.; Abe, K. A pyrazole derivative of curcumin enhances memory. Neurobiol. Aging 2010, 31, 706–709. [Google Scholar] [CrossRef]
- Akaishi, T.; Abe, K. CNB-001, a synthetic pyrazole derivative of curcumin, suppresses lipopolysaccharide-induced nitric oxide production through the inhibition of NF-κB and p38 MAPK pathways in microglia. Eur. J. Pharmacol. 2018, 819, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Hijikuro, I.; Fujita, Y.; Teruya, T.; Kawakami, H.; Takahashi, T.; Sugimoto, H. Design and synthesis of curcumin derivatives as tau and amyloid β dual aggregation inhibitors. Bioorganic Med. Chem. Lett. 2016, 26, 5024–5028. [Google Scholar] [CrossRef] [PubMed]
- Urano, Y.; Takahachi, M.; Higashiura, R.; Fujiwara, H.; Funamoto, S.; Imai, S.; Futai, E.; Okuda, M.; Sugimoto, H.; Noguchi, N. Curcumin Derivative GT863 Inhibits Amyloid-Beta Production via Inhibition of Protein N-Glycosylation. Cells 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battisti, A.; Palumbo Piccionello, A.; Sgarbossa, A.; Vilasi, S.; Ricci, C.; Ghetti, F.; Spinozzi, F.; Marino Gammazza, A.; Giacalone, V.; Martorana, A.; et al. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017, 7, 31714–31724. [Google Scholar] [CrossRef] [Green Version]
- Boländer, A.; Kieser, D.; Voss, C.; Bauer, S.; Schön, C.; Burgold, S.; Bittner, T.; Hölzer, J.; Heyny-Von Haußen, R.; Mall, G.; et al. Bis(arylvinyl)pyrazines, -pyrimidines, and -pyridazines As Imaging Agents for Tau Fibrils and β-Amyloid Plaques in Alzheimer’s Disease Models. J. Med. Chem. 2012, 55, 9170–9180. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunkhamkaew, S.; Athipornchai, A.; Apiratikul, N.; Suksamrarn, A.; Ajavakom, V. Novel racemic tetrahydrocurcuminoid dihydropyrimidinone analogues as potent acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 2880–2882. [Google Scholar] [CrossRef]
- Lo Cascio, F.; Garcia, S.; Montalbano, M.; Puangmalai, N.; McAllen, S.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Modulating Disease-Relevant Tau Oligomeric Strains by Small Molecules. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Endo, H.; Nikaido, Y.; Nakadate, M.; Ise, S.; Konno, H. Structure activity relationship study of curcumin analogues toward the amyloid-beta aggregation inhibitor. Bioorg. Med. Chem. Lett. 2014, 24, 5621–5626. [Google Scholar] [CrossRef]
- Cashman, J.R.; Fiala, M. Diagnostic Methods And genetic Markers for Alzheimer Disease. U.S. Patent Application No. 12/407,756, 22 October 2009. [Google Scholar]
- Batie, S.; Lee, J.H.; Jama, R.A.; Browder, D.O.; Montano, L.A.; Huynh, C.C.; Marcus, L.M.; Tsosie, D.G.; Mohammed, Z.; Trang, V.; et al. Synthesis and biological evaluation of halogenated curcumin analogs as potential nuclear receptor selective agonists. Bioorg. Med. Chem. 2013, 21, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef] [Green Version]
- Bisceglia, F.; Seghetti, F.; Serra, M.; Zusso, M.; Gervasoni, S.; Verga, L.; Vistoli, G.; Lanni, C.; Catanzaro, M.; De Lorenzi, E.; et al. Prenylated Curcumin Analogues as Multipotent Tools to Tackle Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 1420–1433. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, V.; Shankar, R.; Kumar, K.; Rawal, R.K. Synthetic and Medicinal Prospective of Structurally Modified Curcumins. Curr. Top. Med. Chem. 2017, 17, 148–161. [Google Scholar] [CrossRef]
- Nieto, C.I.; Cornago, M.P.; Cabildo, M.P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Martínez Casanova, D.; Sánchez-Alegre, Y.R.; Escudero, E.; et al. Evaluation of the Antioxidant and Neuroprotectant Activities of New Asymmetrical 1,3-Diketones. Molecules 2018, 23, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, J.Y. Total synthesis of Calebin-A, preparation of its analogues, and their neuronal cell protectivity against beta-amyloid insult. Bioorg. Med. Chem. Lett. 2001, 11, 2541–2543. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Majeed, A.; Thomas, S.M. Synthesis of Calebin-A and its Biologically Active Analogs. U.S. Patent No. 9,365,486, 14 June 2016. [Google Scholar]
- Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Coto-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.-X.; Reiter, R.J. Alzheimer’s disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 2012, 52, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.N.; Liu, R.Y.; Kamphorst, W.; Hofman, M.A.; Swaab, D.F. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 2003, 35, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.E.; Liu, K.; Yan, X.; Toldo, S.; Selden, T.; Estrada, M.; Rodríguez-Franco, M.I.; Halquist, M.S.; Ye, D.; Zhang, S. Discovery of 5-(4-Hydroxyphenyl)-3-oxo-pentanoic Acid [2-(5-Methoxy-1H-indol-3-yl)-ethyl]-amide as a Neuroprotectant for Alzheimer’s Disease by Hybridization of Curcumin and Melatonin. ACS Chem. Neurosci. 2014, 5, 690–699. [Google Scholar] [CrossRef]
- Chojnacki, J.E.; Liu, K.; Saathoff, J.M.; Zhang, S. Bivalent ligands incorporating curcumin and diosgenin as multifunctional compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 7324–7331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmegeed, G.A.; Ahmed, H.H.; Hashash, M.A.; Abd-Elhalim, M.M.; El-kady, D.S. Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer’s disease candidates: Evidences-based on in vivo study. Steroids 2015, 101, 78–89. [Google Scholar] [CrossRef]
- Harish, G.; Venkateshappa, C.; Mythri, R.B.; Dubey, S.K.; Mishra, K.; Singh, N.; Vali, S.; Bharath, M.M.S. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: Implications for Parkinson’s disease. Bioorg. Med. Chem. 2010, 18, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Dolai, S.; Shi, W.; Corbo, C.; Sun, C.; Averick, S.; Obeysekera, D.; Farid, M.; Alonso, A.; Banerjee, P.; Raja, K. “Clicked” Sugar–Curcumin Conjugate: Modulator of Amyloid-β and Tau Peptide Aggregation at Ultralow Concentrations. ACS Chem. Neurosci. 2011, 2, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Hu, J.; Liu, A.; He, L.; Li, X.; Wei, H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorg. Med. Chem. 2017, 25, 2946–2955. [Google Scholar] [CrossRef]
- Dias, K.S.; De Paula, C.T.; Dos Santos, T.; Souza, I.N.; Boni, M.S.; Guimarães, M.J.; Da Silva, F.M.; Castro, N.G.; Neves, G.A.; Veloso, C.C.; et al. Design, synthesis and evaluation of novel feruloyl-donepezil hybrids as potential multitarget drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 130, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, L.; Zhang, H.; Gou, S.; Chen, L. Design, synthesis and biological evaluation of multifunctional tacrine-curcumin hybrids as new cholinesterase inhibitors with metal ions-chelating and neuroprotective property. Bioorg. Med. Chem. 2017, 25. [Google Scholar] [CrossRef] [PubMed]

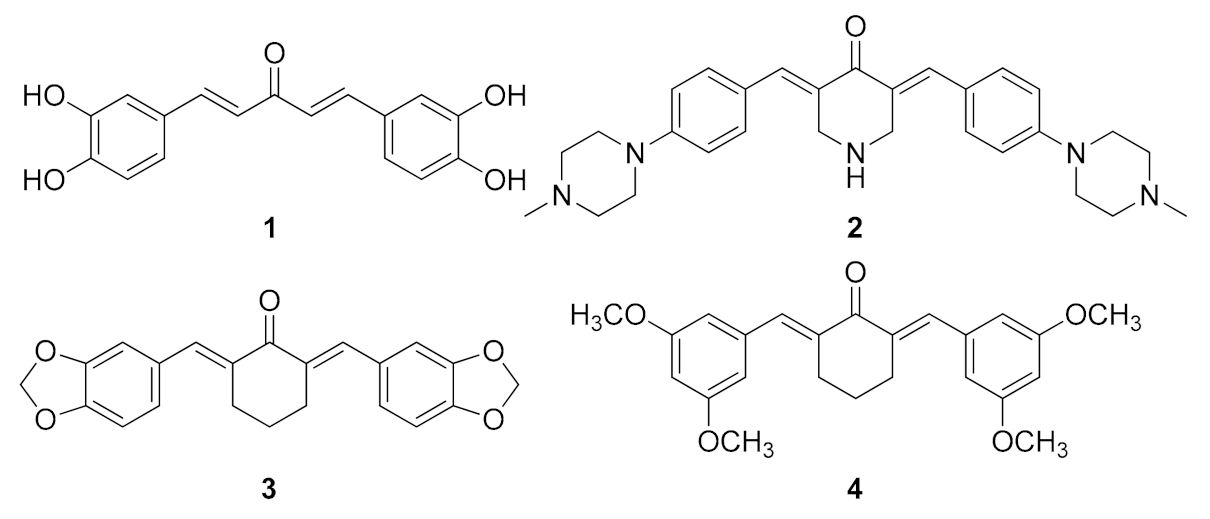










| Compound | Activity | Reference |
|---|---|---|
| MAC 1 (Figure 1) | Aβ oligomerization inhibitor | [111] |
| MAC 2 (Figure 1) | Aβ aggregation inhibitor Antioxidant Metal chelating | [113] |
| MAC 3 (Figure 1) | Aβ aggregation inhibitor Protection against Aβ oligomers toxicity Stabilization of proteins in the native state | [114] |
| MAC 4 (Figure 1) | Antioxidant | [115] |
| K2T21 (Figure 2) | Cu (II)-chelating Antiradical | |
| K2F21 (Figure 2) | Depolymerizing activity of Aβ (1–40) fibrils Antioxidant Antiapoptotic | [116] |
| 5 (Figure 3) | Interaction with Aβ aggregates | [104] |
| 6 (Figure 3) | Depolymerizing activity of tau aggregates Tau aggregation inhibitor γ-secretase activity inhibitor | [104] |
| CNB001 (Figure 3) | Anti-inflammatory | [121] |
| GT863 (Figure 3) | Aβ aggregation inhibitor Tau aggregation inhibitor Inhibitor of glycation of Nicastrin | [123] |
| 7–8a (Figure 3) | Aβ aggregation modulator | [124] |
| 8b (Figure 3) | Tau oligomers modulator | [87] |
| 9–10–11 (Figure 3) | Interaction with Aβ and tau aggregates | [125] |
| THC (Figure 4) | Anti-inflammatory | [126] |
| THDC THBDC THBDC DHPM (Figure 4) | Acetyl cholinesterase inhibitor | [127] |
| CL3–CL8 (Figure 5) | Tau oligomers modulator | [87,128] |
| 12–13 (Figure 6) | Aβ aggregation inhibitor | [111,112,113,114] |
| 14 (Figure 6) | BACE1 inhibitor | [129] |
| 15 (Figure 7) | Aβ aggregation inhibitor Diagnostic tool to detect Aβ amyloid | [118] |
| 16 (Figure 7) | Aβ aggregation inhibitor | [130] |
| 17 (Figure 7) | Antioxidant | [60] |
| 18 (Figure 7) | Aβ fibrillation inhibitor | [133] |
| 19 (Figure 8) | BACE1 inhibitor | [103,134] |
| 20–21 (Figure 9) | Metal chelating Scavenger activity against ROS | [135] |
| 22 (Figure 9) 24–25 (Figure 10) | Modulators of tau oligomers | [87] |
| 23 (Figure 10) | Protection against Aβ (25–35) toxicity | [137] |
| 26 (Figure 11) | Aβ oligomerization inhibitor Antioxidant | [141] |
| 27 (Figure 11) | Aβ aggregation inhibitor Antioxidant | [142] |
| 28–29 (Figure 11) | Increase of acetyl choline levels Increase of GSH levels Antiapoptotic | [143] |
| 30 (Figure 11) | Aβ aggregation inhibitor Tau-aggregation inhibitor | [145] |
| 31–32–33 (Figure 11) | Acetyl cholinesterase inhibitor Antioxidant Metal chelating | [146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Cascio, F.; Marzullo, P.; Kayed, R.; Palumbo Piccionello, A. Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases. Biomedicines 2021, 9, 173. https://doi.org/10.3390/biomedicines9020173
Lo Cascio F, Marzullo P, Kayed R, Palumbo Piccionello A. Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases. Biomedicines. 2021; 9(2):173. https://doi.org/10.3390/biomedicines9020173
Chicago/Turabian StyleLo Cascio, Filippa, Paola Marzullo, Rakez Kayed, and Antonio Palumbo Piccionello. 2021. "Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases" Biomedicines 9, no. 2: 173. https://doi.org/10.3390/biomedicines9020173
APA StyleLo Cascio, F., Marzullo, P., Kayed, R., & Palumbo Piccionello, A. (2021). Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases. Biomedicines, 9(2), 173. https://doi.org/10.3390/biomedicines9020173







