C-Peptide as a Therapy for Type 1 Diabetes Mellitus
Abstract
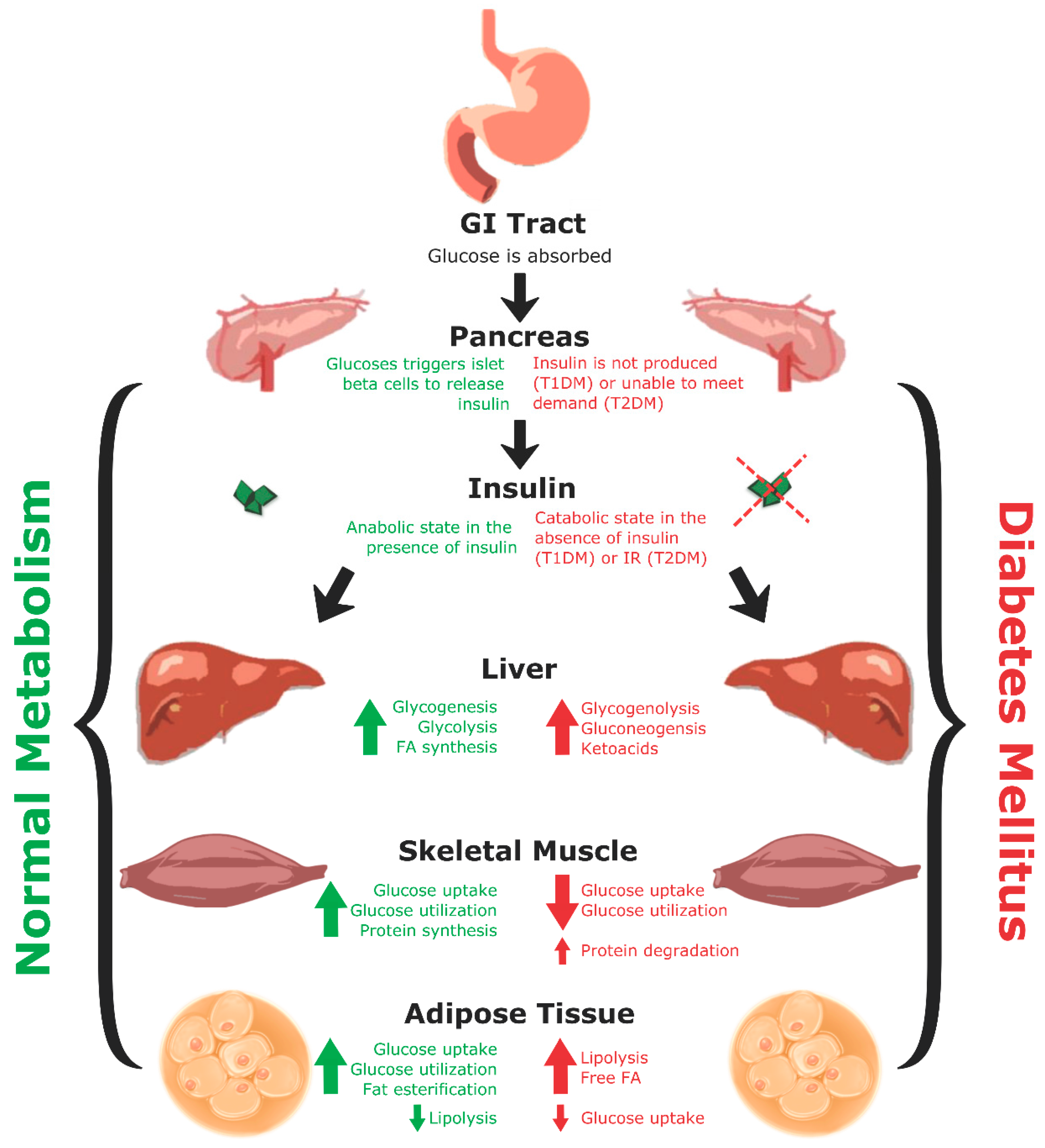
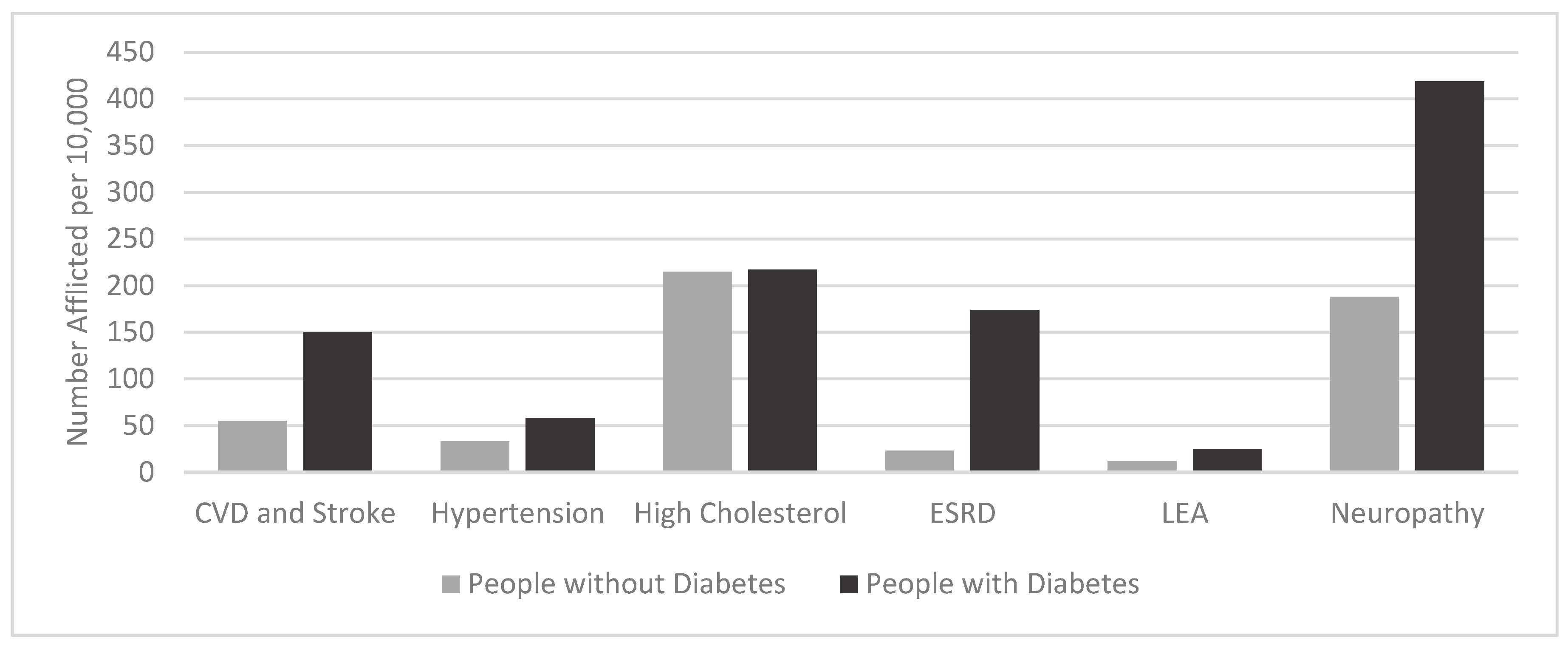
1. Diabetes Mellitus
2. Metabolic Dysregulation
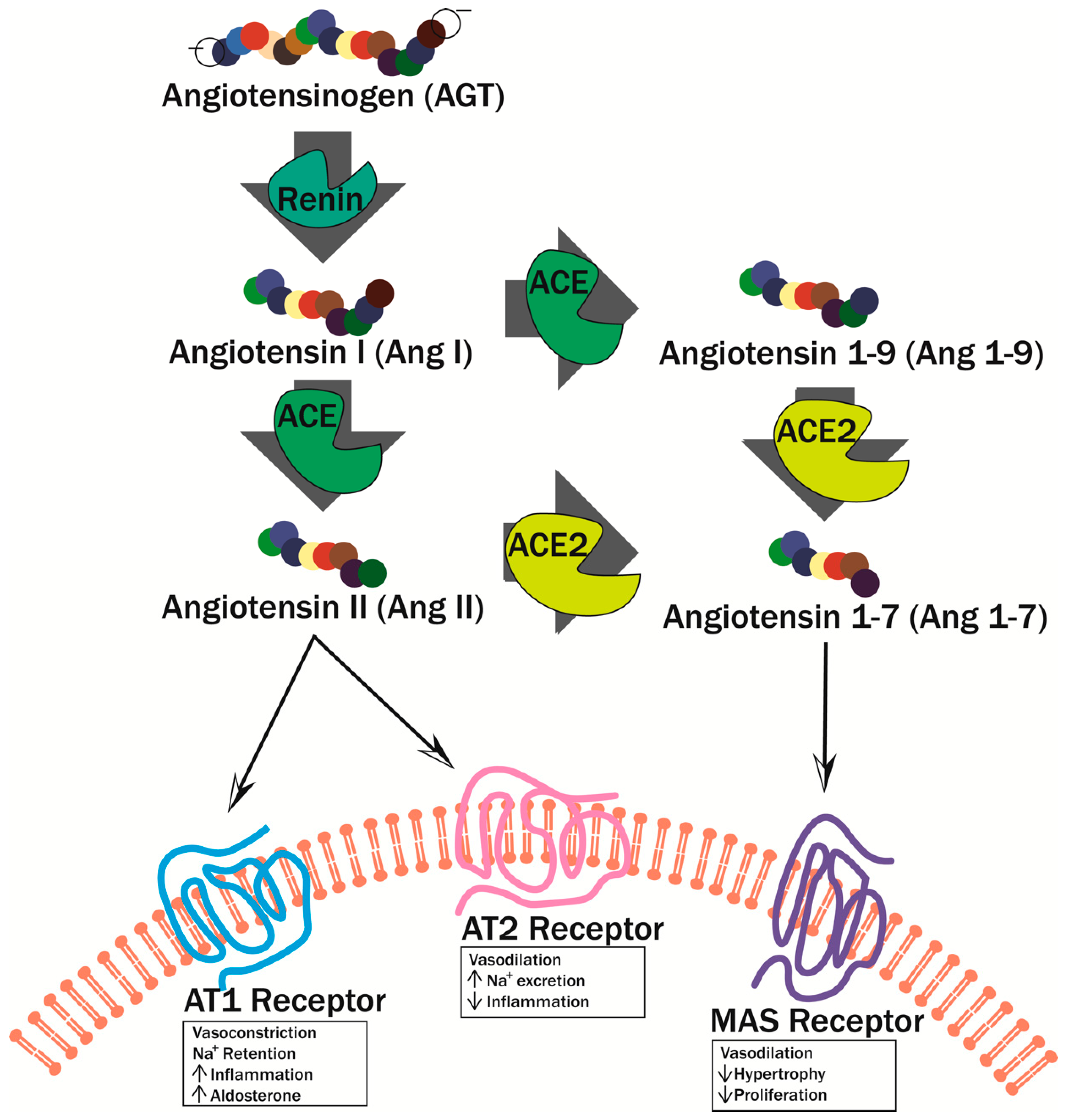
3. Endothelial Damage
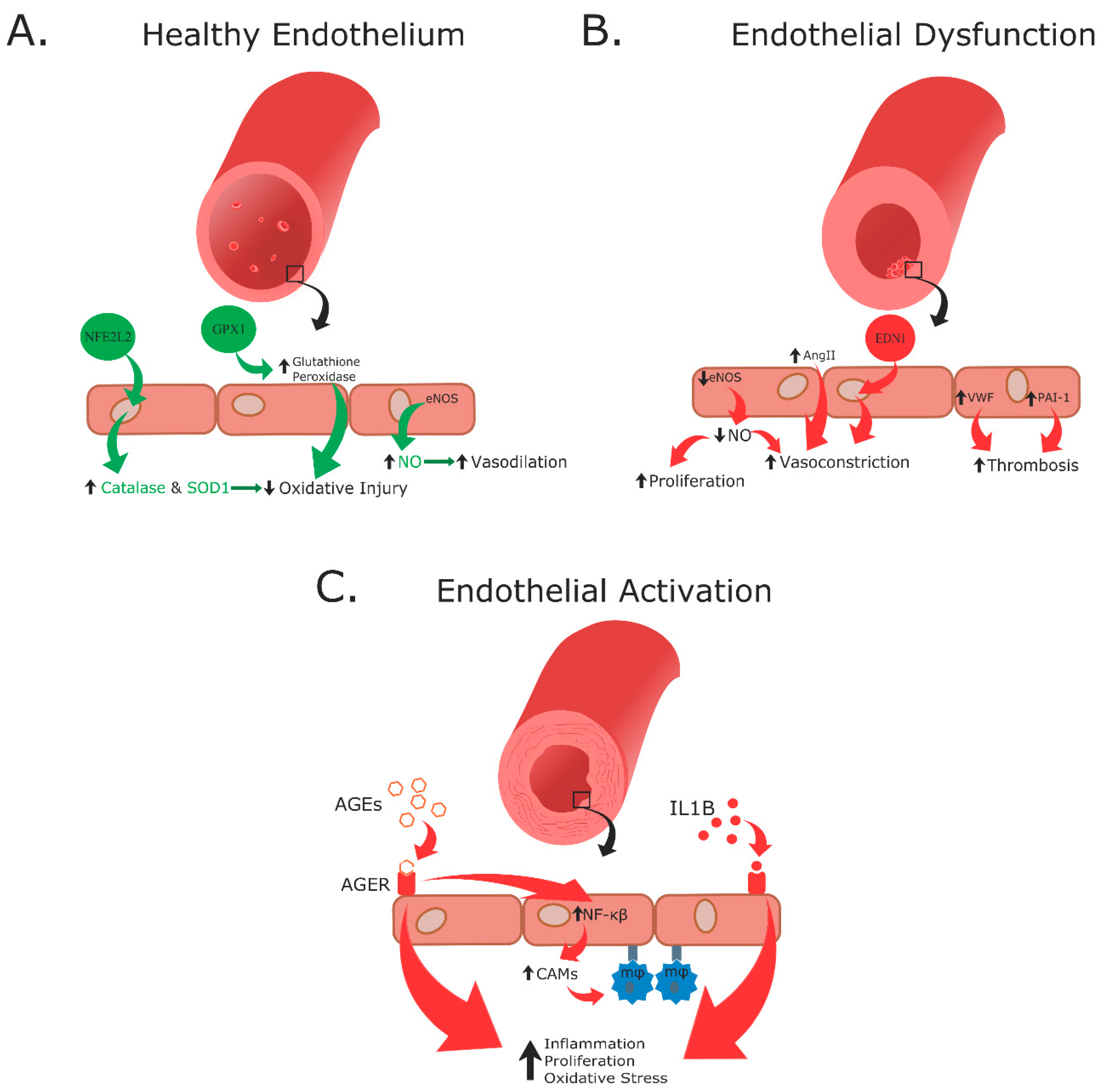
3.1. Endothelial Dysfunction
3.2. Endothelial Activation
3.3. Oxidative Stress
3.4. Cell Death
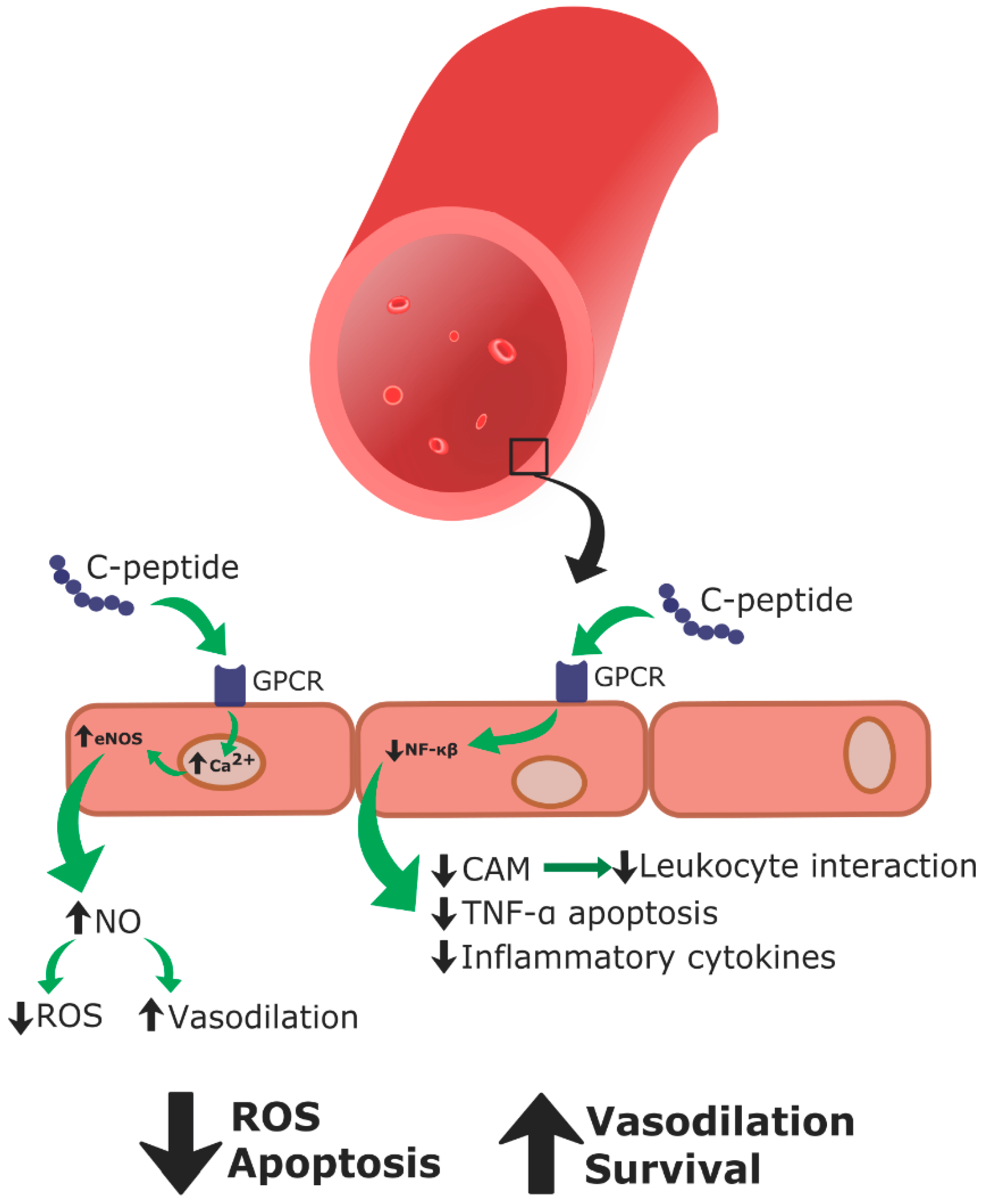
4. C-Peptide
4.1. C-Peptide in T1DM
4.2. C-Peptide in T2DM
4.3. Mechanism of Action
5. Physiological Delivery of C-Peptide
5.1. Dual Hormone Pump Therapy
5.2. Islet or Whole Pancreas Transplantation
5.3. Pancreatic Islet Encapsulation
5.4. Sertoli Cells
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Gregg, E.W.; Ford, E.S.; Geiss, L.S.; Bainbridge, K.E.; Fradkin, J.E. Prevalence of Diabetes and High Risk for Diabetes Using A1C Criteria in the U.S. Population in 1988–2006. Diabetes Care 2010, 33, 562–568. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Bixby, H.; Cowan, M.; Ali, M.; Taddei, C.; et al. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Heron, M. Deaths: Leading Causes for 2015. National Vital Statistics Reports: From the Centers for Disease Control and Prevention; National Center for Health Statistics, National Vital Statistics System: Hyattsville, MD, USA, 2017; Volume 66, pp. 1–76.
- Stokes, A.; Preston, S.H. Deaths Attributable to Diabetes in the United States: Comparison of Data Sources and Estimation Approaches. PLoS ONE 2017, 12, e0170219. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017; Centers for Disease Control and Prevention, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Urrutia-Rojas, X.; Menchaca, J. Prevalence of Risk for Type 2 Diabetes in School Children. J. Sch. Health 2006, 76, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- American Diabetes Association. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S34–S45. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Arana, M.; Radichev, I.; Smith, P.; Huarte, E.; Barajas, M. New Insights into Immunotherapy Strategies for Treating Autoimmune Diabetes. Int. J. Mol. Sci. 2019, 20, 4789. [Google Scholar] [CrossRef]
- Mathieu, C.; Gillard, P.; Benhalima, K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat. Rev. Endocrinol. 2017, 13, 385–399. [Google Scholar] [CrossRef]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef]
- Brown, L.; Edelman, E.R. Optimal Control of Blood Glucose: The Diabetic Patient or the Machine? Sci. Transl. Med. 2010, 2, 27ps18. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I.; Occhioni, S.; Tonolo, G. Sex-gender differences in diabetes vascular complications and treatment. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Dinarello, C.A.; Mandrup-Poulsen, T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019, 19, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-Cell Deficit and Increased beta-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Welch, B.J.; Zib, I. Case Study: Diabetic Ketoacidosis in Type 2 Diabetes: “Look Under the Sheets”. Clin. Diabetes 2004, 22, 198–200. [Google Scholar] [CrossRef][Green Version]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S90–S102. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of Pancreatic beta-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes 2005, 54 (Suppl. 2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E. Proinsulin and C-peptide: A review. Metab. Clin. Exp. 1977, 26, 547–587. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Leslie, R.D.G. Metabolic changes in diabetes. Eye 1993, 7 Pt 2, 205–208. [Google Scholar] [CrossRef]
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Castellano, J.M.; Farkouh, M.E.; Fuster, V. The Relationships between Cardiovascular Disease and Diabetes: Focus on pathogenesis. Endocrinol. Metab. Clin. N. Am. 2014, 43, 41–57. [Google Scholar] [CrossRef]
- Ren, J.; Ceylan-Isik, A.F. Diabetic Cardiomyopathy: Do Women Differ From Men? Endocrine 2004, 25, 73–83. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; De Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 Diabetes Mellitus and Cardiovascular Disease: A scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- USRDS: The United States Renal Data System. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 42, 1–230. [CrossRef]
- Fox, C.S.; Coady, S.; Sorlie, P.D.; Levy, D.; Meigs, J.B.; D’Agostino, R.B.; Wilson, P.W.; Savage, P.J. Trends in Cardiovascular Complications of Diabetes. JAMA 2004, 292, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Vamos, E.P.; Bottle, A.; Edmonds, M.E.; Valabhji, J.; Majeed, A.; Millett, C. Changes in the Incidence of Lower Extremity Amputations in Individuals With and Without Diabetes in England Between 2004 and 2008. Diabetes Care 2010, 33, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Hora, I.; Geiss, L.S.; Gregg, E.W.; Albright, A. Incidence of End-Stage Renal Disease Attributed to Diabetes Among Persons with Diagnosed Diabetes—United States and Puerto Rico, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1165–1170. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic kidney disease: World wide difference of prevalence and risk factors. J. Nephropharmacol. 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2015; NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016; pp. 1–8.
- Roberts, A.C.; Porter, K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes Vasc. Dis. Res. 2013, 10, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Joshua, I.G.; Zhang, Q.; Falcone, J.C.; Bratcher, A.P.; Rodriguez, W.E.; Tyagi, S.C. Mechanisms of endothelial dysfunction with development of type 1 diabetes mellitus: Role of insulin and C-peptide. J. Cell. Biochem. 2005, 96, 1149–1156. [Google Scholar] [CrossRef]
- Oliver, F.J.; de la Rubia, G.; Feener, E.P.; Lee, M.E.; Loeken, M.R.; Shiba, T.; Quertermous, T.; King, G.L. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J. Biol. Chem. 1991, 266, 23251–23256. [Google Scholar] [CrossRef]
- Johansson, J.; Ekberg, K.; Shafqat, J.; Henriksson, M.; Chibalin, A.; Wahren, J.; Jornvall, H. Molecular effects of proinsulin C-peptide. Biochem. Biophys. Res. Commun. 2002, 295, 1035–1040. [Google Scholar] [CrossRef]
- Bhatt, M.P.; Lim, Y.C.; Ha, K.S. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc. Res. 2014, 104, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Larsson, C. C-peptide: New findings and therapeutic possibilities. Diabetes Res. Clin. Pr. 2015, 107, 309–319. [Google Scholar] [CrossRef]
- Beckman, J.A.; Goldfine, A.B.; Gordon, M.B.; Garrett, L.A.; Creager, M.A. Inhibition of Protein Kinase Cbeta Prevents Impaired Endothelium-Dependent Vasodilation Caused by Hyperglycemia in Humans. Circ. Res. 2002, 90, 107–111. [Google Scholar] [CrossRef]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture). Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Schmidt, A.M.; Zhang, C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in Type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H491–H498. [Google Scholar] [CrossRef]
- Garcia, J.G.; Pavalko, F.M.; Patterson, C.E. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 1995, 6, 609–626. [Google Scholar] [CrossRef]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.; Lemieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Lazos, S.A.; Tong, K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J. Clin. Investig. 1995, 95, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Feener, E.P.; Northrup, J.M.; Aiello, L.P.; King, G.L. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J. Clin. Investig. 1995, 95, 1353–1362. [Google Scholar] [CrossRef]
- Shimizu, T.; Uematsu, M.; Yoshizaki, T.; Obata, J.E.; Nakamura, T.; Fujioka, D.; Watanabe, K.; Watanabe, Y.; Kugiyama, K. Myocardial Production of Plasminogen Activator Inhibitor-1 is Associated with Coronary Endothelial and Ventricular Dysfunction after Acute Myocardial Infarction. J. Atheroscler. Thromb. 2016, 23, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 Is Proinflammatory in the Diabetic Retina. Investig. Opthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Hori, O.; Chen, J.X.; Li, J.F.; Crandall, J.; Zhang, J.; Cao, R.; Yan, S.D.; Brett, J.; Stern, D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Investig. 1995, 96, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte Migration into Inflamed Tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Potenza, M.A.; Gagliardi, S.; Nacci, C.; Carratu’, M.R.; Montagnani, M. Endothelial Dysfunction in Diabetes: From Mechanisms to Therapeutic Targets. Curr. Med. Chem. 2009, 16, 94–112. [Google Scholar] [CrossRef]
- Morita, M.; Yano, S.; Yamaguchi, T.; Sugimoto, T. Advanced glycation end products-induced reactive oxygen species generation is partly through NF-kappa B activation in human aortic endothelial cells. J. Diabetes Complicat. 2013, 27, 11–15. [Google Scholar] [CrossRef]
- Sun, Q.A.; Runge, M.S.; Madamanchi, N.R. Oxidative stress, NADPH oxidases, and arteries. Hamostaseologie 2016, 36, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Hink, U.; Tsilimingas, N.; Wendt, M.; Munzel, T. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus: Therapeutic implications. Treat. Endocrinol. 2003, 2, 293–304. [Google Scholar] [CrossRef]
- Jørgensen, P.L. Structure, function and regulation of Na,K-ATPase in the kidney. Kidney Int. 1986, 29, 10–20. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Shapiro, J.I.; Sodhi, K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Obesity and Cardiovascular Disease. Molecules 2016, 21, 1172. [Google Scholar] [CrossRef]
- Scarpini, E.; Bianchi, R.; Moggio, M.; Sciacco, M.; Fiori, M.G.; Scarlato, G. Decrease of nerve Na+,K+-ATPase activity in the pathogenesis of human diabetic neuropathy. J. Neurol. Sci. 1993, 120, 159–167. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Bao, M.; Yang, Y.; Jun, H.S.; Yoon, J.W. Molecular Mechanisms for Gender Differences in Susceptibility to T Cell-Mediated Autoimmune Diabetes in Nonobese Diabetic Mice. J. Immunol. 2002, 168, 5369–5375. [Google Scholar] [CrossRef]
- Ekberg, K.; Brismar, T.; Johansson, B.L.; Lindstrom, P.; Juntti-Berggren, L.; Norrby, A.; Berne, C.; Arnqvist, H.J.; Bolinder, J.; Wahren, J. C-Peptide Replacement Therapy and Sensory Nerve Function in Type 1 Diabetic Neuropathy. Diabetes Care 2007, 30, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Forst, T.; Kunt, T.; Wilhelm, B.; Weber, M.M.; Pfutzner, A. Role of C-Peptide in the Regulation of Microvascular Blood Flow. Exp. Diabetes Res. 2008, 2008, 176245. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.P.; Zhang, F.; DiMarchi, R.D. Insulin structure and function. Biopolymers 2007, 88, 687–713. [Google Scholar] [CrossRef]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2013, 30, 803–817. [Google Scholar] [CrossRef]
- Matthews, D.R.; Rudenski, A.S.; Burnett, M.A.; Darling, P.; Turner, R.C. The half-life of endogenous insulin and c-peptide in man assessed by Somatostatin suppression. Clin. Endocrinol. 1985, 23, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wahren, J.; Ekberg, K.; Johansson, J.; Henriksson, M.; Pramanik, A.; Johansson, B.L.; Rigler, R.; Jornvall, H. Role of C-peptide in human physiology. Am. J. Physiol. Metab. 2000, 278, E759–E768. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Vindigni, A.; Chang, K.; Stramm, L.; Chance, R.; Heath, W.F.; DiMarchi, R.D.; Di Cera, E.; Williamson, J.R. Prevention of Vascular and Neural Dysfunction in Diabetic Rats by C-Peptide. Science 1997, 277, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Messina, E.J. C-peptide induces a concentration-dependent dilation of skeletal muscle arterioles only in presence of insulin. Am. J. Physiol. 1999, 276, H1223–H1228. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Kunt, T.; Forst, T.; Closs, E.I.; Lehmann, R.; Flohr, T.; Gabriel, M.; Schafer, D.; Gopfert, A.; Pfutzner, A.; et al. Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide Biol. Chem. 2003, 9, 95–102. [Google Scholar] [CrossRef]
- Giebink, A.W.; Vogel, P.A.; Medawala, W.; Spence, D.M. C-peptide-stimulated nitric oxide production in a cultured pulmonary artery endothelium is erythrocyte mediated and requires Zn(2+). Diabetes Metab. Res. Rev. 2013, 29, 44–52. [Google Scholar] [CrossRef]
- Forst, T.; Kunt, T.; Pohlmann, T.; Goitom, K.; Engelbach, M.; Beyer, J.; Pfutzner, A. Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J. Clin. Investig. 1998, 101, 2036–2041. [Google Scholar] [CrossRef][Green Version]
- Scalia, R.; Coyle, K.M.; Levine, B.J.; Booth, G.; Lefer, A.M. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 2357–2364. [Google Scholar] [CrossRef]
- De La Tour, D.D.; Raccah, D.; Jannot, M.F.; Coste, T.; Rougerie, C.; Vague, P. Erythrocyte Na/K ATPase activity and diabetes: Relationship with C-peptide level. Diabetologia 1998, 41, 1080–1084. [Google Scholar] [CrossRef]
- Kunt, T.; Schneider, S.; Pfutzner, A.; Goitum, K.; Engelbach, M.; Schauf, B.; Beyer, J.; Forst, T. The effect of human proinsulin C-peptide on erythrocyte deformability in patients with Type I diabetes mellitus. Diabetologia 1999, 42, 465–471. [Google Scholar] [CrossRef][Green Version]
- Hach, T.; Forst, T.; Kunt, T.; Ekberg, K.; Pfutzner, A.; Wahren, J. C-Peptide and Its C-Terminal Fragments Improve Erythrocyte Deformability in Type 1 Diabetes Patients. Exp. Diabetes Res. 2008, 2008, 730594. [Google Scholar] [CrossRef]
- Lim, Y.C.; Bhatt, M.P.; Kwon, M.H.; Park, D.; Lee, S.; Choe, J.; Hwang, J.; Kim, Y.M.; Ha, K.S. Prevention of VEGF-mediated microvascular permeability by C-peptide in diabetic mice. Cardiovasc. Res. 2014, 101, 155–164. [Google Scholar] [CrossRef]
- Slinko, S.; Piraino, G.; Hake, P.W.; Ledford, J.R.; O’Connor, M.; Lahni, P.; Solan, P.D.; Wong, H.R.; Zingarelli, B. Combined Zinc Supplementation With Proinsulin C-Peptide Treatment Decreases the Inflammatory Response and Mortality in Murine Polymicrobial Sepsis. Shock 2014, 41, 292–300. [Google Scholar] [CrossRef]
- Sjoquist, M.; Huang, W.; Johansson, B.L. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int. 1998, 54, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Samnegard, B.; Jacobson, S.H.; Jaremko, G.; Johansson, B.L.; Sjoquist, M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int. 2001, 60, 1258–1265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samnegard, B.; Jacobson, S.H.; Jaremko, G.; Johansson, B.L.; Ekberg, K.; Isaksson, B.; Eriksson, L.; Wahren, J.; Sjoquist, M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrol. Dial. Transplant. 2005, 20, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.L.; Sjoberg, S.; Wahren, J. The influence of human C-peptide on renal function and glucose utilization in Type 1 (insulin-dependent) diabetic patients. Diabetologia 1992, 35, 121–128. [Google Scholar] [CrossRef]
- Johansson, B.L.; Borg, K.; Fernqvist-Forbes, E.; Kernell, A.; Odergren, T.; Wahren, J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabet. Med. J. Bri. Diabet. Assoc. 2000, 17, 181–189. [Google Scholar] [CrossRef]
- Cotter, M.A.; Ekberg, K.; Wahren, J.; Cameron, N.E. Effects of proinsulin C-peptide in experimental diabetic neuropathy: Vascular actions and modulation by nitric oxide synthase inhibition. Diabetes 2003, 52, 1812–1817. [Google Scholar] [CrossRef]
- Ghorbani, A.; Omrani, G.R.; Hadjzadeh, M.A.; Varedi, M. Effects of Rat C-peptide-II on Lipolysis and Glucose Consumption in Cultured Rat Adipose Tissue. Exp. Clin. Endocrinol. Diabetes 2011, 119, 343–347. [Google Scholar] [CrossRef]
- Sima, A.A.; Zhang, W.; Sugimoto, K.; Henry, D.; Li, Z.; Wahren, J.; Grunberger, G. C-peptide prevents and improves chronic Type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia 2001, 44, 889–897. [Google Scholar] [CrossRef]
- Johansson, B.L.; Borg, K.; Fernqvist-Forbes, E.; Odergren, T.; Remahl, S.; Wahren, J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia 1996, 39, 687–695. [Google Scholar] [CrossRef]
- Ekberg, K.; Brismar, T.; Johansson, B.L.; Jonsson, B.; Lindstrom, P.; Wahren, J. Amelioration of Sensory Nerve Dysfunction by C-Peptide in Patients With Type 1 Diabetes. Diabetes 2003, 52, 536–541. [Google Scholar] [CrossRef]
- Walcher, D.; Aleksic, M.; Jerg, V.; Hombach, V.; Zieske, A.; Homma, S.; Strong, J.; Marx, N. C-peptide induces chemotaxis of human CD4-positive cells: Involvement of pertussis toxin-sensitive G-proteins and phosphoinositide 3-kinase. Diabetes 2004, 53, 1664–1670. [Google Scholar] [CrossRef]
- Marx, N.; Walcher, D.; Raichle, C.; Aleksic, M.; Bach, H.; Grb, M.; Hombach, V.; Libby, P.; Zieske, A.; Homma, S.; et al. C-Peptide Colocalizes with Macrophages in Early Arteriosclerotic Lesions of Diabetic Subjects and Induces Monocyte Chemotaxis In Vitro. Arter. Thromb. Vasc. Biol. 2004, 24, 540–545. [Google Scholar] [CrossRef]
- Kitazawa, M.; Shibata, Y.; Hashimoto, S.; Ohizumi, Y.; Yamakuni, T. Proinsulin C-Peptide Stimulates a PKC/IκB/NF-κB Signaling Pathway to Activate COX-2 Gene Transcription in Swiss 3T3 Fibroblasts. J. Biochem. 2006, 139, 1083–1088. [Google Scholar] [CrossRef]
- Ohtomo, Y.; Bergman, T.; Johansson, B.L.; Jörnvall, H.; Wahren, J. Differential effects of proinsulin C-peptide fragments on Na+, K+-ATPase activity of renal tubule segments. Diabetologia 1998, 41, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Medawala, W.; McCahill, P.; Giebink, A.; Meyer, J.; Ku, C.J.; Spence, D.M. A Molecular Level Understanding of Zinc Activation of C-peptide and its Effects on Cellular Communication in the Bloodstream. Rev. Diabet. Stud. 2009, 6, 148–158. [Google Scholar] [CrossRef]
- Hills, C.E.; Brunskill, N.J.; Squires, P.E. C-Peptide as a Therapeutic Tool in Diabetic Nephropathy. Am. J. Nephrol. 2010, 31, 389–397. [Google Scholar] [CrossRef]
- Lim, Y.C.; Bhatt, M.P.; Kwon, M.H.; Park, D.; Na, S.; Kim, Y.M.; Ha, K.S. Proinsulin C-Peptide Prevents Impaired Wound Healing by Activating Angiogenesis in Diabetes. J. Investig. Dermatol. 2015, 135, 269–278. [Google Scholar] [CrossRef]
- Lindenblatt, N.; Braun, B.; Menger, M.D.; Klar, E.; Vollmar, B. C-peptide exerts antithrombotic effects that are repressed by insulin in normal and diabetic mice. Diabetologia 2006, 49, 792–800. [Google Scholar] [CrossRef]
- Nordquist, L.; Johansson, M. Proinsulin C-peptide: Friend or foe in the development of diabetes-associated complications? Vasc. Health Risk Manag. 2008, 4, 1283–1288. [Google Scholar] [CrossRef]
- Yosten, G.L.; Kolar, G.R.; Redlinger, L.J.; Samson, W.K. Evidence for an interaction between proinsulin C-peptide and GPR146. J. Endocrinol. 2013, 218, B1–B8. [Google Scholar] [CrossRef] [PubMed]
- Kolar, G.R.; Grote, S.M.; Yosten, G.L. Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146. J. Intern. Med. 2017, 281, 25–40. [Google Scholar] [CrossRef]
- Timimi, F.K.; Ting, H.H.; Haley, E.A.; Roddy, M.A.; Ganz, P.; Creager, M.A. Vitamin C Improves Endothelium-Dependent Vasodilation in Patients with Insulin-Dependent Diabetes Mellitus. J. Am. Coll. Cardiol. 1998, 31, 552–557. [Google Scholar] [CrossRef]
- Yosten, G.L.C.; Maric-Bilkan, C.; Luppi, P.; Wahren, J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am. J. Physiol. Metab. 2014, 307, E955–E968. [Google Scholar] [CrossRef]
- Nerelius, C.; Alvelius, G.; Jornvall, H. N-terminal segment of proinsulin C-peptide active in insulin interaction/desaggregation. Biochem. Biophys. Res. Commun. 2010, 403, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.; Kann, P.; Pfutzner, A. Influence of C-Peptide on Glucose Utilisation. Exp. Diabetes Res. 2008, 2008, 769483. [Google Scholar] [CrossRef]
- O’Connor, M.R.; Carlin, K.; Coker, T.; Zierler, B.; Pihoker, C. Disparities in Insulin Pump Therapy Persist in Youth With Type 1 Diabetes Despite Rising Overall Pump Use Rates. J. Pediatr. Nurs. 2019, 44, 16–21. [Google Scholar] [CrossRef]
- Fiorina, P.; Shapiro, A.M.; Ricordi, C.; Secchi, A. The Clinical Impact of Islet Transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2008, 8, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, R.W.; Gruessner, A.C. Pancreas Transplant Alone: A procedure coming of age. Diabetes Care 2013, 36, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, A.C.; Gill, J.S. Financial Incompatibility and Paired Kidney Exchange: Walking a Tightrope or Blazing a Trail? Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2017, 17, 597–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, T.C.; Barshes, N.R.; Agee, E.E.; O’Mahoney, C.A.; Brunicardi, F.C.; Goss, J.A. The effect of whole organ pancreas transplantation and PIT on diabetic complications. Curr. Diabetes Rep. 2006, 6, 323–327. [Google Scholar] [CrossRef]
- Fioretto, P.; Steffes, M.W.; Sutherland, D.E.; Goetz, F.C.; Mauer, M. Reversal of Lesions of Diabetic Nephropathy after Pancreas Transplantation. N. Engl. J. Med. 1998, 339, 69–75. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Lakey, J.R.T.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Ryan, E.A.; Lakey, J.R.; Paty, B.W.; Imes, S.; Korbutt, G.S.; Kneteman, N.M.; Bigam, D.; Rajotte, R.V.; Shapiro, A.M. Successful Islet Transplantation: Continued Insulin Reserve Provides Long-Term Glycemic Control. Diabetes 2002, 51, 2148–2157. [Google Scholar] [CrossRef]
- Senior, P.; Kin, T.; Shapiro, A.M.J.; Koh, A. Islet Transplantation at the University of Alberta: Status Update and Review of Progress over the Last Decade. Can. J. Diabetes 2012, 36, 32–37. [Google Scholar] [CrossRef]
- Shapiro, A.M. Islet Transplantation in Type 1 Diabetes: Ongoing Challenges, Refined Procedures, and Long-Term Outcome. Rev. Diabet. Stud. 2012, 9, 385–406. [Google Scholar] [CrossRef]
- Fiorina, P.; Folli, F.; Zerbini, G.; Maffi, P.; Gremizzi, C.; Di Carlo, V.; Socci, C.; Bertuzzi, F.; Kashgarian, M.; Secchi, A. Islet Transplantation Is Associated with Improvement of Renal Function among Uremic Patients with Type I Diabetes Mellitus and Kidney Transplants. J. Am. Soc. Nephrol. 2003, 14, 2150–2158. [Google Scholar] [CrossRef]
- Plesner, A.; Verchere, C.B. Advances and Challenges in Islet Transplantation: Islet Procurement Rates and Lessons Learned from Suboptimal Islet Transplantation. J. Transplant. 2011, 2011, 979527. [Google Scholar] [CrossRef]
- HRSA; UNOS; URREA. 2018 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplanted Recipients: Transplant Data 2007–2018; Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation: Rockville, MD, USA; United Network for Organ Sharing: Richmond, VA, USA; University Renal Research and Education Association: Ann Arbor, MI, USA, 2018.
- Marcen, R. Immunosuppressive Drugs in Kidney Transplantation: Impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009, 69, 2227–2243. [Google Scholar] [CrossRef]
- Vaithilingam, V.; Tuch, B.E. Islet Transplantation and Encapsulation: An Update on Recent Developments. Rev. Diabet. Stud. 2011, 8, 51–67. [Google Scholar] [CrossRef]
- Dimitrioglou, N.; Kanelli, M.; Papageorgiou, E.; Karatzas, T.; Hatziavramidis, D. Paving the way for successful islet encapsulation. Drug Discov. Today 2019, 24, 737–748. [Google Scholar] [CrossRef]
- Jacobs-Tulleneers-Thevissen, D.; Chintinne, M.; Ling, Z.; Gillard, P.; Schoonjans, L.; Delvaux, G.; Strand, B.L.; Gorus, F.; Keymeulen, B.; Pipeleers, D. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 2013, 56, 1605–1614. [Google Scholar] [CrossRef]
- Korsgren, O. Islet Encapsulation: Physiological Possibilities and Limitations. Diabetes 2017, 66, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Dhabuwala, C.B.; Shichi, H. Immunotolerance induced by intratesticular antigen priming: Expression of TGF-beta, Fas and Fas ligand. Ocul. Immunol. Inflamm. 1997, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Vadala, S.; Dufour, J.M. An overview of a Sertoli cell transplantation model to study testis morphogenesis and the role of the Sertoli cells in immune privilege. Environ. Epigenet. 2017, 3, dvx012. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J. Therapeutic potential of immune privileged Sertoli cells. Anim. Reprod. 2015, 12, 105–117. [Google Scholar]
- Dufour, J.M.; Dass, B.; Halley, K.R.; Korbutt, G.S.; Dixon, D.E.; Rajotte, R.V. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 2008, 17, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Halley, K.; Dyson, E.L.; Kaur, G.; Mital, P.; Uong, P.M.; Dass, B.; Crowell, S.N.; Dufour, J.M. Delivery of a Therapeutic Protein by Immune-Privileged Sertoli Cells. Cell Transplant 2010, 19, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Mital, P.; Kaur, G.; Bowlin, B.; Paniagua, N.J.; Korbutt, G.S.; Dufour, J.M. Nondividing, Postpubertal Rat Sertoli Cells Resumed Proliferation after Transplantation. Biol. Reprod. 2014, 90, 13. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Babcock, R.L.; Mueller, K.; Dufour, J.M. Sertoli Cells Engineered to Express Insulin to Lower Blood Glucose in Diabetic Mice. DNA Cell Biol. 2018, 37, 680–690. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Pasham, M.; Tessanne, K.; Long, C.R.; Dufour, J.M. Sustained expression of insulin by a genetically engineered sertoli cell line after allotransplantation in diabetic BALB/c mice. Biol. Reprod. 2014, 90, 109. [Google Scholar] [CrossRef]
- Dufour, J.M.; Gores, P.; Hemendinger, R.; Emerich, D.F.; Halberstadt, C.R. Transgenic Sertoli cells as a vehicle for gene therapy. Cell Transplant 2004, 13, 1–6. [Google Scholar] [CrossRef]

| Study | Model | C-Peptide Delivery | Results | Ref. |
|---|---|---|---|---|
| In vivo | STZ 1-induced diabetic rats | Injection with biosynthetic human C-peptide 2×/day for 5 weeks | Improvement of vascular and neural dysfunction by preventing sodium potassium ATPase disruption | [78] |
| In vitro | Rat arterioles from cremaster muscles | Biosynthetic human C-peptide (0.3–1000 ng/mL doses) | Increased arteriolar dilation through NO-mechanism | [79] |
| In vitro | Bovine aortic endothelial cells | Human C-peptide (0.033–66.6 nM) | Increased eNOS activity and NO synthesis through increased calcium concentrations intracellularly; increased blood flow to extremities and skin | [80] |
| In vitro | Bovine pulmonary aortic endothelial cells and human erythrocytes | Human C-peptide (20 nM) | C-peptide plus zinc stimulated NO production through erythrocyte mediation | [81] |
| Clinical Study | T1DM patients | Intravenous administration of C-peptide with insulin for one hour | Increased capillary blood flow velocity to extremities and skin | [82] |
| In vivo | Male Sprague Dawley rats | Intravenous administration of bolus of biosynthetic human C-peptide (7 or 70 nmol/kg) | Decreased inflammation by inhibiting endothelial-leukocyte interaction through decreased endothelial cell surface expressions of P-selectin and ICAM-1 by a NO-dependent mechanism | [83] |
| Clinical Study | T1DM and T2DM patients | Comparison of ATPase activity and C-peptide levels between T1DM and T2DM patient groups | Lower C-peptide levels correlated with lower erythrocyte sodium potassium ATPase activity | [84] |
| Ex vivo | T1DM patient and healthy control blood samples | Preincubation of erythrocytes with proinsulin C-peptide (0–66.6 ng/L) | Improvement of erythrocyte deformability | [85] |
| Ex vivo | T1DM patient and healthy control blood samples | Incubation of blood samples with human C-peptide or C-peptide fragments (6.6 nM) | Improvement of erythrocyte deformability | [86] |
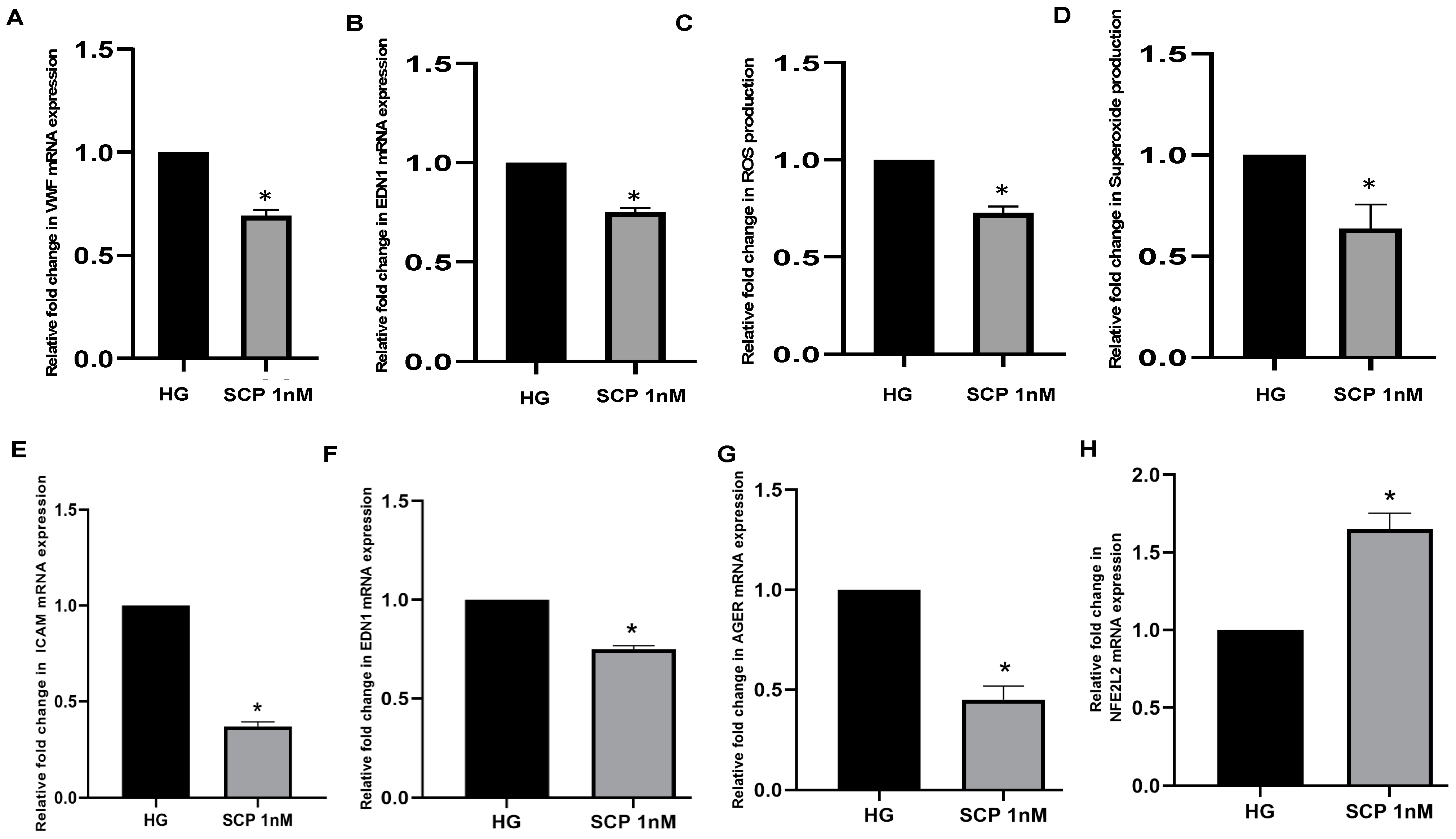
| In vitro | Human umbilical vein endothelial cells | Incubation with C-peptide (0.5 nM) | Decreased ROS production through inhibition of intracellular VEGF mechanism | [87] |
| In vivo | STZ 1-induced diabetic mice | Injected with C-peptide (2 μL) into eye | Decreased vascular permeability; decreased microvascular leakage in back skin and retina | [87] |
| In vivo | Male C57BL/6 mice | Injected with zinc gluconate (1.3 mg/kg) daily for three days before infection, then injected with of C-peptide (280 nmol/kg) | Zinc availability before polymicrobial infection is necessary for C-peptide’s anti-inflammatory functions through management of NF-κB pathways | [88] |
| In vivo | STZ 1-induced diabetic rats | Intravenous administration of human C-peptide (0.5 nmol/kg per minute) for 140 min | Reduced glomerular hyperfiltration rate, reduced glomerular protein leakage, and restored half of normal renal functional protein reserve | [89] |
| In vivo | STZ 1-induced diabetic rats | Intravenous administration of rat C-peptide II (50 pmol/kg per minute) for 14 days | Prevented glomerular hypertrophy, reduced glomerular hyperfiltration rate, prevented albuminuria | [90] |
| In vivo | STZ 1-induced diabetic rats | Subcutaneous infusion of rat C-peptide II (50 pmol/kg per minute) for four weeks | Prevented glomerular hypertrophy, reduced mesangial matrix expansion of diabetic nephropathy | [91] |
| Clinical Study | T1DM patients | Initial intravenous administration of C-peptide overnight, then two infusions of C-peptide (5 and 30 pmol/kg per minute) for one hour | Reduced glomerular filtration rate, increased effective renal plasma flow, increased whole-body glucose utilization | [92] |
| Clinical Study | Normotensive patients having micro- albuminuria | Daily subcutaneous injection of human C-peptide (600 nmol) with regular insulin treatment for three months | Improved glycemic control, decreased urinary albumin excretion, decreased nerve dysfunction | [93] |
| In vivo | STZ 1-induced diabetic rats | Subcutaneous osmotic minipump implants with rat C-peptide II (50 pmol/kg per minute) | Increased sciatic and saphenous nerve conduction velocity; improved nerve function | [94] |
| Ex vivo | Retroperitoneal adipose tissue from male rats | Incubated with C-peptide, insulin, or both (6nM C-peptide, 10 nM insulin) | Reduced basal lipolysis, decreased isoproterenol-stimulated lipolysis, modulated some insulin metabolic mechanisms | [95] |
| In vivo | Diabetic BB/Wor rats | Subcutaneous osmopump administration of rat C-peptide II (75 nmol/kg daily) | Increased neural sodium potassium ATPase activity, decreased paranodal swelling, decreased acute and chronic nerve conduction issues | [96] |
| Clinical Study | T1DM patients with diabetic polyneuropathy symptoms | Intravenous administration of human C-peptide for 3 h (0.11–1.73 nmol/L) | Increase of respiratory heart rate variability; improved autonomic nerve function | [97] |
| Clinical Study | T1DM patients without peripheral neuropathy symptoms | Four daily doses of C-peptide (600 nmol/day) | Increased function of sensory nerve conduction velocity; improved vibration perception | [98] |
| Ex vivo | CD4 T cells from healthy individuals | CD4 T cells were incubated with recombinant C-peptide (10 nM for 2.5 h) | Stimulation of T cell chemotaxis involving proinflammatory pathways | [99] |
| Ex vivo | Thoracic artery tissue from T2DM patients | Immunohistochemical staining for C-peptide and macrophages | Accumulation of C-peptide colocalized with monocytes and macrophages in thoracic arterial blood vessel wall of T2DM patients in early atherogenesis; | [100] |
| In vitro | Swiss 373 mouse fibroblast cell line | Incubated with mouse C-peptide (1 nM for 24 h) | Activation of PKC/IκB/NF-κB inflammatory signaling pathways | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washburn, R.L.; Mueller, K.; Kaur, G.; Moreno, T.; Moustaid-Moussa, N.; Ramalingam, L.; Dufour, J.M. C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines 2021, 9, 270. https://doi.org/10.3390/biomedicines9030270
Washburn RL, Mueller K, Kaur G, Moreno T, Moustaid-Moussa N, Ramalingam L, Dufour JM. C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines. 2021; 9(3):270. https://doi.org/10.3390/biomedicines9030270
Chicago/Turabian StyleWashburn, Rachel L., Karl Mueller, Gurvinder Kaur, Tanir Moreno, Naima Moustaid-Moussa, Latha Ramalingam, and Jannette M. Dufour. 2021. "C-Peptide as a Therapy for Type 1 Diabetes Mellitus" Biomedicines 9, no. 3: 270. https://doi.org/10.3390/biomedicines9030270
APA StyleWashburn, R. L., Mueller, K., Kaur, G., Moreno, T., Moustaid-Moussa, N., Ramalingam, L., & Dufour, J. M. (2021). C-Peptide as a Therapy for Type 1 Diabetes Mellitus. Biomedicines, 9(3), 270. https://doi.org/10.3390/biomedicines9030270









