Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Peptides from Sea Anemone Heteractis Crispa
2.2. Production of Recombinant Peptides
2.3. Mass Spectrometry Analysis
2.4. Amino Acid Sequence Determination
2.5. CD Spectroscopy
2.6. Trypsin Inhibition Constant Determination
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. 6-Hydroxydopamine-Induced Cytotoxicity
2.10. Evaluation of Intracellular ROS Level
2.11. DPPH Radical Scavenging Assay
2.12. Bioassays Data Evaluation
2.13. Expression of Voltage-Gated Ion Channels in Xenopus Laevis Oocytes
2.14. Electrophysiological Studies
3. Results
3.1. Production and Characterization of the Peptides
3.2. CD Spectra of the Recombinant InhVJ
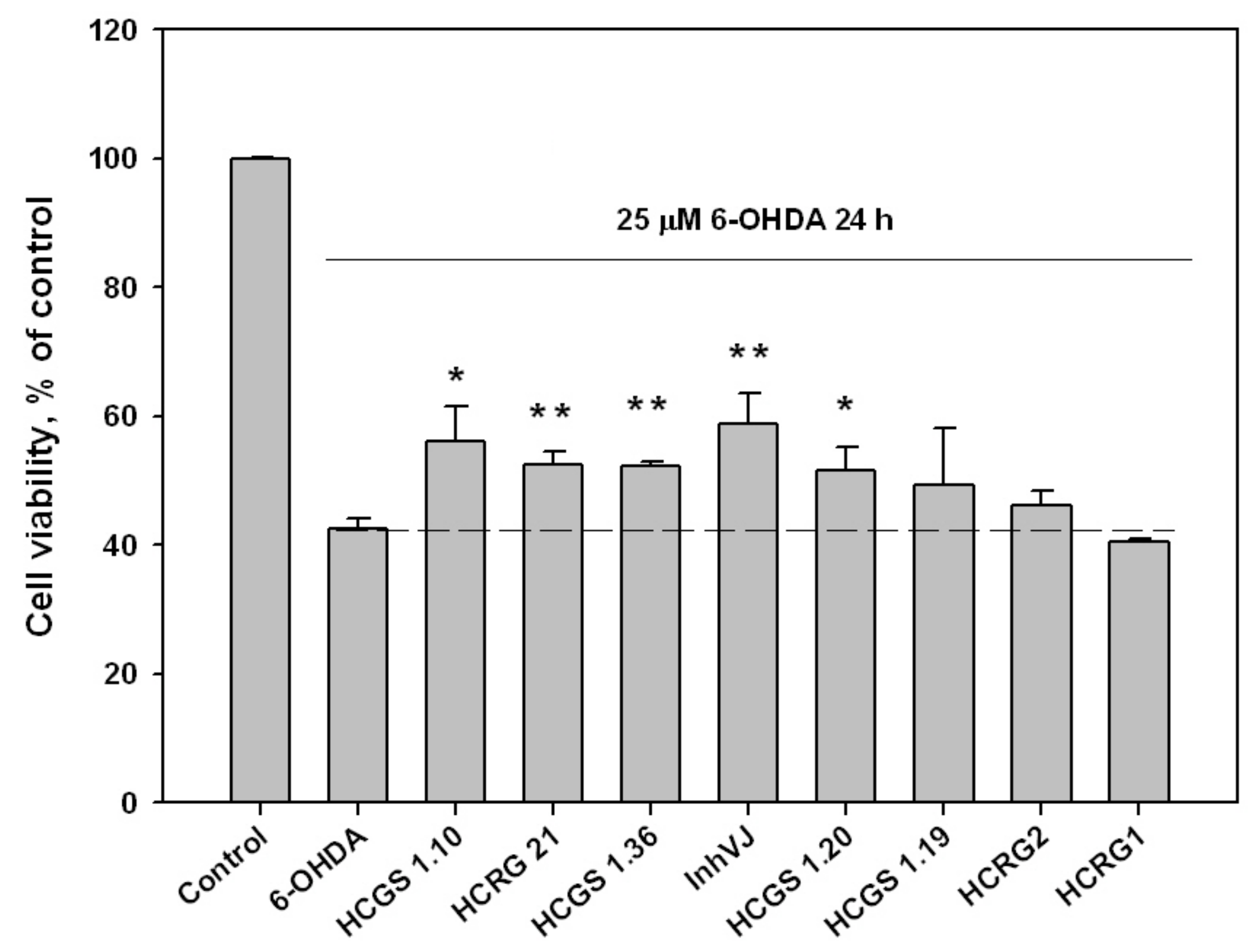
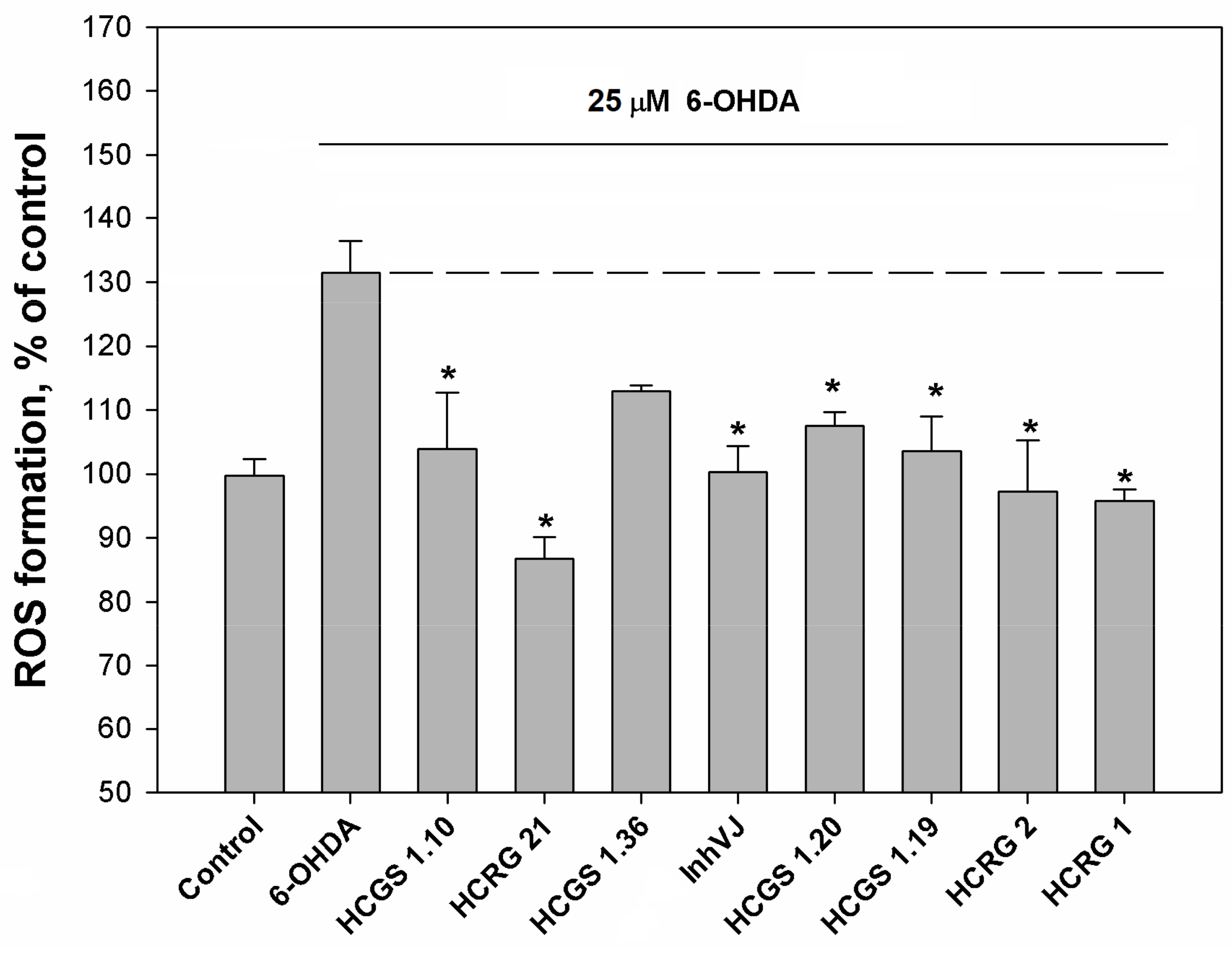
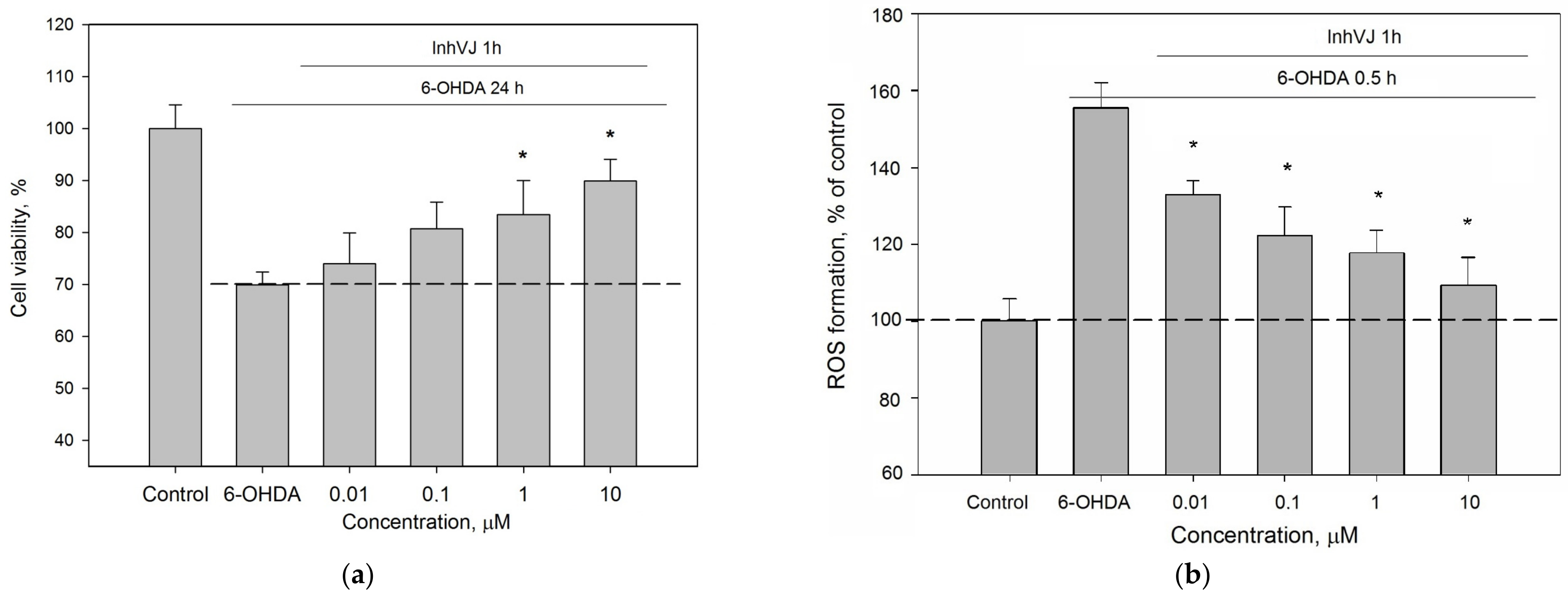
3.3. Neuroprotective Activity of Kunitz-Type Peptides and Influence on Reactive Oxygen Species Production
3.4. Antioxidant Activity of Kunitz-Type Peptides
3.5. Effect of Kunitz-Type Peptides on TRPV1 and Kv Channels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA a.a. | 6-hydroxydopamine amino acid |
| ASICs | acid-sensing ion channels |
| BBB | blood-brain barrier |
| BPTI | bovine pancreatic trypsin inhibitor |
| Cav | voltage-gated calcium channel |
| CD | circular dichroism |
| Da | Dalton |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| HPLC | High-Pressure Liquid Chromatography |
| IL-6 | interleukin-6 |
| Kv | voltage-gated potassium channel |
| LRP | lipoprotein receptor-related protein |
| MALDI-TOF | Matrix-Assisted Laser Desorption Ionization |
| MW | molecular weight |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) |
| Nav | voltage-gated sodium channel |
| PD | Parkinson’s disease |
| ROS | reactive oxygen species |
| TRPV1 | transient receptor potential cation channel subfamily V member 1 |
| proIL-1β | precursor of interleukin 1 beta |
| TFA | trifluoro acetic acid |
| TNF-α | tumor necrosis factor alpha |
References
- Ranasinghe, S.; McManus, D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef]
- Mans, B.J.; Louw, A.I.; Neitz, A.W.H. Savignygrin, a Platelet Aggregation Inhibitor from the Soft Tick Ornithodoros savignyi, Presents the RGD Integrin Recognition Motif on the Kunitz-BPTI Fold. J. Biol. Chem. 2002, 277, 21371–21378. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 activation power can switch an action mode for its polypeptide ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.-M.M.; Béress, L.; Lazdunski, M.; Beress, L.; Lazdunski, M.; Béress, L.; et al. Two different classes of sea anemone toxins for voltage sensitive K+ cannels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stotz, S.C.; Spaetgens, R.L.; Zamponi, G.W. Block of voltage-dependent calcium channel by the green mamba toxin calcicludine. J. Membr. Biol. 2000, 174, 157–165. [Google Scholar] [CrossRef]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Béress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharm. 2011, 82, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Báez, A.; Salceda, E.; Fló, M.; Graña, M.; Fernández, C.; Vega, R.; Soto, E. α-Dendrotoxin inhibits the ASIC current in dorsal root ganglion neurons from rat. Neurosci. Lett. 2015, 606, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Twenty years of dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Ciolek, J.; Reinfrank, H.; Sigismeau, S.; Mouillac, B.; Peigneur, S.; Tytgat, J.; Droctov, L.; Mourier, G.; De Pauw, E.; Servent, D.; et al. Green mamba peptide targets type-2 vasopressin receptor against polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2017, 114, 7154–7159. [Google Scholar] [CrossRef] [Green Version]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type peptide HCRG21 from the sea anemone Heteractis crispa is a full antagonist of the TRPV1 receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, R.; Peigneur, S.; Pons, T.; Alvarez, C.; González, L.; Chávez, M.A.; Tytgat, J. The kunitz-type protein ShPI-1 inhibits serine proteases and voltage-gated potassium channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokotun, I.N.; Il’ina, A.P.; Monastyrnaya, M.M.; Leychenko, E.V.; Es’kov, A.A.; Anastuk, S.D.; Kozlovskaya, E.P. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: Isolation and characteristic. Biochem. 2007, 72, 301–306. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Zelepuga, E.; Sintsova, O.; Tabakmakher, V.; Gnedenko, O.; Ivanov, A.; Hua, K.-F.; Kozlovskaya, E. New Kunitz-type HCRG polypeptides from the sea anemone Heteractis crispa. Mar. Drugs 2015, 13, 6038–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintsova, O.V.; Pislyagin, E.A.; Gladkikh, I.N.; Monastyrnaya, M.M.; Menchinskaya, E.S. Kunitz-Type Peptides of the Sea Anemone Heteractis crispa: Potential Anti-Inflammatory Compounds. Russ. J. Bioorg. Chem. 2017, 43, 91–97. [Google Scholar] [CrossRef]
- De Almeida Nogueira, N.P.; Morgado-Díaz, J.A.; Menna-Barreto, R.F.S.; Paes, M.C.; da Silva-López, R.E. Effects of a marine serine protease inhibitor on viability and morphology of Trypanosoma cruzi, the agent of Chagas disease. Acta Trop. 2013, 128, 27–35. [Google Scholar] [CrossRef]
- Dai, S.-X.; Zhang, A.-D.; Huang, J.-F. Evolution, expansion and expression of the Kunitz/BPTI gene family associated with long-term blood feeding in Ixodes scapularis. BMC Evol. Biol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaeva, M.P.; Chausova, V.E.; Zelepuga, E.A.; Guzev, K.V.; Tabakmakher, V.M.; Monastyrnaya, M.M.; Kozlovskaya, E.P. A new multigene superfamily of Kunitz-type protease inhibitors from sea anemone Heteractis crispa. Peptides 2012, 34, 88–97. [Google Scholar] [CrossRef]
- Župunski, V.; Kordiš, D.; Gubenšek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [Green Version]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Chausova, V.; Monastyrnaya, M.; Anastyuk, S.; Chernikov, O.; Yurchenko, E.; Aminin, D.; Isaeva, M.; Leychenko, E.; et al. Peptide fingerprinting of the sea anemone Heteractis magnifica mucus revealed neurotoxins, Kunitz-type proteinase inhibitors and a new β-defensin α-amylase inhibitor. J. Proteom. 2018, 173, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [Green Version]
- Sintsova, O.V.; Monastyrnaya, M.M.; Pislyagin, E.A.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Anti-inflammatory activity of a polypeptide from the Heteractis crispa sea anemone. Russ. J. Bioorg. Chem. 2015, 41, 590–596. [Google Scholar] [CrossRef]
- Tabakmakher, V.M.; Sintsova, O.V.; Krivoshapko, O.N.; Zelepuga, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P. Analgesic effect of novel Kunitz-type polypeptides of the sea anemone Heteractis crispa. Dokl. Biochem. Biophys. 2015, 461, 232–235. [Google Scholar] [CrossRef]
- Kvetkina, A.N.; Leychenko, E.V.; Yurchenko, E.A.; Pislyagin, E.A.; Peigneur, S.; Tytgat, J.; Isaeva, M.; Aminin, D.; Kozlovskaya, E.P. A New Iq-Peptide of the Kunitz Type from the Heteractis magnifica Sea Anemone Exhibits Neuroprotective Activity in a Model of Alzheimer’s Disease. Russ. J. Bioorg. Chem. 2018, 44, 416–423. [Google Scholar] [CrossRef]
- Kvetkina, A.; Leychenko, E.; Chausova, V.; Zelepuga, E.; Chernysheva, N.; Guzev, K.; Pislyagin, E.; Yurchenko, E.; Menchinskaya, E.; Aminin, D.; et al. A new multigene HCIQ subfamily from the sea anemone Heteractis crispa encodes Kunitz-peptides exhibiting neuroprotective activity against 6-hydroxydopamine. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvetkina, A.N.; Kaluzhskiy, L.A.; Leychenko, E.V.; Isaeva, M.P. New Targets of Kunitz-Type Peptide from Sea Anemone Heteractis magnifica. Dokl. Biochem. Biophys. 2019, 487, 1–4. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic compound from sea anemone Heteractis crispa is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [Green Version]
- Sintsova, O.V.; Palikov, V.A.; Palikova, Y.A.; Klimovich, A.A.; Gladkikh, I.N.; Andreev, Y.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Dyachenko, I.A.; Kozlov, S.A.; et al. Peptide Blocker of Ion Channel TRPV1 Exhibits a Long Analgesic Effect in the Heat Stimulation Model. Dokl. Biochem. Biophys. 2020, 493, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Peigneur, S.; Sintsova, O.; Lopes Pinheiro-Junior, E.; Klimovich, A.; Menshov, A.; Kalinovsky, A.; Isaeva, M.; Monastyrnaya, M.; Kozlovskaya, E.; et al. Kunitz-Type Peptides from the Sea Anemone Heteractis crispa Demonstrate Potassium Channel Blocking and Anti-Inflammatory Activities. Biomedicines 2020, 8, 473. [Google Scholar] [CrossRef]
- Liao, Q.; Li, S.; Siu, S.W.I.; Yang, B.; Huang, C.; Chan, J.Y.W.; Morlighem, J.É.R.L.; Wong, C.T.T.; Rádis-Baptista, G.; Lee, S.M.Y. Novel Kunitz-like Peptides Discovered in the Zoanthid Palythoa caribaeorum through Transcriptome Sequencing. J. Proteome Res. 2018, 17, 891–902. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Vassilevski, A.A.; Grishin, E.V. Cyanogen bromide cleavage of proteins in salt and buffer solutions. Anal. Biochem. 2010, 407, 144–146. [Google Scholar] [CrossRef]
- Provencher, S.W.; Glöckner, J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 1981, 20, 33–37. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of Protein Secondary Structure from Circular Dichroism Spectra: Comparison of CONTIN, SELCON, and CDSSTR Methods with an Expanded Reference Set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J. 1953, 55, 170–171. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Kolesnikova, S.A.; Lyakhova, E.G.; Menchinskaya, E.S.; Pislyagin, E.A.; Chingizova, E.A.; Aminin, D.L. Lanostane Triterpenoid Metabolites from a Penares sp. Marine Sponge Protect Neuro-2a Cells against Paraquat Neurotoxicity. Molecules 2020, 25, 5397. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Am. Assoc. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective activity of some marine fungal metabolites in the 6-hydroxydopamin- and paraquat-induced Parkinson’s disease models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liman, E.R.; Tytgat, J.; Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992, 9, 861–871. [Google Scholar] [CrossRef]
- Kalina, R.S.; Peigneur, S.; Zelepuga, E.A.; Dmitrenok, P.S.; Kvetkina, A.N.; Kim, N.Y.; Leychenko, E.V.; Tytgat, J.; Kozlovskaya, E.P.; Monastyrnaya, M.M.; et al. New insights into the type II toxins from the sea anemone Heteractis crispa. Toxins 2020, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintsova, O.; Gladkikh, I.; Kalinovskii, A.; Zelepuga, E.; Monastyrnaya, M.; Kim, N.; Shevchenko, L.; Peigneur, S.; Tytgat, J.; Kozlovskaya, E.; et al. Magnificamide, a β-Defensin-Like Peptide from the Mucus of the Sea Anemone Heteractis magnifica, Is a Strong Inhibitor of Mammalian α-Amylases. Mar. Drugs 2019, 17, 542. [Google Scholar] [CrossRef] [Green Version]
- Bové, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Ghatak, S.; Trudler, D.; Dolatabadi, N.; Ambasudhan, R. Parkinson’s disease: What the model systems have taught us so far. J. Genet. 2018, 97, 729–751. [Google Scholar] [CrossRef]
- Bové, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharm. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Mechanisms of Neuroprotective Effects of Peptides Derived from Natural Materials and Their Production and Assessment. Compr. Rev. Food Sci. Food Saf. 2019, 18, 923–935. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Ryu, B.; Himaya, S.; Kim, S.K. Optimization of hydrolysis conditions, isolation, and identification of neuroprotective peptides derived from seahorse Hippocampus trimaculatus. Amino Acids 2013, 45, 369–381. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, S. Potential Beneficial Effects of Marine Peptide on Human Neuron Health. Curr. Protein Pept. Sci. 2013, 14, 173–176. [Google Scholar] [CrossRef]
- Vaidya, B.; Sharma, S.S. Transient Receptor Potential Channels as an Emerging Target for the Treatment of Parkinson’s Disease: An Insight Into Role of Pharmacological Interventions. Front. Cell Dev. Biol. 2020, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Hoge, S.G.; Grahn, S.W.; Kim, E.; Divino, L.A.; Grady, E.F.; Bunnett, N.W.; Kirkwood, K.S. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G959–G969. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Kaneko, S. Physiological and pathophysiological roles of transient receptor potential channels in microglia-related CNS inflammatory diseases. Biol. Pharm. Bull. 2018, 41, 1152–1157. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, D.Y.; Chung, E.S.; Oh, U.T.; Kim, S.U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons In vivo and In vitro. J. Neurosci. 2005, 25, 662–671. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Xu, Q.; Ding, F.; Tian, Y.; Zhang, M. Sensation of TRPV1 via 5-hydroxytryptamine signaling modulates pain hypersensitivity in a 6-hydroxydopamine induced mice model of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2019, 521, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Escelsior, A.; Sterlini, B.; Murri, M.B.; Valente, P.; Amerio, A.; di Brozolo, M.R.; da Silva, B.P.; Amore, M. Transient receptor potential vanilloid 1 antagonism in neuroinflammation, neuroprotection and epigenetic regulation: Potential therapeutic implications for severe psychiatric disorders treatment. Psychiatr. Genet. 2020, 30, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Pollastro, F.; Bramanti, P.; Mazzon, E. Cannabidiol exerts protective effects in an in vitro model of Parkinson’s disease activating AKT/mTOR pathway. Fitoterapia 2020, 143, 104553. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, K.; Li, Z.; Zhao, Y.; Wang, Y.; Hu, B.; Xu, P.; Shi, X.; Zhou, B.; Pennington, M.; et al. Blockade of Kv1.3 channels ameliorates radiation-induced brain injury. Neuro. Oncol. 2014, 16, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Redman, P.T.; Jefferson, B.S.; Ziegler, C.B.; Mortensen, O.V.; Torres, G.E.; Levitan, E.S.; Aizenman, E. A vital role for voltage-dependent potassium channels in dopamine transporter-mediated 6-hydroxydopamine neurotoxicity. Neuroscience 2006, 143, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xue, B.; Wang, J.; Liu, H.; Shi, L.; Xie, J. Potassium channels: A potential therapeutic target for Parkinson’s Disease. Neurosci. Bull. 2017, 34, 341–348. [Google Scholar] [CrossRef]
- Sokotun, I.N.; Gnedenko, O.V.; Leychenko, E.V.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Molnar, A.A.; Ivanov, A.S. Study of the interaction of trypsin inhibitor from the sea anemone Radianthus macrodactylus with proteases. Biochem. Suppl. Ser. B Biomed. Chem. 2007, 1, 139–142. [Google Scholar] [CrossRef]
- Kounnas, M.Z.; Moir, R.D.; Rebeck, G.W.; Bush, A.I.; Argraves, W.S.; Tanzi, R.E.; Hyman, B.T.; Strickland, D.K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 1995, 82, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Demeule, M.; Regina, A.; Che, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.-P.; Beliveau, R. Identification and Design of Peptides as a New Drug Delivery System for the Brain. J. Pharmacol. Exp. Ther. 2007, 324, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Zong, Z.; Hua, L.; Wang, Z.; Xu, H.; Ye, C.; Pan, B.; Zhao, Z.; Zhang, L.; Lu, J.; Liu, H.; et al. Self-assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy. Drug Deliv. 2019, 26, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Liu, T.; Jiang, N.; Zhou, K.; Yang, K.; Ning, W.; Yu, Y. A novel delivery system of cyclovirobuxine D for brain targeting: Angiopep-conjugated polysorbate 80-coated liposomes via intranasal administration. J. Biomed. Nanotechnol. 2018, 14, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, Z.; Liu, Z.; Huang, X.; Jiang, X. Angiopep-2/IP10-EGFRvIIIscFv modified nanoparticles and CTL synergistically inhibit malignant glioblastoma. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]

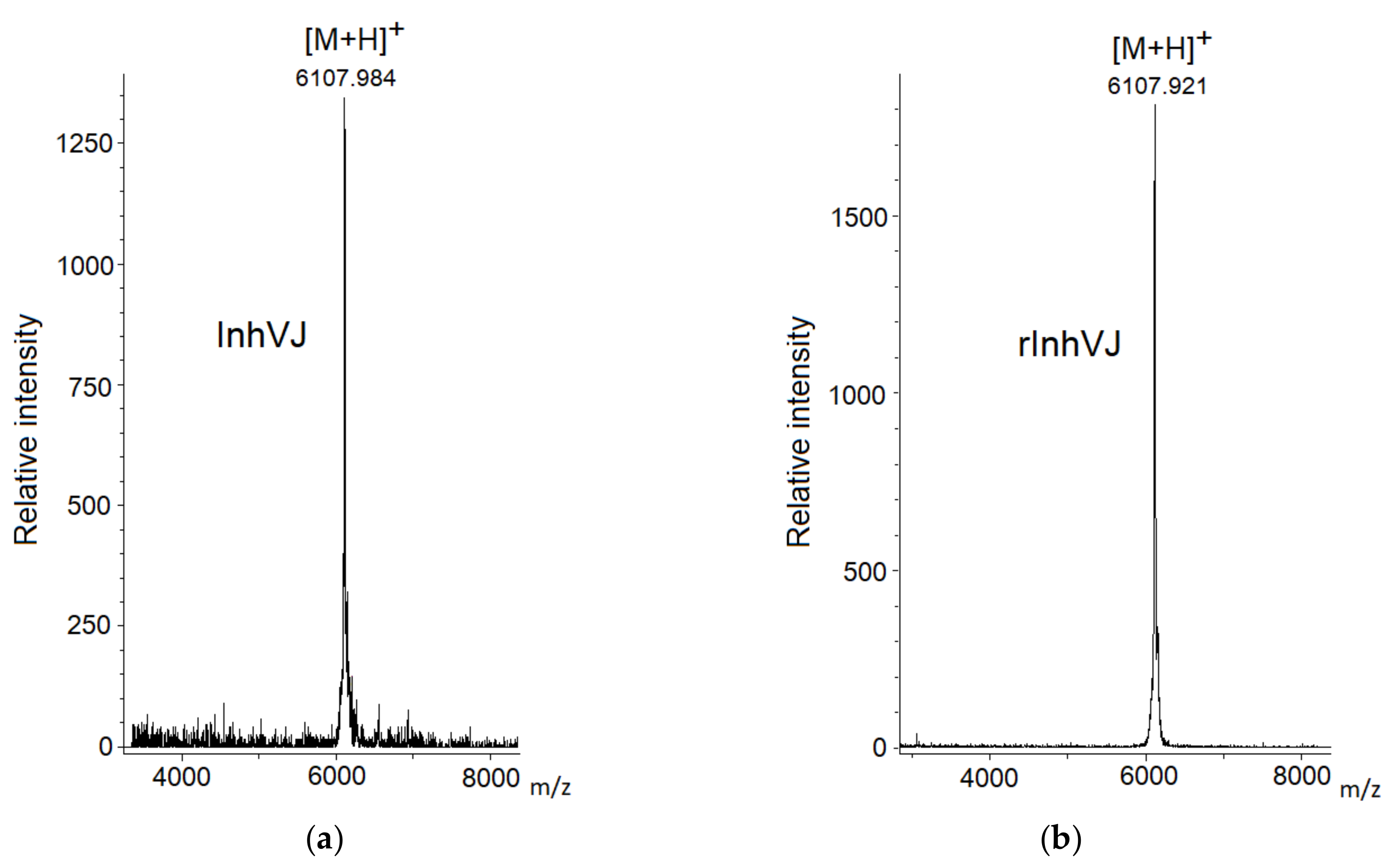

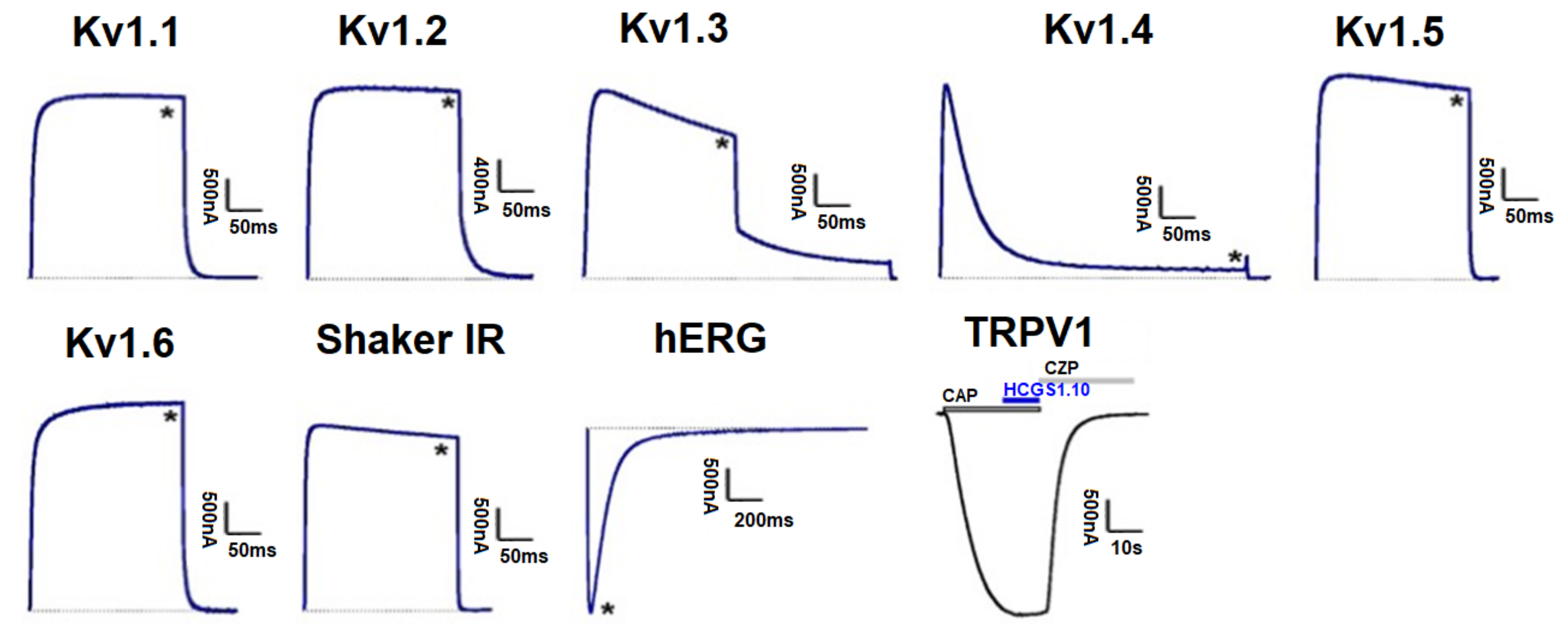
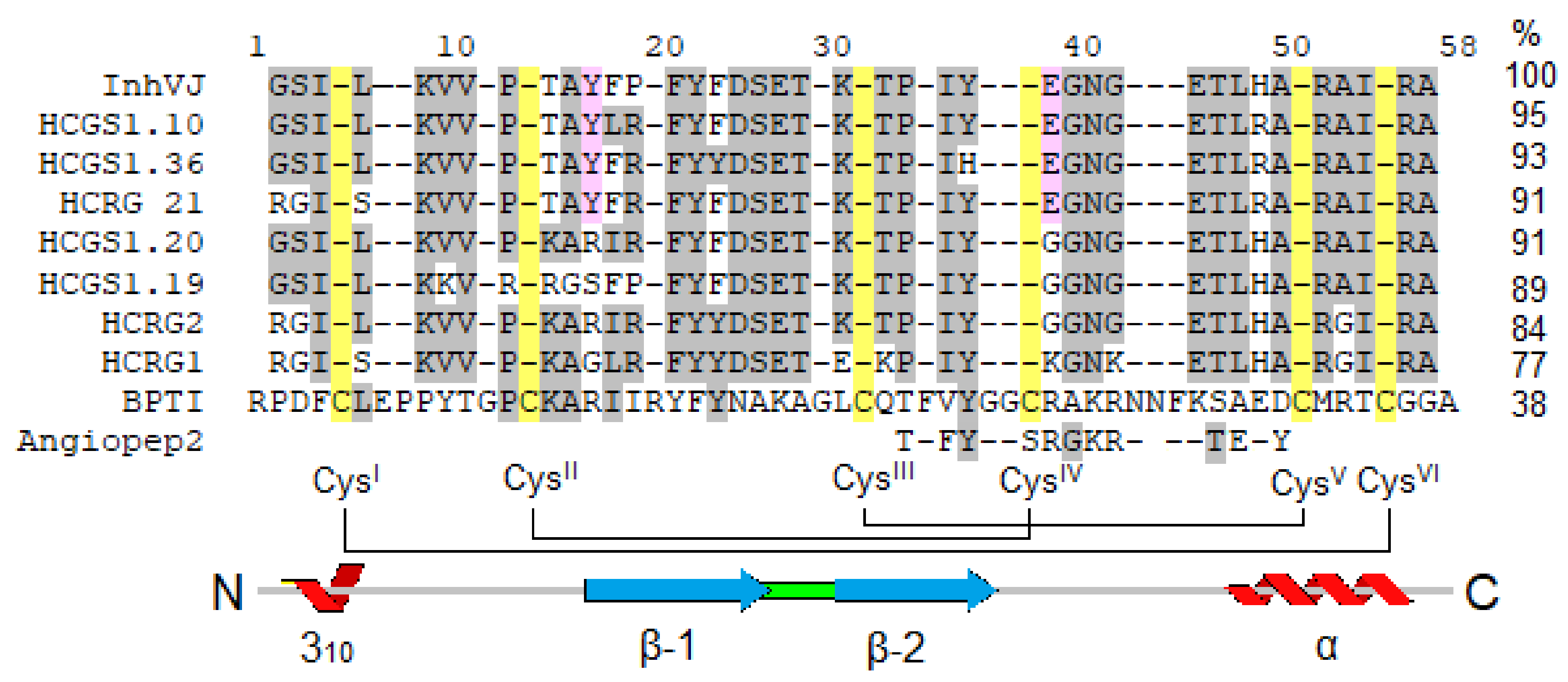
| № | Peptide | MW Calculated, Da | MW Measured by MS, Da | Ki Trypsin, M |
|---|---|---|---|---|
| 1 | HCRG1 | 6195.6 | 6196 [14] | 2.8 × 10−8 [14] |
| 2 | HCRG2 | 6150.6 | 6148 [14] | 5.0 × 10−8 [14] |
| 3 | HCGS1.19 | 6087.5 | 6188.7 | 3.0 × 10−8 [15] |
| 4 | HCGS1.20 | 6079.6 | 6078.9 | 2.1 × 10−8 [24] |
| 5 | HCGS1.36 | 6174.5 | 6174.8 | 1.0 × 10−7 [15] |
| 6 | HCGS1.10 | 6150.5 | 6150.5 | 2.1 × 10−7 [15] |
| 7 | HCRG21 | 6227.5 | 6228.5 | 1.0 × 10−7 [10] |
| 8 | InhVJ | 6106.4 | 6107.9 | 7.8 × 10−8 |
| InhVJ | Helixes | β-Sheet | Turns | Others | ||||
|---|---|---|---|---|---|---|---|---|
| α | 310 | Sum | β-Turn | PP2 | Sum | |||
| Recombinant | 12.4 | 8.7 | 21.1 | 10.1 | 18.0 | 6.5 | 24.5 | 44.3 |
| Native | 6.9 | 8.8 | 15.7 | 16.3 | 15.7 | 9.3 | 25.0 | 43.0 |
| Compounds, 10 µM | Scavenging of DPPH Radicals, % | Compounds, 10 µM | Scavenging of DPPH Radicals, % |
|---|---|---|---|
| HCGS1.10 | 8.9 ± 3.4 * | HCGS 1.20 | 11.7 ± 2.4 * |
| HCRG21 | 14.5 ± 4.1 * | HCGS 1.19 | 2.4 ± 1.0 |
| HCGS1.36 | 6.2 ± 2.6 | HCRG2 | 12.9 ± 3.0 * |
| InhVJ | 8.1 ± 1.9 * | HCRG1 | 10.9 ± 3.5 * |
| Ascorbic acid | 33.8 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sintsova, O.; Gladkikh, I.; Monastyrnaya, M.; Tabakmakher, V.; Yurchenko, E.; Menchinskaya, E.; Pislyagin, E.; Andreev, Y.; Kozlov, S.; Peigneur, S.; et al. Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model. Biomedicines 2021, 9, 283. https://doi.org/10.3390/biomedicines9030283
Sintsova O, Gladkikh I, Monastyrnaya M, Tabakmakher V, Yurchenko E, Menchinskaya E, Pislyagin E, Andreev Y, Kozlov S, Peigneur S, et al. Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model. Biomedicines. 2021; 9(3):283. https://doi.org/10.3390/biomedicines9030283
Chicago/Turabian StyleSintsova, Oksana, Irina Gladkikh, Margarita Monastyrnaya, Valentin Tabakmakher, Ekaterina Yurchenko, Ekaterina Menchinskaya, Evgeny Pislyagin, Yaroslav Andreev, Sergey Kozlov, Steve Peigneur, and et al. 2021. "Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model" Biomedicines 9, no. 3: 283. https://doi.org/10.3390/biomedicines9030283
APA StyleSintsova, O., Gladkikh, I., Monastyrnaya, M., Tabakmakher, V., Yurchenko, E., Menchinskaya, E., Pislyagin, E., Andreev, Y., Kozlov, S., Peigneur, S., Tytgat, J., Aminin, D., Kozlovskaya, E., & Leychenko, E. (2021). Sea Anemone Kunitz-Type Peptides Demonstrate Neuroprotective Activity in the 6-Hydroxydopamine Induced Neurotoxicity Model. Biomedicines, 9(3), 283. https://doi.org/10.3390/biomedicines9030283









