Immunotherapy in Adrenocortical Carcinoma: Predictors of Response, Efficacy, Safety, and Mechanisms of Resistance
Abstract
:1. Introduction
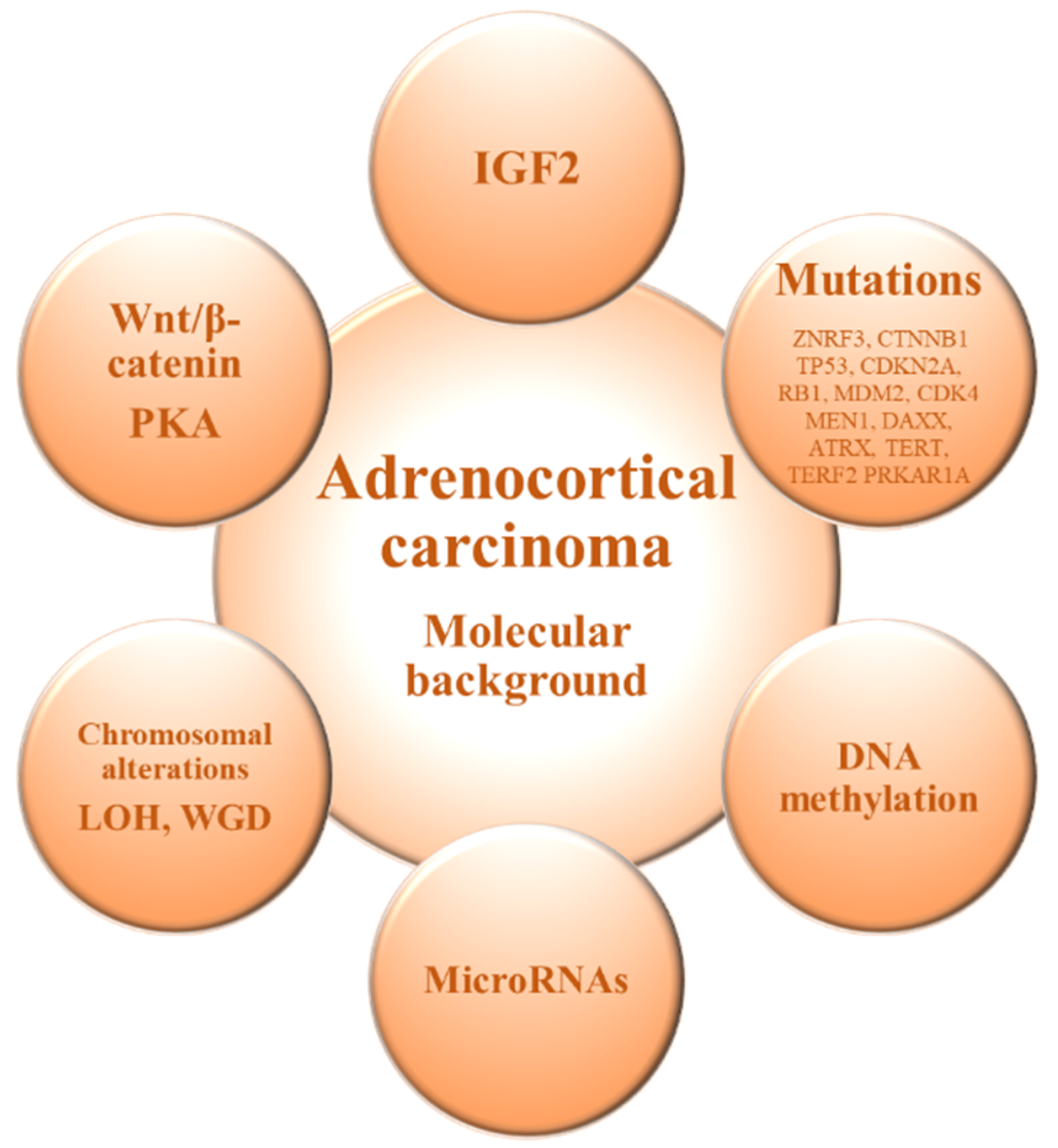
2. Molecular Background of Adrenocortical Carcinoma
3. Markers of Response to Immunotherapy in ACC
| Molecular Markers of Prognosis | ||
| Molecular Marker | Most Common Alteration/s | Clinical Implications |
| IGF2 | IGF2 overexpressed in 90% of ACCs | Targeting IGF2 system as a potential therapeutic approach [15]. |
| [12,13,15,41] | Differential diagnosis of ACC and ACA [41]. | |
| DNA methylation | Hypomethylated intergenic regions and hypermethylated CpG islands | Hypermethylated profile is associated with a poorer prognosis of ACC [49,50]. |
| [12,13,47,48,49,50] | Differential diagnosis of ACC and ACA (ACC are globally hypomethylated) [47,48]. | |
| microRNA | miR-483-5p and miR-483-3p overexpressed and miR-195 downregulated | Downregulation of miR-195 and upregulation of miR-483-5p are associated with poorer disease-specific survival [55,58]. |
| [55,56,57,58] | Differential diagnosis of ACC and ACA (upregulation of miR-483-5p is a marker of ACC) [56,58]. | |
| Chromosomal alterations | Amplification in chromosomal regions of TERT and CDK4 genes, and deletions in ZNRF3, CDKN2A and RB1 genes. | Chromosomal alterations are more common in ACC than in ACA [62]. |
| [12,13,38,62] | LOH and WGD | Copy number phenotype and WGD are hallmarks of disease progression [13]. |
| Wnt/b-catenin pathway [12,13,38] | Abnormal cytoplasmic and nuclear accumulation of beta-catenin and somatic activating mutations of CTNNB1 and ENC1 upregulation | Activating mutations of CTNNB1 are typical of aggressive ACC [43,83]. |
| PKA pathway | Somatic mutations in PKA regulatory subunit PRKAR1A | PRKAR1A gene mutations are typical of ACA [84]. |
| [13,63] | Somatic activating mutations in the PKA catalytic subunit alpha gene (PRKACA) are observed in cortisol-secreting ACA [85]. | |
| Immunological Markers OD Response to Immunotherapy | ||
| Immunological Marker | Most Common Alteration/s | Clinical Implications |
| PD-1/PD-L1 [27,28,29,30,31,69,71,74] | 10.7% of ACCs are PD-L1 positive on tumor cell membrane, 70.4% on tumor-infiltrating lymphocytes. | Levels of PD-L1 expression as a potential predictor of response to immunomodulatory agents [69,71]. |
| MSI [33,36,77] | 3% of all ACC are associated to Lynch syndrome and 4.4% have MSI. | MSI may causing a “hypermutator” phenotype that presents a greater response to immunological treatments [36] |
| TMB | High TMB status in ACC. | ACC metastatic tumors had 2.8-fold higher median mutation rate compared to primary ACC [34]. |
| [30,34,82] | Higher TMB in conventional and myxoid variants than in oncocytic ACC [82]. | |
4. Immunotherapy in Adrenocortical Carcinoma: Efficacy
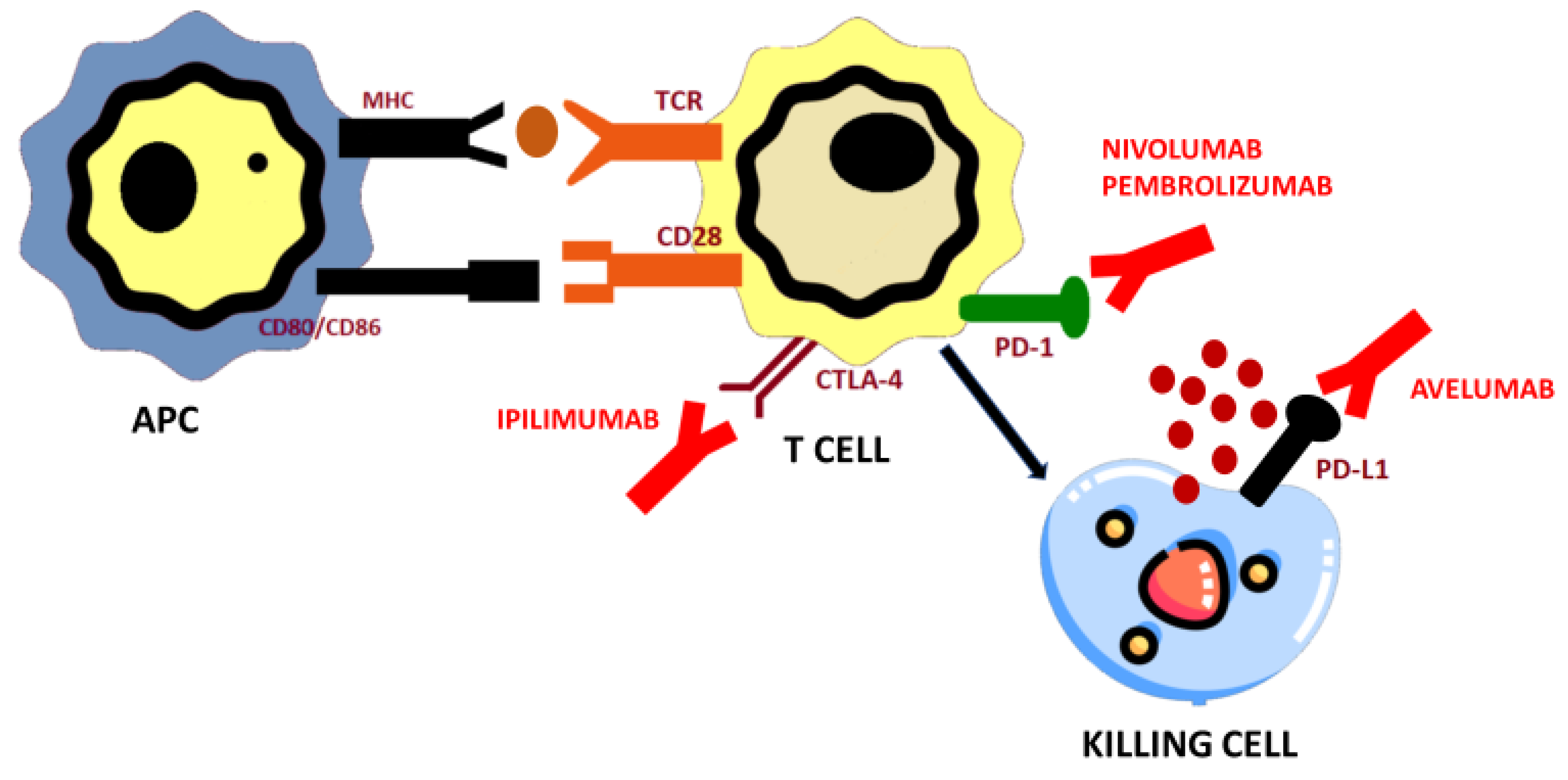
4.1. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Protein Ligand (PD-L1) Axis and PD-1/PD-L1 Blockade
4.1.1. Pembrolizumab
4.1.2. Nivolumab
4.1.3. Avelumab
4.2. Cytotoxic T-Lymphocyte-Associated Antigen 4 (CTLA-4) Blockade
Ipilimumab
4.3. Potential Immune Related Targets under Research in ACC
| Drug | Study Design | Population | Number of Patients | PD-L1 Status (IHC) | Primary Endpoint | Other Main Endpoints |
|---|---|---|---|---|---|---|
| Pembrolizumab 200 mg every 3 weeeks during 24 months (35 cycles) | Phase II-single arm [30] | Prior systemic therapy: 28 patients (31% with ≥1 prior line) | 39 | 7/34 | ORR RECIST 1.1 = 23% | DoR = NR PFS = 2.1 months OS = 24.9 months |
| Pembrolizumab 200 mg every 3 weeeks during 24 months (35 cycles) | Phase II-single arm [29] | Prior systemic therapy: median number of prior lines = 2 (1–5) | 16 | 0/14 | Non-progression rate at 27 weeks = 36% | ORR = 14% |
| Pembrolizumab 200 mg every 3 weeeks + Mitotane | Retrospective [88] | Prior 1 line of systemic therapy | 6 | NA | NA | Two patients PR and four SD |
| Pembrolizumab 200 mg every 3 weeeks + Lenvatinib | Retrospective [89] | Prior systemic therapy | 8 | NA | ORR = 25% | PFS = 5.5 months |
| Nivolumab 240 mg every 2 weeks | Phase II-single arm [28] | Prior 0—≥1 cisplatin-based chemotherapy | 10 | 6/10 | ORR RECIST 1.1 = 11% | PFS = 1.8 months |
| Nivolumab 3 mg/kg plus Ipilimumab 1mg/kg | Phase II—multicohort [31] | Prior 0—≥1 cisplatin-based chemotherapy | 16 | NA | ORR RECIST 1.1 = 6% | PFS = 4.5 months |
| Avelumab 10 mg/kg every 2 weeks | Phase Ib expansion cohort [27] | Prior cisplatin-based chemotherapy. Concomitant mitotane allowed. | 50 | 12/42 | ORR RECIST 1.1 = 6% | PFS = 2.6 months OS = 10.6 months |
| Study Design | NCT Identifier | Treatment | Estimated N | Primary Endpoint |
|---|---|---|---|---|
| DART trial Phase 2 multicohort | NCT02834013 | Nivolumab + Ipilimumab | 818 (all cohorts) | ORR RECIST 1.1 in subsets |
| Phase 2 multicohort | NCT02721732 | Pembrolizumab | 225 (all cohorts) | Non-progression rate Incidence adverse events |
| Phase I/II | NCT04187404 | EO2401 + Nivolumab | 60 | Incidence adverse events |
| Phase I/Ib first-in-human multicohort | NCT02637531 | Nivolumab + Eganelisib | 219 (all cohorts) | Dose limiting toxicities Adverse Events |
| Phase II multicohort | NCT04400474 | Cabozantinib + Atezolizumab | 144 (all cohorts) | ORR RECIST 1.1 |
5. Safety of Immunotherapy
6. Mechanisms of Resistance to Immunotherapy in Adrenocortical Carcinoma
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varghese, J.; Habra, M.A. Update on adrenocortical carcinoma management and future directions. Curr. Opin. Endocrinol. Diabetes Obes. 2017. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.A.; Verhoeven, R.H.A.; Van Der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van De Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 2013. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Jasim, S.; Feng, L.; Ejaz, S.; Deniz, F.; Busaidy, N.; Waguespack, S.G.; Naing, A.; Sircar, K.; Wood, C.G.; et al. Adrenocortical carcinoma: Clinical outcomes and prognosis of 330 patients at a tertiary care Center. Eur. J. Endocrinol. 2013. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; De Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the study of adrenal tumors. Eur. J. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.; Baudin, E.; Terzolo, M.; Allolio, B.; Chadarevian, R.; Mueller, H.H.; Skogseid, B.; Leboulleux, S.; Mantero, F.; Haak, H.R.; et al. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013. [Google Scholar] [CrossRef] [Green Version]
- Berruti, A.; Terzolo, M.; Sperone, P.; Pia, A.; Della Casa, S.; Gross, D.J.; Carnaghi, C.; Casali, P.; Porpiglia, F.; Mantero, F.; et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr. Relat. Cancer 2005. [Google Scholar] [CrossRef] [PubMed]
- Megerle, F.; Kroiss, M.; Hahner, S.; Fassnacht, M. Advanced Adrenocortical Carcinoma-What to do when First-Line Therapy Fails? Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, T.J.; Gillis, A.; Alobuia, W.M.; Wild, H.; Kebebew, E. Surgery for adrenocortical carcinoma: When and how? Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101408. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Al-Assaf, H.; Xu, Y.; El-Sayed, S. Adrenocortical carcinoma: Patterns of care and role of adjuvant radiation therapy— a population-based study and review of the literature. Curr. Oncol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Armignacco, R.; Cantini, G.; Canu, L.; Poli, G.; Ercolino, T.; Mannelli, M.; Luconi, M. Adrenocortical carcinoma: The dawn of a new era of genomic and molecular biology analysis. J. Endocrinol. Investig. 2018. [Google Scholar] [CrossRef]
- Assié, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; René-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagneré, A.M.; René-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of β-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, T.J.; Kuick, R.; Else, T.; Gauger, P.G.; Vinco, M.; Bauersfeld, J.; Sanders, D.; Thomas, D.G.; Doherty, G.; Hammer, G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; Carneiro, B.A.; Tavora, F.; Pai, S.G.; Kaplan, J.B.; Chae, Y.K.; Chandra, S.; Kopp, P.A.; Giles, F.J. The challenge of developmental therapeutics for adrenocortical carcinoma. Oncotarget 2016. [Google Scholar] [CrossRef] [Green Version]
- De Martino, M.C.; Al Ghuzlan, A.; Aubert, S.; Assié, G.; Scoazec, J.Y.; Leboulleux, S.; Do Cao, C.; Libè, R.; Nozières, C.; Lombès, M.; et al. Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. J. Clin. Endocrinol. Metab. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creemers, S.G.; Hofland, L.J.; Korpershoek, E.; Franssen, G.J.H.; Van Kemenade, F.J.; De Herder, W.W.; Feelders, R.A. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr. Relat. Cancer 2016. [Google Scholar] [CrossRef]
- Konda, B.; Kirschner, L.S. Novel targeted therapies in adrenocortical carcinoma. Curr. Opin. Endocrinol. Diabetes Obes. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperone, P.; Ferrero, A.; Daffara, F.; Priola, A.; Zaggia, B.; Volante, M.; Santini, D.; Vincenzi, B.; Badalamenti, G.; Intrivici, C.; et al. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: A multicenter phase II study. Endocr. Relat. Cancer 2010. [Google Scholar] [CrossRef] [Green Version]
- Henning, J.E.K.; Deutschbein, T.; Altieri, B.; Steinhauer, S.; Kircher, S.; Sbiera, S.; Wild, V.; Schlötelburg, W.; Kroiss, M.; Perotti, P.; et al. Gemcitabine-based chemotherapy in adrenocortical carcinoma: A multicenter study of efficacy and predictive factors. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.S.; Imam, H.; Juhlin, C.; Skogseid, B.; Gröndal, S.; Tibblin, S.; Wilander, E.; Öberg, K.; Eriksson, B. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: Long-term survival in its adjuvant use. Ann. Oncol. 2000. [Google Scholar] [CrossRef]
- Berruti, A.; Sperone, P.; Ferrero, A.; Germano, A.; Ardito, A.; Priola, A.M.; De Francia, S.; Volante, M.; Daffara, F.; Generali, D.; et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur. J. Endocrinol. 2012. [Google Scholar] [CrossRef] [Green Version]
- De Martino, M.C.; Feelders, R.A.; Pivonello, C.; Simeoli, C.; Papa, F.; Colao, A.; Pivonello, R.; Hofland, L.J. The role of mTOR pathway as target for treatment in adrenocortical cancer. Endocr. Connect. 2019, 8, R144–R156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015. [Google Scholar] [CrossRef] [Green Version]
- De Martino, M.C.; van Koetsveld, P.M.; Feelders, R.A.; de Herder, W.W.; Dogan, F.; Janssen, J.A.M.J.L.; Hofste op Bruinink, D.; Pivonello, C.; Waaijers, A.M.; Colao, A.; et al. IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Tourneau, C.; Hoimes, C.; Zarwan, C.; Wong, D.J.; Bauer, S.; Claus, R.; Wermke, M.; Hariharan, S.; Von Heydebreck, A.; Kasturi, V.; et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J. Immunother. Cancer 2018. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, B.A.; Konda, B.; Costa, R.B.; Costa, R.L.B.; Sagar, V.; Gursel, D.B.; Kirschner, L.S.; Chae, Y.K.; Abdulkadir, S.A.; Rademaker, A.; et al. Nivolumab in Metastatic Adrenocortical Carcinoma: Results of a Phase 2 Trial. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Habra, M.A.; Stephen, B.; Campbell, M.; Hess, K.; Tapia, C.; Xu, M.; Rodon Ahnert, J.; Jimenez, C.; Lee, J.E.; Perrier, N.D.; et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. Immunother. Cancer 2019. [Google Scholar] [CrossRef]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 blockade in advanced adrenocortical carcinoma. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Campbell, M.T.; Xie, W.; Farah, S.; Bilen, M.A.; Schmidt, A.L.; Sonpavde, G.P.; Kilbridge, K.L.; Choudhury, A.D.; Mortazavi, A.; et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 2020, 1–10. [Google Scholar] [CrossRef]
- Billon, E.; Finetti, P.; Bertucci, A.; Niccoli, P.; Birnbaum, D.; Mamessier, E.; Bertucci, F. PDL1 expression is associated with longer postoperative, survival in adrenocortical carcinoma. Oncoimmunology 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, V.M.; Everett, J.N.; Furtado, L.V.; Gustafson, S.L.; Jungbluth, C.R.; Gruber, S.B.; Hammer, G.D.; Stoffel, E.M.; Greenson, J.K.; Giordano, T.J.; et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J. Clin. Oncol. 2013, 31, 3012. [Google Scholar] [CrossRef]
- Gara, S.K.; Lack, J.; Zhang, L.; Harris, E.; Cam, M.; Kebebew, E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentini, D.; Grisanti, S.; Volta, A.D.; Laganà, M.; Fiorentini, C.; Perotti, P.; Sigala, S.; Berruti, A. Immunotherapy failure in adrenocortical cancer: Where next? Endocr. Connect. 2018. [Google Scholar] [CrossRef] [Green Version]
- Fiorentini, C.; Grisanti, S.; Cosentini, D.; Abate, A.; Rossini, E.; Berruti, A.; Sigala, S. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J. Oncol. 2019. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Goh, G.; Healy, J.M.; Fonseca, A.L.; Scholl, U.I.; Stenman, A.; Kunstman, J.W.; Brown, T.C.; Overton, J.D.; Mane, S.M.; et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2015. [Google Scholar] [CrossRef]
- Assié, G.; Guillaud-Bataille, M.; Ragazzon, B.; Bertagna, X.; Bertherat, J.; Clauser, E. The pathophysiology, diagnosis and prognosis of adrenocortical tumors revisited by transcriptome analyses. Trends Endocrinol. Metab. 2010. [Google Scholar] [CrossRef]
- Giordano, T.J.; Thomas, D.G.; Kuick, R.; Lizyness, M.; Misek, D.E.; Smith, A.L.; Sanders, D.; Aljundi, R.T.; Gauger, P.G.; Thompson, N.W.; et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003. [Google Scholar] [CrossRef] [Green Version]
- De Fraipont, F.; El Atifi, M.; Cherradi, N.; Le Moigne, G.; Defaye, G.; Houlgatte, R.; Bertherat, J.; Bertagna, X.; Plouin, P.F.; Baudin, E.; et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 2005. [Google Scholar] [CrossRef] [Green Version]
- Heaton, J.H.; Wood, M.A.; Kim, A.C.; Lima, L.O.; Barlaskar, F.M.; Almeida, M.Q.; Fragoso, M.C.B.V.; Kuick, R.; Lerario, A.M.; Simon, D.P.; et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am. J. Pathol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Ragazzon, B.; Libé, R.; Gaujoux, S.; Assié, G.; Fratticci, A.; Launay, P.; Clauser, E.; Bertagna, X.; Tissier, F.; De Reyniès, A.; et al. Transcriptome analysis reveals that p53 and β-catenin alterations occur in a group of aggressive adrenocortical cancers. Cancer Res. 2010. [Google Scholar] [CrossRef] [Green Version]
- De Reyniès, A.; Assié, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; René-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA Methylation and Cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar]
- Kalari, S.; Pfeifer, G.P. Identification of Driver and Passenger DNA Methylation in Cancer by Epigenomic Analysis. Adv. Genet. 2010, 70, 277–308. [Google Scholar] [PubMed] [Green Version]
- Rechache, N.S.; Wang, Y.; Stevenson, H.S.; Killian, J.K.; Edelman, D.C.; Merino, M.; Zhang, L.; Nilubol, N.; Stratakis, C.A.; Meltzer, P.S.; et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J. Clin. Endocrinol. Metab. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, A.L.; Kugelberg, J.; Starker, L.F.; Scholl, U.; Choi, M.; Hellman, P.; Åkerström, G.; Westin, G.; Lifton, R.P.; Björklund, P.; et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosom. Cancer 2012. [Google Scholar] [CrossRef] [PubMed]
- Barreau, O.; Assié, G.; Wilmot-Roussel, H.; Ragazzon, B.; Baudry, C.; Perlemoine, K.; René-Corail, F.; Bertagna, X.; Dousset, B.; Hamzaoui, N.; et al. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 2013. [Google Scholar] [CrossRef] [Green Version]
- Jouinot, A.; Assie, G.; Libe, R.; Fassnacht, M.; Papathomas, T.; Barreau, O.; De La Villeon, B.; Faillot, S.; Hamzaoui, N.; Neou, M.; et al. DNA methylation is an independent prognostic marker of survival in adrenocortical cancer. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef]
- Creemers, S.G.; Van Koetsveld, P.M.; Van Kemenade, F.J.; Papathomas, T.G.; Franssen, G.J.H.; Dogan, F.; Eekhoff, E.M.W.; Van Der Valk, P.; De Herder, W.W.; Janssen, J.A.M.J.L.; et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinomas. Endocr. Relat. Cancer 2016. [Google Scholar] [CrossRef] [Green Version]
- Cherradi, N. MicroRNAs as potential biomarkers in adrenocortical cancer: Progress and challenges. Front. Endocrinol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Lerario, A.M.; Moraitis, A.; Hammer, G.D. Genetics and epigenetics of adrenocortical tumors. Mol. Cell. Endocrinol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Igaz, P.; Igaz, I.; Nagy, Z.; Nyíro, G.; Szabó, P.M.; Falus, A.; Patócs, A.; Rácz, K. MicroRNAs in adrenal tumors: Relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2015. [Google Scholar] [CrossRef]
- Soon, P.S.H.; Tacon, L.J.; Gill, A.J.; Bambach, C.P.; Sywak, M.S.; Campbell, P.R.; Yeh, M.W.; Wong, S.G.; Clifton-Bligh, R.J.; Robinson, B.G.; et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin. Cancer Res. 2009. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.E.; Holloway, A.K.; Weng, J.; Fojo, T.; Kebebew, E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011. [Google Scholar] [CrossRef] [Green Version]
- Özata, D.M.; Caramuta, S.; Velázquez-Fernández, D.; Akçakaya, P.; Xie, H.; Höög, A.; Zedenius, J.; Bäckdahl, M.; Larsson, C.; Lui, W.O. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabre, O.; Libé, R.; Assie, G.; Barreau, O.; Bertherat, J.; Bertagna, X.; Feige, J.J.; Cherradi, N. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer 2013. [Google Scholar] [CrossRef]
- Tömböl, Z.; Szabó, P.M.; Molnár, V.; Wiener, Z.; Tölgyesi, G.; Horányi, J.; Riesz, P.; Reismann, P.; Patócs, A.; Likó, I.; et al. Integrative molecular bioinformatics study of human adrenocortical tumors: MicroRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.J.; Helwig, J.; Bertram, S.; Sheu, S.Y.; Suttorp, A.C.; Seggewiß, J.; Willscher, E.; Walz, M.K.; Worm, K.; Schmid, K.W. Differential expression of microRNA-675, microRNA-139-3p and microRNA-335 in benign and malignant adrenocortical tumours. J. Clin. Pathol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.R.; Luconi, M.; Szabó, P.M.; Tóth, M.; Szücs, N.; Horányi, J.; Nagy, Z.; Mannelli, M.; Patócs, A.; Rácz, K.; et al. Analysis of circulating microRNAs in adrenocortical tumors. Lab. Investig. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assié, G.; Jouinot, A.; Bertherat, J. The “omics” of adrenocortical tumours for personalized medicine. Nat. Rev. Endocrinol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Lerario, A.M.; Rainey, W.; Hammer, G.D. Development of Adrenal Cortex Zonation. Endocrinol. Metab. Clin. N. Am. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finco, I.; Lerario, A.M.; Hammer, G.D. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 2018. [Google Scholar] [CrossRef] [Green Version]
- Ragazzon, B.; Assié, G.; Bertherat, J. Transcriptome analysis of adrenocortical cancers: From molecular classification to the identification of new treatments. Endocr. Relat. Cancer 2011. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Peng, Y.; Song, Y.; Ding, J.; Li, N.; Zhang, Z.; Wang, H. Identification of immune-related biomarkers in adrenocortical carcinoma: Immune-related biomarkers for ACC. Int. Immunopharmacol. 2020, 88, 106930. [Google Scholar] [CrossRef]
- Sbiera, S.; Kroiss, M.; Thamm, T.; Beyer, M.; Majidi, F.; Kuehner, D.; Wobser, M.; Becker, J.C.; Adam, P.; Ronchi, C.; et al. Survivin in adrenocortical tumors—Pathophysiological implications and therapeutic potential. Horm. Metab. Res. 2013. [Google Scholar] [CrossRef]
- Mcdermott, D.F.; Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2013. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying cancers basedon T-cell infiltration and PD-L1. Cancer Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fay, A.P.; Signoretti, S.; Callea, M.; Tel, G.H.; McKay, R.R.; Song, J.; Carvo, I.; Lampron, M.E.; Kaymakcalan, M.D.; Poli-de-Figueiredo, C.E.; et al. Programmed death ligand-1 expression in adrenocortical carcinoma: An exploratory biomarker study. J. Immunother. Cancer 2015. [Google Scholar] [CrossRef] [Green Version]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016. [Google Scholar] [CrossRef]
- Connell, C.M.; Raby, S.; Beh, I.; Flint, T.R.; Williams, E.H.; Fearon, D.T.; Jodrell, D.I.; Janowitz, T. Cancer immunotherapy trial registrations increase exponentially but chronic immunosuppressive glucocorticoid therapy may compromise outcomes. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017. [Google Scholar] [CrossRef]
- Petr, E.J.; Else, T. Genetic predisposition to endocrine tumors: Diagnosis, surveillance and challenges in care. Semin. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.M.; Sousa, L.G.; Braghiroli, M.I.; Siqueira, L.T.; Neto, J.E.B.; Chapchap, P.; De Oliveira Hoff, A.A.; Hoff, P.M. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden Two case reports. Medicine 2018. [Google Scholar] [CrossRef]
- Altieri, B.; Ronchi, C.L.; Kroiss, M.; Fassnacht, M. Next-generation therapies for adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101434. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Vatrano, S.; Volante, M.; Duregon, E.; Giorcelli, J.; Izzo, S.; Rapa, I.; Votta, A.; Germano, A.; Scagliotti, G.; Berruti, A.; et al. Detailed genomic characterization identifies high heterogeneity and histotype-specific genomic profiles in adrenocortical carcinomas. Mod. Pathol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Gaujoux, S.; Grabar, S.; Fassnacht, M.; Ragazzon, B.; Launay, P.; Libé, R.; Chokri, I.; Audebourg, A.; Royer, B.; Sbiera, S.; et al. β-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin. Cancer Res. 2011. [Google Scholar] [CrossRef] [Green Version]
- Bertherat, J.; Groussin, L.; Sandrini, F.; Matyakhina, L.; Bei, T.; Stergiopoulos, S.; Papageorgiou, T.; Bourdeau, I.; Kirschner, L.S.; Vincent-Dejean, C.; et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase a expression and activity. Cancer Res. 2003, 63, 5308–5319. [Google Scholar] [PubMed]
- Beuschlein, F.; Fassnacht, M.; Assié, G.; Calebiro, D.; Stratakis, C.A.; Osswald, A.; Ronchi, C.L.; Wieland, T.; Sbiera, S.; Faucz, F.R.; et al. Constitutive Activation of PKA Catalytic Subunit in Adrenal Cushing’s Syndrome. N. Engl. J. Med. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.C.; Chintakuntlawar, A.V.; Hilger, C.; Bancos, I.; Morris, J.C.; Ryder, M.; Smith, C.Y.; Jenkins, S.M.; Bible, K.C. Salvage Therapy With Multikinase Inhibitors and Immunotherapy in Advanced Adrenal Cortical Carcinoma. J. Endocr. Soc. 2020. [Google Scholar] [CrossRef]
- Head, L.; Kiseljak-Vassiliades, K.; Clark, T.J.; Somerset, H.; King, J.; Raeburn, C.; Albuja-Cruz, M.; Weyant, M.; Cleveland, J.; Wierman, M.E.; et al. Response to Immunotherapy in Combination with Mitotane in Patients with Metastatic Adrenocortical Cancer. J. Endocr. Soc. 2019. [Google Scholar] [CrossRef]
- Bedrose, S.; Miller, K.C.; Altameemi, L.; Ali, M.S.; Nassar, S.; Garg, N.; Daher, M.; Eaton, K.D.; Yorio, J.T.; Daniel, D.B.; et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J. Immunother. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [Green Version]
- Rotte, A.; Jin, J.Y.; Lemaire, V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann. Oncol. 2018. [Google Scholar] [CrossRef] [Green Version]
- McGregor, B.A.; McKay, R.R.; Braun, D.A.; Werner, L.; Gray, K.; Flaifel, A.; Signoretti, S.; Hirsch, M.S.; Steinharter, J.A.; Bakouny, Z.; et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Molina-Cerrillo, J.; Alonso-Gordoa, T.; Gajate, P.; Grande, E. Bruton’s tyrosine kinase (BTK) as a promising target in solid tumors. Cancer Treat. Rev. 2017. [Google Scholar] [CrossRef]
- Chifu, I.; Heinze, B.; Fuss, C.T.; Lang, K.; Kroiss, M.; Kircher, S.; Ronchi, C.L.; Altieri, B.; Schirbel, A.; Fassnacht, M.; et al. Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma. Front. Endocrinol. 2020. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Labbé, C.; Doherty, M.K.; Young, K.; Albaba, H.; Leighl, N.B. Monitoring and Management of Immune-Related Adverse Events Associated With Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, B.L.; Bailey, E.; Gill, D.M.; Agarwal, N. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front. Oncol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Barquín-García, A.; Molina-Cerrillo, J.; Garrido, P.; Garcia-Palos, D.; Carrato, A.; Alonso-Gordoa, T. New oncologic emergencies: What is there to know about inmunotherapy and its potential side effects? Eur. J. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Villadolid, J.; Amin, A. Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl. Lung Cancer Res. 2015. [Google Scholar] [CrossRef]
- Elia, G.; Ferrari, S.M.; Galdiero, M.R.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Varricchi, G.; Fallahi, P.; Antonelli, A. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract. Res. Clin. Endocrinol. Metab. 2020. [Google Scholar] [CrossRef] [PubMed]
- Spiers, L.; Coupe, N.; Payne, M. Toxicities associated with checkpoint inhibitors-An overview. Rheumatology 2019, 58, vii7–vii16. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousin, S.; Seneschal, J.; Italiano, A. Toxicity profiles of immunotherapy. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018. [Google Scholar] [CrossRef]
- Kronfol, Z.; Starkman, M.; Schteingart, D.E.; Singh, V.; Zhang, Q.; Hill, E. Immune regulation in Cushing’s syndrome: Relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology 1996. [Google Scholar] [CrossRef]
- Landwehr, L.S.; Altieri, B.; Schreiner, J.; Sbiera, I.; Weigand, I.; Kroiss, M.; Fassnacht, M.; Sbiera, S. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J. Immunother. Cancer 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Castro, M.; Pascual-Corrales, E.; Molina-Cerrillo, J.; Alonso-Gordoa, T. Immunotherapy in Adrenocortical Carcinoma: Predictors of Response, Efficacy, Safety, and Mechanisms of Resistance. Biomedicines 2021, 9, 304. https://doi.org/10.3390/biomedicines9030304
Araujo-Castro M, Pascual-Corrales E, Molina-Cerrillo J, Alonso-Gordoa T. Immunotherapy in Adrenocortical Carcinoma: Predictors of Response, Efficacy, Safety, and Mechanisms of Resistance. Biomedicines. 2021; 9(3):304. https://doi.org/10.3390/biomedicines9030304
Chicago/Turabian StyleAraujo-Castro, Marta, Eider Pascual-Corrales, Javier Molina-Cerrillo, and Teresa Alonso-Gordoa. 2021. "Immunotherapy in Adrenocortical Carcinoma: Predictors of Response, Efficacy, Safety, and Mechanisms of Resistance" Biomedicines 9, no. 3: 304. https://doi.org/10.3390/biomedicines9030304







